Abstract
Sympathetic ophthalmia is a rare bilateral diffuse granulomatous panuveitis that usually results from surgical or penetrating trauma to one eye. The symptoms range from impaired near vision to pain, photophobia, and loss of visual acuity. Anterior segment manifestations include bilateral acute uveitis with mutton-fat keratic precipitates and posterior segment findings include vitritis, multifocal neurosensory retinal detachment, choroiditis, optic nerve edema, and Dalen–Fuchs nodules. The diagnosis is clinical. Ancillary investigations include fundus fluorescein angiography, indocyanine green angiography, optical coherence tomography (OCT), ultrasound B scan, and autofluorescence imaging. The management options include corticosteroids (topical and systemic) as the first line along with immunomodulatory therapy started at the presentation of the disease. Recent advances include imaging with OCT-angiography, enhanced depth imaging-OCT (EDI-OCT, choroidal vascular index/CVI), targeting IL-23/IL-17 pathway, and use of biologics for the management of this rare entity. Recent advances in early diagnosis and prompt treatment has led to improved final visual outcomes in both the sympathizing and exciting eye. This review is aimed at giving a comprehensive overview of sympathetic ophthalmia along with a special emphasis on current treatment strategies and recent advances.
Introduction
Sympathetic ophthalmia (SO) also known as “sympathetic ophthalmitis” or “sympathetic uveitis” is a rare inflammatory condition caused by a delayed hypersensitivity reaction. It results in bilateral diffuse granulomatous panuveitis, which usually occurs following surgical or penetrating trauma to one eye with uveal tissue incarceration. The traumatized or operated eye is called the exciting or inciting eye, whereas the non-injured contralateral eye is called the sympathizing eye.Citation1 The time interval between ocular trauma to the development of symptoms is unpredictable and may vary from 5 daysCitation2 to even several (up to 66) yearsCitation3 following injury; however, 80% of the cases of SO develop within 3 months and 90% of the cases present within 1 year of trauma to the exciting eye.Citation4 Most cases were seen in childhood and young adults owing to the higher incidence of accidental injuries in previously published reports.Citation5 The increased incidence in the age group of 60–70 years may be due to a higher incidence of surgical procedures.Citation4 Though a rare condition, SO is one of the most dreadful ophthalmic conditions, which can cause bilateral blindness.Citation6
History
The concept of SO dates back to the writings of Hippocrates.Citation5 The clinical description was first given by William Mackenzie in 1840Citation7 who also coined the term SO. He hypothesized that SO was an inflammatory process that spreads from the exciting to the sympathizing eye via the optic nerve and optic chiasma. The histopathologic description of the disease was given by Ernst FuchsCitation8 who described it as a mixed-cell inflammatory infiltration of the uveal tract, particularly affecting the choroid, and then along with Dalen, he described the inflammatory nodular aggregates as Dalen–Fuchs (DF) nodules.Citation9
Epidemiology
It has been proposed that SO is a hypersensitivity reaction triggered by melanin.Citation10 Correlation of uveal injury to SO was further confirmed by the presence of anti-uveal antibodies in many patients, suggesting the pathogenic role of uvea-retina.Citation11 However, the most convincing theory is the cell-mediated immune response to antigens (type 4 hypersensitivity reaction) from the retinal photoreceptor layer.Citation12 The exact target antigen in SO is still being investigated, but skin immunization in experimental animals with various antigens including retinal antigens (retinal S-antigen, now identified as arrestin; interphotoreceptor retinoid-binding protein, rhodopsin), melanocyte-associated tyrosinase, and retinal pigment epithelium (RPE)-associated antigens result in autoimmune uveitis very similar or indistinguishable from SO.Citation11,Citation13–Citation16
The commonest causes of SO include penetrating injury to the eye from trauma or surgery (). Vitreoretinal surgery (pars plana vitrectomy/PPV),Citation17 in particular, is becoming one of the most prominent causes of SO in current practice and some authors suggest that risk of SO should be discussed before considering such interventions as the estimated risk of SO after PPV may be as high as 1 in 799.Citation18 However, in a large series of SO from India, SO was noted to occur in 0.038% of all cases undergoing PPV.Citation19
Table 1 Triggering Events in SO Patients
Penetrating surgical interventions causing SO include cataract surgery,Citation20 intravitreal injection,Citation21 iridencleisis,Citation22 and iris/uveal inclusion in the wound.
Corneal infections by various organisms including acanthamoeba,Citation23 and fungus (Aspergillus fumigatus, leading to corneal perforation and evisceration)Citation24 can predispose to SO.
Few cases of SO have also been reported following closed globe injuries or blunt trauma,Citation25–Citation27 intraocular foreign body,Citation28 chemical injuries,Citation29 and even thermal eye injuries.Citation30 Various surgical procedures can cause SO, including transscleral cyclophotocoagulation,Citation31 scleral buckling,Citation32 proton beam radiation,Citation33 helium ion therapy for choroidal melanoma,Citation34 ruthenium plaque for iris melanoma,Citation35 enucleation,Citation36 penetrating keratoplasty,Citation37 conjunctival flap procedure for perforated corneal ulcer,Citation38 phakic intraocular lens implantation,Citation39 evisceration,Citation40 iridectomy,Citation41 trabeculectomy,Citation42 repair of cyclodialysis cleft,Citation43 and paracentesis.Citation44
Gomi et alCitation45 suggested the incidence of SO to be approximately 0.3% of all uveitis cases. The overall incidence of SO may be up to 0.2% to 1%Citation46 after penetrating ocular trauma and 0.01% after intraocular surgery.Citation46 Liddy and Stuart reported the occurrence of SO in 0.2% of nonsurgical woundsCitation47 of which 0.19% were following penetrating injuries and 0.007% were following intraocular surgery, whereas Holland reported SO in 0.5% of the eyes with trauma.Citation48 Gass et al, reported the prevalence of SO following routine pars plana vitrectomy to be 0.01% but prevalence increased to 0.06% when vitrectomy was performed for penetrating ocular trauma.Citation49 The prevalence of post-traumatic SO is higher in males, with men being affected 1.8 times more than women.Citation50 This is due to a higher incidence of accidental trauma and penetrating ocular injuries in men attributable to occupational hazards and more outdoor activities. However, the ratio for post-surgical SO is equal for males as well as females, and the incidence of post-surgical SO is increasing recently.Citation51 Castiblanco et alCitation52 found that 44% of the cases were contributed by post-surgical trauma of which 21% occurred after pars plana vitrectomy. A common link is the presence of a penetrating injury in which wound healing is complicated by incarceration of the iris, ciliary body, or choroid.Citation4
Pathophysiology
The exact cause of SO is unknown. The predominant predisposing factors such as accidental penetrating trauma and penetrating surgical trauma contribute to 60–70% and 30% of the cases, respectively.Citation53 Although it has been believed in the past that the SO was aided by infectious etiology, it has been proven that most cases of SO develop in the absence of intraocular infection.Citation1
MarakCitation46,Citation54 and Wong et alCitation11 demonstrated that patients with SO have lymphocytes that are sensitized to some components of uveal-retinal antigen. Genetic factors play an important role in the development of SO. Proposed Human Leukocyte Antigen (HLA) associations include HLA-A11, HLA-B40, HLA-DR4/DRw53, and HLA-DR4/DQw3 haplotypes.Citation55,Citation56 A significant correlation exists between HLA-DRB1*4 and HLA-DQB1*04 and the development of SO.Citation57–Citation59
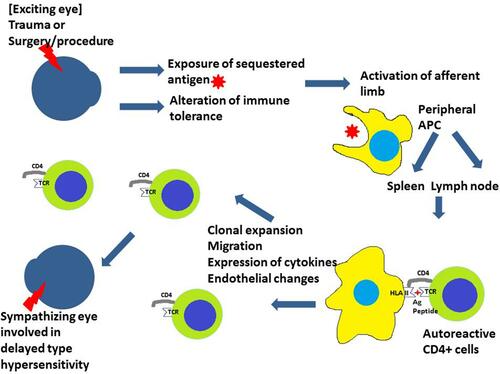
It is thought that the penetrating trauma or surgery activates the afferent phase of an autoimmune reaction. This may be due to the exposure of sequestered ocular antigens to the immune system after trauma or an alteration of the immune tolerance to ocular antigens ().Citation60 The eye does not have a lymphatic system. The antigen-presenting cells (APCs, usually macrophage or dendritic cells) process the antigen and present the antigen peptide to abnormal (autoreactive) CD4+ T cells at lymph nodes or spleen via HLA II molecules. The antigen peptide is recognized by the T cell receptor (TCR) and this leads to the activation of CD4+ cells (mainly Th1 cells). Clonal expansion of Th1 cell happens and these cells migrate. There are endothelial changes, change in cytokine milieu, and activation of multiple effector pathways causing damage to the sympathizing eye.Citation61
Figure 1 After the inciting event, the antigen-presenting cells (APC) present the antigen peptide to autoreactive CD4+ cells. This activates the CD4+ cells and brings about the autoimmune reaction damaging the other (sympathizing) eye.
Immunopathology
The immunopathological alterations consist of a diffuse granulomatous inflammation of the uveal tract, made up of lymphocytes, plasma cells, and nests of epithelioid histiocytes.Citation1,Citation11 Pigment is often present within these epithelioid cells and also within the giant cells.Citation62 The inflammatory process does not involve the choriocapillaris or the retina in histopathology.Citation63,Citation64 Rao showed that although SO and Vogt–Koyanagi–Harada (VKH) syndrome are different entities, these conditions share similar clinical features, immunohistochemistry, histology, HLA associations, and T cell response to different antigens in vitro.Citation64 The sparing of choriocapillaris in both these diseases was hypothesized to be due to anti-inflammatory factors (including transforming growth factor-beta) released from the RPE.Citation64 However, in chronic recurrent VKH syndrome, granulomatous inflammation is noted and the choriocapillaris is involved.Citation65
The absence of necrosis is another characteristic feature.Citation4 The choroid is the main seat of activity, while the retina is mostly spared.Citation4 Dalen–Fuchs (DF) nodules are clinically seen as yellow-white lesions in the peripheral retina, which may extend towards the optic disc or even involve the posterior pole. Sometimes, these lesions can be confluent.Citation66
DF nodules have been reported in around 25%−35% of the cases with SO.Citation1 As DF nodules may occur early in the peripheral retina, incidence increases to 41.5% if evidence of at least one peripheral DF nodule is taken into account.Citation1 Involvement of the RPE in SO may be of three different morphologies.Citation67 The first type showed focal RPE hyperplasia and aggregation. The second type or typical DF nodule is characterized by intact RPE overlying lymphocytes and epithelioid cells. The third type showed degeneration of RPE and disorganized DF nodule with a possible subretinal release of the contents of the DF nodule.Citation67
Initially, DF nodules have “substance” in them which gives an elevated appearance of the overlying retina, and with time atrophy ensues. DF nodules are nodular clusters of epithelioid cells containing pigment lying between the RPE and Bruch’s membrane.Citation68 Font et alCitation69 found that the nodules were visible as minute (130–160 µm), round, greyish-white mounds elevating the RPE. Under electron microscopy, the “epithelioid” cells had round to oval nuclei with abundant, relatively lucent cytoplasm containing parallel profiles of rough-surfaced endoplasmic reticulum, prominent Golgi lamellae, clusters of polyribosomes, and scattered mitochondria.Citation69 Under ultraviolet light, they appeared as myriad autofluorescent yellowish-orange dots consistent with lipofuscin.Citation69 This provided support to the concept that the “epithelioid” cells in DF nodules represent transformed retinal pigment epithelial cells forming a cage-like framework within the nodule.Citation70
Although the retina is spared, few enucleated eyes of SO show collections of mononuclear cells around the blood vessels and in the areas overlying the DF nodules.Citation1
The sclera is involved along the emissary veins and inflammatory exudates involve the optic nerve along with the meninges.Citation70 Along with breaks in the lens capsule, features of phacoanaphylaxis, with zonal granulomatous inflammation around the lens material may be seen as an atypical histopathological feature.Citation70
Immunohistochemical studies have revealed infiltration of predominantly T lymphocytes in the uveal tract.Citation68,Citation71 B lymphocytes are seen in long-standing cases and patients receiving corticosteroids.Citation72,Citation73 Helper (CD4+) T cells predominate early in the disease driving a T helper 1(Th1) response with the secretion of proinflammatory cytokines such as interferon-γ, interleukin 2 (IL-2), and interleukin 17 (IL-17).Citation74–Citation76 Suppressor/cytotoxic (CD8+) T cells are observed in chronic cases.Citation72,Citation73 M1 macrophages have been reported to predominate in the granulomas, with significant expression of interleukin 23 (IL-23), chemokine ligand 19 (CCL19), and C-X-C motif chemokine 11 (CXCL11).Citation74,Citation64
Tumour necrosis factor-alpha (TNF-α)-mediated mitochondrial oxidative stress in the outer retina of enucleated human globes leading to the apoptosis of photoreceptors, could be an early mechanism leading to vision loss in SO.Citation77,Citation78 However, higher levels of protective molecules like α-A-crystallin have also been detected in the outer retinal layers.Citation79 The expression of Fas and Fas ligand (FasL) is increased in eyes with SO and it has been suggested that apoptosis may play a role in limiting ocular inflammation.Citation76,Citation80
Zhong et alCitation81 described the role of the interleukin-23/interleukin-17 signalling pathway in autoinflammatory and autoimmune uveitis pathologies like SO. They showed that IL-17 targets RPE cells and disturbs their retinal barrier function, thereby promoting intraocular inflammation. They proposed that future studies focusing on drugs targeting the IL-23/IL-17 pathway may help in the improvement of diagnosis and treatment of this autoimmune uveitis entity.[81
Downregulation of microRNAs (hsa-miR-1, hsa-let-7e, hsa-miR-9, and hsa-miR182) has been associated with the expression of proinflammatory cytokines, particularly TNF-α and nuclear factor-kappa B1 (NF-κB), that are crucial to the pathogenesis of the disease.Citation82
Clinical Features
SO is described as a rare, bilateral, granulomatous uveitis that follows ocular trauma or surgical procedures to one eye (inciting eye) threatening sight in the fellow eye (sympathizing eye).
The most common event that can trigger the development of SO is penetrating eye injuries ().Citation83 The usual period between inciting event and SO is 2 weeks and 3 months, but cases have been described as early as 5 days and as late as 66 years after the initial incident.Citation84,Citation85
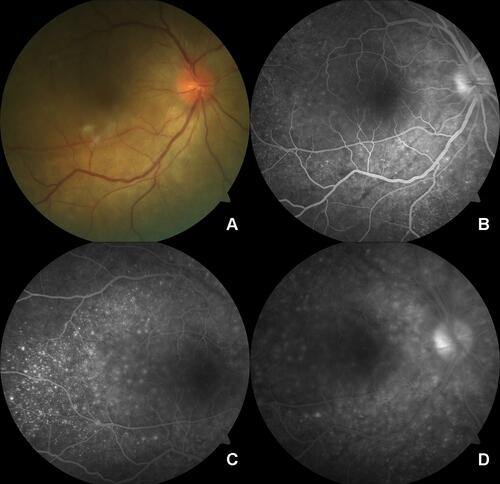
Figure 2 (A) Fundus photo of the posterior pole of the sympathizing eye shows a blurred margin of the optic disc, radial retinal folds around the optic disc, and subretinal fluid at the posterior pole. (B) FFA image at early venous phase shows disc leak, choroidal folds, and pinpoint leaks. (C) FFA image at mid-venous phase shows the typical multiple pinpoint leaks more clearly. (D) FFA image at late venous phase shows disc leak and blurred margins of pinpoint leaks.
Patients may present with symptoms like diminution of vision, ranging from mild visual disturbance to significant visual loss, pain, redness, photophobia, photopsia, and floaters.Citation86 Due to changes in accommodation, near vision may be compromised in the early course of the disease.Citation85
Anterior segment examination often reveals bilateral acute anterior uveitis with mutton-fat keratic precipitates on the corneal endothelium.Citation66,Citation87 However, non-granulomatous reaction involving the anterior segment may also be noted in some cases of SO.Citation88 Lymphocytic infiltration of the iris may result in thickening and synechiae while variation in the intraocular pressure may be attributed to ciliary body shutdown or blockage of the trabecular meshwork.Citation66
Posterior segment examination often reveals multifocal serous retinal detachment, exudative retinal detachment, moderate-to-severe vitritis, choroiditis, thickening of the peripapillary choroid, and edema or hyperemia of the optic nerve in the acute phase ().Citation66,Citation87 Yellow-white lesions beneath the level of RPE signify DF nodules that are not specific to SO and they may not be present in 30–50% of the cases.Citation89
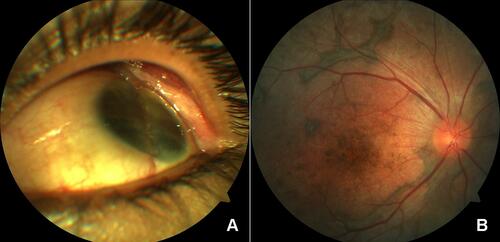
The degree of intraocular inflammation is found to be similar in the exciting eye and the sympathizing eye. In the chronic phase of the SO, the most common presentation is panuveitis or anterior uveitis with extensive chorioretinal damage. Subretinal fibrosis () and choroidal neovascularizationCitation90 may be noted in chronic cases. Depigmentation of the choroid in pigmented individuals and the appearance of multiple nummular chorioretinal scars located mostly in the periphery of the fundus are seen.Citation91,Citation92
Figure 3 (A) Anterior segment photo shows a phthisical eye with evidence of repair of globe rupture. (B) Posterior segment photo of the other eye shows depigmented fundus (sunset glow) with areas of fibrotic pigmentary changes.
The co-existing extra-ocular findings documented in some cases of SO include headache, meningismus, deafness, poliosis, vitiligo, and cerebrospinal fluid (CSF) pleocytosis.Citation89 The association of SO and hearing loss is rare but is possible as both uvea and auditory labyrinth share antigens owing to their common origin from the neural crest cells.Citation89 The spectrum of varying clinical presentations in cases of SO is summarized in .Citation52,Citation1,Citation6,Citation19,Citation20,Citation127
Table 2 Clinical Features of Patients with Sympathetic Ophthalmia (SO)
Complications that may follow the chronic phase of SO are cataract, glaucoma, choroidal atrophy, subretinal fibrosis, choroidal neovascularization (CNV), hypotony, and phthisis.Citation93
Investigations
Key criteria to consider the diagnosis of SO in the case of bilateral uveitis include
Antecedent history of unilateral ocular trauma or surgery, and
An intraocular inflammation (anterior chamber and vitreous) or a panuveitis with choroidal involvement.Citation94
The ancillary investigations work as an adjunct to support the diagnosis, to determine the severity of the disease, to assess the response to treatment during follow-up, and to detect possible secondary complications like CNV.Citation91
In patients who present only with posterior segment manifestations, the most commonly used imaging modality is fundus fluorescein angiography (FFA).Citation95 Other tests that can offer useful information include indocyanine green angiography (ICGA), optical coherence tomography (OCT), ultrasound B scan, and autofluorescence imaging.
FFA (Fundus Fluorescein Angiography)
Abnormal angiographic features seen in SO are of two distinct types: initial pinpoint hyperfluorescent leaks and multiple hypofluorescent spots with pooling of the dye in the later phases. These pinpoint leaks slowly increase in size and coalesce under focal retinal detachments. This results in multilobular pooling of the dye with staining of the subretinal fluid.Citation95 Early angiographic features of the acute phase of Vogt–Koyanagi–Harada (VKH) disease are also similar to this. The second type of angiographic feature is less common and comprises hypofluorescent foci during the early phase of angiography. These become hyperfluorescent subsequently. This pattern is identical to that seen in patients with acute posterior multifocal placoid pigment epitheliopathy (APMPPE).Citation96 In the chronic phase of SO, the nummular scars show window defects from chorioretinal adhesions, and focal RPE and inner choroidal damage. The subretinal fibrotic changes give rise to a gradual increase in hyper fluorescence and staining.Citation97 The presence of choroidal neovascularization shows leakage of the dye at the macula.
ICGA (Indocyanine Green Angiography)
ICGA is the modality of choice specifically to assess the choroid and to search for the presence of choroidal infiltrates.Citation98,Citation99 Multiple hypocyanescent spots are the common findings on ICGA in patients with SO. These spots occur due to the presence of cellular infiltration of the choroid, DF nodules, or overlying edema. Casella et alCitation100 showed that these hypocyanescent spots persist throughout the phases of ICGA. These hypocyanescent spots are noted to lessen or vanish once the treatment with corticosteroid is started and are consistent with clinical improvement.Citation99,Citation101
Autofluorescence Imaging (AF)
Autofluorescence imaging (AF) is a simple and non-invasive procedure. It is based on the detection of fluorophores denoted mainly by lipofuscin in the RPE.Citation102 The amount of lipofuscin is thought to be an indicator of the activity and viability of the RPE. AF can not only aid in the early detection of disease activity but also permits for the evaluation of the extent of damage and identification of sequelae such as choroidal neovascularization secondary to inflammation.Citation103,Citation104 Fleischman et alCitation104 described a petaloid area of hyperautofluorescence centered on the optic nerve corresponding to areas of exudative retinal detachment before initiation of treatment. Following treatment, speckled areas of hyper- and hypoautofluorescence similar to leopard spots were seen in the previous areas of exudative detachment.Citation104 However, owing to limited studies reporting the use of AF in SO, further studies are necessary to establish the pattern of AF in posterior uveitis, especially that of SO.Citation104,Citation105
Ultrasound B-Scan
B-scan ultrasonography in SO characteristically demonstrates diffuse choroidal thickening (60% of the cases) and the presence of serous retinal detachment at the posterior pole.Citation106 In the presence of posterior synechiae or dense cataracts where the retinal examination is not possible, B-scan ultrasonography can provide vital information towards the diagnosis.Citation107
Optical Coherence Tomography
In time-domain optical coherence tomography (TD-OCT), DF nodules appear as “discrete nodules at the RPE level with shallow serous detachments of the neurosensory retina overlying some of these areas”.Citation108 A hyperreflectivity of the overlying photoreceptor layer may denote inflammation of the overlying retina as has been demonstrated in histological studies.Citation108
Spectral-domain optical coherence tomography (SD-OCT) with or without enhanced depth imaging (EDI-OCT) and swept-source optical coherence tomography (SS-OCT) are non-invasive imaging modalities. These are widely used in the diagnosis and the management of several retinal diseases and uveitides. Multiple serous detachments of the neurosensory retina can be documented using OCT. The distinctive feature in SO and VKH is the presence of hyperreflective septa crossing dividing the fluid into pockets, also called bacillary layer detachment.Citation109
Bacillary layer detachment is a newly described SD-OCT feature that denotes an intraretinal split at the photoreceptor inner segment myoid. Polyak SL described the bacillary layer in 1941 as the combination of inner segment and outer segment of photoreceptors.Citation109 Mehta et al proposed the term presumed bacillary layer detachment in SD-OCT to describe an intraretinal split at the level of myoid of photoreceptor inner segment, which separates the cell body from the outer segment and ellipsoid zone.Citation109
Other SD-OCT features of SO include RPE folds and fluctuations of the internal limiting membrane.
EDI-OCT helps in demonstrating the presence of choroidal thickening, subretinal choroidal folds, and the loss of the physiologic choroidal vascular pattern. Photoreceptors’ outer segments seem elongated as a result of the photoreceptors’ disconnection from the RPE and the resultant interruption in the shedding of the outer segments.Citation107
SD-OCT shows “multiple hyperreflective lesions at the level of the RPE with associated disruption of the photoreceptor inner segment/outer segment (IS/OS) junction” at the location of DF nodules at the active stage.Citation110 On the resolution of the inflammation, disruptions of the IS/OS junction are noted with disruption or disturbance of the RPE.Citation110
Rogaczewska et alCitation111 noted a minimal disintegration of the RPE, the interdigitation zone (IZ), and the ellipsoid zone (EZ) before the appearance of any obvious signs of uveitis in SO. Agrawal et alCitation112 described choroidal structural changes in SO on swept-source optical coherence tomography (SS-OCT). They showed that choroidal thickness (CT) and choroidal vascularity index (CVI) were significantly increased in SO, representing a novel non-invasive biomarker of disease activity that can be potentially explored in the diagnosis and follow-up of patients with SO.
Optical Coherence Tomography Angiography
Optical coherence tomography angiography (OCTA) is used to non-invasively study the retinochoroidal vasculature without the need for intravenous dye. It is used to detect multiple small foci of choriocapillaris flow-void that correlate with ICGA findings.Citation113 These areas of flow void in choriocapillaris can be used to monitor the treatment response to therapy as these resolve following successful therapy.Citation114
OCTA has also been stated to be more sensitive than conventional imaging techniques like fluorescein angiography and OCT in denoting choroidal neovascularization.Citation115 . Brar et alCitation114 reported the treatment response in SO as assessed by widefield OCT angiography. They highlighted that OCTA can detect possible ischemic changes within the choriocapillaris in SO and demonstrated that these can be used as an anatomic marker to monitor treatment response.Citation114
Differential Diagnosis
Vogt–Koyanagi–Harada Syndrome
Vogt–Koyanagi–Harada syndrome is a bilateral granulomatous panuveitis. It is most commonly seen in between the second and fifth decadeCitation116 with a racial predisposition towards Asians.Citation116 It is often associated with a prodrome of meningeal and auditory symptoms.Citation4 In acute as well as chronic phases, the clinical and histopathologic features of the disease are identical to SO, except the absence of a history of trauma. However, some findings such as vitiligo and alopecia,Citation116 and cerebrospinal fluid (CSF) pleocytosisCitation117 are more closely associated with Vogt–Koyanagi–Harada syndrome than with SO. History of penetrating trauma is helpful in differentiating between SO and VKH syndrome.Citation4,Citation65
Ocular Sarcoidosis, Tuberculosis (TB), Syphilis, Multifocal Choroiditis, and Panuveitis
Sympathetic ophthalmia must be differentiated from several infectious and non-infectious uveitides, including syphilis, tuberculosis, sarcoidosis, multifocal choroiditis, and panuveitis. Other bacterial and fungal infections can also produce granulomatous anterior and/or posterior uveitis. They are usually differentiated from each other by history and associated clinical findings.Citation4 Tuberculosis associated with uveitis is commonly observed in Southeast Asian, Western Pacific, and Eastern Mediterranean regions.Citation118–Citation120 Idiopathic multifocal choroiditis is mostly seen in Caucasians,Citation121 during the second and sixth decades of life.Citation122,Citation123
Phacoanaphylactic Uveitis
It is the immunological response to lens proteins due to a rupture (surgical or traumatic) of the lens capsule as severe granulomatous uveitis. It can simulate the clinical picture of sympathetic ophthalmia, and these two entities may even coexist in the same eye. Although phacoanaphylactic endophthalmitis is mostly unilateral, there have also been rare reports of bilateral cases.Citation124 In contrast to SO, in bilateral phacoanaphylaxis, the timing of inflammation in each eye is different and choroidal thickening is minimal even in very severe forms of phacoanaphylactic endophthalmitis.Citation125 Evidence of rupture of lens capsule helps in clinching the diagnosis and should be carefully looked for. Lens extraction is the mainstay of management in phacoanaphylactic uveitis.Citation125
Treatment
Medical Treatment
A retrospective study conducted by the National Eye institute in 1995 showed good visual outcomes following SO when early treatment with high doses of steroids and immunosuppressive agents was instituted.Citation126
Corticosteroids
Although corticosteroids are the mainstay of treatment of SO, they may not prevent the occurrence of SO when given prophylactically.Citation52 It is recommended to start additional immunomodulator therapy at the initial presentation itself.Citation52 An initial high dose of oral prednisone is used with a dose ranging from 1 mg/kg/day to 2 mg/kg/day.Citation127 Intravenous (IV) pulse steroid therapy with methylprednisolone at 1g/day for 3 days has been used for severe cases.Citation6 Pulse dexamethasone therapyCitation127 (100 mg dexamethasone in 250 mL of 5% dextrose per day for 3 days) is a cheaper and safer alternative to pulse intravenous methylprednisolone.Citation128 In severe cases, intravenous pulse steroid is used daily for 3 days, followed by the use of high-dose oral steroids from the fourth day. Oral proton pump inhibitors (including omeprazole or pantoprazole or rabeprazole) and calcium supplements should also be started from the first day.
The anterior chamber reaction needs to be treated with topical steroids and cycloplegics according to the severity. Response to therapy is clinically monitored by visual acuity, anterior chamber cells, media haze/vitritis, optic disc hyperemia, choroiditis, and subretinal fluid. Optical coherence tomography helps in monitoring by objectively documenting subretinal fluid, bacillary layer detachment, the convex appearance of the RPE, and choroidal thickness. Once the inflammation is adequately controlled, very gradual tapering of steroids over 3–6 months is started, with a close watch on clinical features and OCT.Citation127 SO is a chronic disease with a very high chance of recurrence on reducing steroids/immunomodulators. Thus, the aim is to maintain remission either on a low daily dose of steroids (maintenance dose of oral prednisolone of ≤10mg daily), or by using a low daily dose of steroids in combination with immunomodulators, or only immunomodulator/s. If the disease recurs, the acute phase needs to be managed by increasing the oral dose of prednisolone (depending on the severity of recurrence, the dose may be generally adjusted to 0.5–1mg/kg).
Foster et al have proposed that for most cases of uveitis if a patient has been in remission for at least 2 years on immunomodulator/s without steroids, weaning the patient of immunomodulatory therapy should be tried in a very gradual process.Citation129 Such patients may then go into long-term remission. However, some cases of SO may be an exception to this and would require lifelong therapy with immunomodulators to prevent relapses.
Complications of systemic steroid therapy include worsening of diabetes mellitus, adrenal insufficiency, obesity, susceptibility to infections, aseptic necrosis of the hip, and osteoporosis.Citation130
The intravitreal route helps in reducing the systemic doses of steroids and other immunosuppressive agents necessary to maintain remission.Citation131 The intravitreal approach lessens some of the steroid-induced systemic side effects and enables the delivery of high concentrations of drug directly at the site of illness.Citation132 Reduction of intraocular inflammation, improvement in the visual field, visual acuity, and lessening the doses of systemic therapy have been reported with the use of IVTA (intravitreal triamcinolone acetonide).Citation133 Mahajan et alCitation134 described eight patients treated with an implantable fluocinolone acetonide implant device (Retisert, Bausch & Lomb, Rochester, NY). Potential risks associated with the use of intravitreal steroid injections include cataract formation, secondary glaucoma, retinal detachment, retinal tears, and endophthalmitis.Citation66
Meira et alCitation135 showed that 0.19 mg fluocinolone acetonide intravitreal implant was effective in controlling recurrent non-infectious uveitic macular edema in a patient with 'presumptive diagnosis of idiopathic panuveitis and sympathethic ophthalmia'. They also suggested that sustained control of inflammation enables better control of macular edema, and benefits of intraocular implant may persist even after cessation of the direct anti-inflammatory effect.
Wocker et alCitation136 demonstrated the effective use of intravitreal dexamethasone implant in cystoid macular edema cases secondary to SO.
MansourCitation137 reported the use of multiple dexamethasone implants (Ozurdex, Allergan Inc, Irvine, CA) as sole therapy in a 46-year-old woman who denied systemic immunosuppression. The intraocular pressure was controlled medically. This is an exclusive report of SO treated merely with slow-release dexamethasone implants without systemic therapies. However, this is not the accepted standard therapy till now.Citation137
Immunomodulators
Earlier indications of immunomodulators in SO included the cases where there is a contraindication for steroid use, the occurrence of significant steroid-induced side effects, flare-ups on tapering or stopping of steroid therapy, or cases who do not respond adequately to steroid therapy.Citation104 A large multicenter retrospective case series noted that 70.7% of 130 SO patients needed immunosuppressive therapy.Citation138
However, many authors recommend that SO is one of the few indications in which early initiation of immunomodulators should be considered.Citation19,Citation129,Citation139,Citation140 This would enable achieving an adequate effect of immunomodulators before the steroids are tapered to low levels. Owing to the significant toxicities of these agents, close monitoring is necessary preferably in consultation with a rheumatologist or internist.Citation66
Azathioprine
Azathioprine is a prodrug of 6-mercaptopurine. It inhibits purine synthesis and affects the maturation of the B-lymphocytes and T-lymphocytes. Thiopurine methyltransferase (TPMT) metabolizes tche drug, and azathioprine should be avoided in patients with low or absent TPMT activity to avoid toxicity.
Azathioprine has been used at 50–150 mg per day (1–3 mg/kg/day)Citation129 dose with regular monitoring of complete blood count. Azathioprine is very effective in the management of SOCitation141 and also has a steroid-sparing effect; hence, it can be given in case of intolerance to steroids or with decompensated diabetes.Citation142 Tan et al noted that azathioprine was used in around 80% of 130 patients with SO.Citation138
Methotrexate
Methotrexate is a folic acid analogue and prevents thymidylate production by inhibiting dihydrofolate reductase and also affects the rapidly dividing leucocytes. Anti-inflammatory effects may be related to adenosine release. The usual dose of methotrexate is 7.5–25 mg/week.Citation143 Methotrexate or azathioprine is usually used as the initial choice of immunomodulatory therapy in our practice for such cases. Methotrexate is a preferred choice for pediatric disease. The advantages of methotrexate include low cost, once-weekly dosage, efficacy, relative safety, and long experience with the drug. Side effects include gastric upset, weakness, hepatic compromise, and pneumonitis. However, it is teratogenic and must be avoided if pregnancy/conception is being planned. In our series of 14 patients with SO, 5 patients were initially started on methotrexate, and 3 patients were started with azathioprine along with systemic steroids. At the final follow-up, five patients were on methotrexate, and azathioprine was being used in one patient.Citation127 Eight percent of patients were treated with methotrexate in a large case series.Citation138
Mycophenolate Mofetil
Mycophenolate mofetil is a prodrug of mycophenolic acid and inhibits inosine monophosphate dehydrogenase resulting in inhibition of purine synthesis. It has to be taken in an empty stomach.
Mycophenolate is given 1–3 g once daily.Citation129 It can be used to treat refractory panuveitis or posterior uveitis unresponsive to high-dose maintenance steroidsCitation144 with fewer/manageable side effectsCitation145 even when other immunosuppressive drugs fail or are not well tolerated.Citation146
Cyclosporine
Cyclosporine is a T-cell inhibitor (calcineurin inhibitor). The recommended dose for cyclosporine is 2.5–5 mg/kg/day,Citation147 tapering to a maintenance dose of 1mg/kg/day slowly over a 1–2 month period.Citation148 The drug is wboth ofell tolerated by children and adolescentsCitation149 and can give a complete response in even progressive and severe inflammation caused by sympathetic ophthalmia.Citation150 Additionally, cyclosporine may help in controlling inflammation in cases resistant to systemic steroids and cytotoxic agents.Citation151
Regular monitoring of blood pressure and renal function is important, while the patient is on cyclosporine therapy. Combination of cyclosporine and azathioprine along with steroids controls the intraocular inflammation at a lower dose of corticosteroids, thereby reducing the steroid-related side effects.Citation152 An analysis of 130 cases with SO showed that cyclosporine was used in 13% of the patients and mycophenolate was used in 12% of the cases.Citation138
Alkylating Agents
Alkylating agent like cyclophosphamide is used in the dose of 1–3 mg/kg/day.Citation153 The usual dose of chlorambucil is 2–12 mg/kg/day orally. Patel et al noted that high-dose short-term chlorambucil was effective in controlling inflammation in 16 patients with SO.Citation86,Citation154 Hence, chlorambucil is an alternative to steroids in the case of steroid-resistant sympathetic ophthalmia but has serious side effects demanding a long-term follow-up.Citation155
Biologic Therapy
The term biologic agents or biologic response modifiers includes the class of drugs that inhibit various cytokines. They were first utilized in the treatment of uveitis in the year 1990.Citation156 They are considered to play an important role in the treatment of uveitis because of their ability to provide targeted immunosuppression. Unlike the evidence of the use of biologic agents in various systemic disorders, the mainstream literature of these therapies in uveitis treatment is either in the form of uncontrolled trials or case series.Citation157
Elevated levels of TNF-α, a key pro-inflammatory cytokine, are found in serum and ocular fluids in patients with SO.Citation158 This shows credible evidence that the therapy with TNF-α antagonists may be useful for the treatment of SO.Citation159
Of the available anti-TNF-α agents, the currently recognized ones for the treatment of SO are infliximab and adalimumab. Infliximab is a mouse-human chimeric monoclonal IgG1 antibody against TNF-α, whereas adalimumab is a fully humanized monoclonal IgG1 antibody against TNF-α. Both of them bind efficiently to the soluble and transmembrane forms of TNF-α.Citation160 The usual administered dose of infliximab is 5–20 mg/kg/day and adalimumab is 40 mg once every 2 weeks.Citation161 Adalimumab has been approved by the US Food and Drug Administration in 2016 for non-infectious intermediate, posterior, and pan-uveitis.Citation162 Gupta et alCitation163 reported the successful treatment of refractory SO in a child using infliximab. The child had not responded to therapy with high-dose oral prednisone, methotrexate, cyclosporine, mycophenolate, and daclizumab. Infliximab was administered at a dosage of 10mg/kg every 4 weeks. The inflammation was controlled within 2 weeks of starting therapy. The quiescence was maintained for more than 2 years, permitting for discontinuation of all systemic treatment. Infliximab can also be used earlier in sympathetic ophthalmia when treatment with corticosteroids is to be shortened.Citation164 Soheilian et alCitation39 documented the use of adalimumab in treating a patient with refractory SO after phakic intraocular lens implantation. Kim et alCitation165 successfully used adalimumab therapy in the treatment-refractory pediatric SO after trauma. Vallet et alCitation166 demonstrated that adalimumab and infliximab are equally efficacious for the treatment of non-infectious uveitis.
Hiyama et alCitation42 demonstrated the efficacy of adalimumab in steroid or immunosuppressant refractory SO, particularly for glaucoma subjects, in whom long-term steroid therapy should be avoided. They achieved control of intraocular inflammation with the use of adalimumab and also showed that intraocular pressure (IOP) stayed within the target range for 7 months.
A combination of adalimumab and methotrexate was needed in around 70% of the patients with VKH/SO compared to around 20% of the patients with Behçet disease to control inflammation in a study by Hiyama et al.Citation167
However, as for infliximab, a nonsignificant trend towards a higher rate of serious side effects was noted, including infections, hypersensitivity reactions, autoimmune disease, and neoplasia.Citation166 Also, patients need to be tested for tuberculosis exposure before starting infliximab therapy. The study conducted by Simonini et alCitation168 showed a higher rate of long-term quiescence with the use of adalimumab compared with infliximab. They also depicted that adalimumab is less expensive, can be administered subcutaneously, and can provide more comfort for patients. This alleviates the need for hospitalization required in case of intravenous administration of infliximab.Citation168
Thus, in non-infectious uveitis entities such as SO, where TNF-α is considered to play a role in the pathogenesis, TNF-α antagonists, particularly adalimumab may be a strong contender as a first-line corticosteroid-sparing agent. Dosage and route of administration of various immunomodulatory agents including biologics used to treat sympathetic ophthalmia are mentioned in .Citation147,Citation161
Table 3 The Spectrum of Various Immunomodulatory Agents Used in the Treatment of Sympathetic Ophthalmia (SO)
Mesquida et alCitation169 reported the 24-month efficacy and safety of the interleukin-6 receptor antagonist tocilizumab (TCZ) for refractory uveitis-related macular edema (ME) in various non-infectious uveitides including SO. This study showed that TCZ was effective and had a comparable safety profile when used for the treatment of refractory uveitis-related ME.
Other Medical Therapy
Saatci et alCitation90 successfully used intravitreal aflibercept as an adjunct to systemic therapy in the case of choroidal neovascular membrane associated with SO.
Graham et alCitation170 studied apheresis therapy in the treatment of ophthalmologic diseases including SO. Apheresis affects the disease by modifying blood plasma and modulating disease-causing agents.
Surgical Treatment
In situations where the exciting eye is blind or severely traumatized, the indicated surgical options are evisceration or enucleation of the exciting eye. However, there is debate about which procedure is the most suitable and when the procedure should be carried out if at all needed. The classic dictum regarding the prevention of sympathetic ophthalmia is by enucleation of the injured eye within 2 weeks of its injury.Citation171 Lubin et alCitation1 showed that if enucleation took place within 2 weeks of SO onset then 74% of their patients had retained a visual acuity of 20/70 or better. Controversy remains regarding the benefit of enucleating the exciting eye once the disease process has started. Some studies stated that early enucleation of the exciting eye aid in improving the visual potential of the sympathizing eye.Citation1 Another review showed no benefit to the sympathizing eye from enucleation of the exciting eye at any time during the disease course.Citation63 Since in the course of the disease, the exciting eye may have better visionCitation127; therefore, enucleation should be considered only in cases where there is little or no prognosis for useful visual function.
Dogra et alCitation172 stated that surgical intervention in the inciting eye including keratoplasty, glaucoma surgery, and pars plana vitrectomy is a viable option for improving anatomic and functional outcomes in these eyes. However, the essential prerequisite for this is adequate control of inflammation with the use of oral steroids and immunosuppressive agents.
Prognosis
Galor et alCitation173 analyzed the findings of 85 patients with SO and stated in this series that about 60% of sympathizing eyes sustained a visual acuity of at least 20/50 while 75% retained a visual acuity better than 20/100. Payal and FosterCitation174 showed that with a minimum follow‑up of 2.5 years about 57.9% achieved at least 20/50 visual acuity in the sympathizing eye.
Chawla et alCitation127 demonstrated that with the initiation of treatment within 15 days of onset of symptoms of SO, they were able to achieve a visual acuity of 20/40 or better in 85.7% sympathizing eyes, and 21.4% exciting eyes maintained visual acuity of at least 20/80.
Dutta Majumdar et al found that a final visual acuity of at least 20/40 could be maintained in 70% of sympathizing eyes of 20 cases with pediatric SO.Citation175 Three exciting eyes also had a good visual outcome. Seventeen of 20 patients received immunomodulatory therapy in addition to systemic steroids.Citation175
Steroid and immunosuppressives were used in all cases in a series with SO after PPV, and significant improvement in vision was noted in 15 of 16 patients.Citation19
Yang et al noted that the final visual outcome was worse in patients with SO compared to patients with VKH.Citation88
Method of Literature Search
Search engines used included Google Scholar, PubMed, and Google books. The search phrases included sympathetic ophthalmia and sympathetic ophthalmitis. We reviewed English articles and articles in other languages were also reviewed using their English abstract or English translation.
Conclusion
All the past and recent literature on SO reinforce the importance of prompt diagnosis and treatment in achieving better visual outcomes in these patients. Immunomodulatory therapy has revolutionized the treatment of SO patients by helping them achieve remission without or on low-dose steroids.Citation127 Hence, the key to success in the management of this blinding disease is early recognition of SO, a rapid institution of therapy, and further titration of the dosage of immunomodulatory agents to minimize their side effects. Biologics may further improve upon the outcomes in selected cases of SO. Advances in imaging modalities including OCT, OCTA, and ICGA help in detecting subclinical inflammation and may guide immunosuppression leading to better visual outcomes.
Most physicians now prefer to avoid enucleation or evisceration of the exciting eye as useful vision can be maintained in some of these eyes with early treatment. SO after vitreoretinal surgery should be kept in mind as more and more such procedures are being performed. SO is increasingly being seen in the older population likely related to ocular surgical exposure. Also, both genders are now equally involved in developed countries, which should be an impetus for the development of injury prevention programs.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas: took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- Lubin JR, Albert DM, Weinstein M. Sixty-five years of sympathetic ophthalmia. A clinicopathologic review of 105 cases (1913–1978). Ophthalmology. 1980;87(2):109–121. doi:10.1016/s0161-6420(80)35270-6
- Williams AM, Shepler AM, Chu CT, Nischal KK. Sympathetic ophthalmia presenting 5 days after penetrating injury. Am J Ophthalmol Case Rep. 2020;19:100816. doi:10.1016/j.ajoc.2020.100816
- Zaharia MA, Lamarche J, Laurin M. Sympathetic uveitis 66 years after injury. Can J Ophthalmol. 1984;19(5):240–243.
- Goto H, Rao NA. Sympathetic ophthalmia and Vogt-Koyanagi-Harada syndrome. Int Ophthalmol Clin. 1990;30(4):279–285. doi:10.1097/00004397-199030040-00014
- Albert DM, Diaz-Rohena R. A historical review of sympathetic ophthalmia and its epidemiology. Surv Ophthalmol. 1989;34(1):1–14. doi:10.1016/0039-6257(89)90125-2
- Arevalo JF, Garcia RA, Al-Dhibi HA, Sanchez JG, Suarez-Tata L. Update on sympathetic ophthalmia. Middle East Afr J Ophthalmol. 2012;19(1):13–21. doi:10.4103/0974-9233.92111
- Mackenzie W. A Practical Treatise on the Diseases of the Eye. Blanchard and Lea; 1855.
- Fuchs E. Ueber sympathisierende Entzundung (nebst Bemerkungen ueber serose traumatische Iritis. Graef Arch Clin Exp. 1905;3:365–456. doi:10.1007/BF01969717
- Varghese M, Raghavendra R. Dalen Fuch’s nodules and serous retinal detachment on optical coherence tomography in sympathetic ophthalmitis. Indian J Ophthalmol. 2013;61(5):245–246. doi:10.4103/0301-4738.113320
- Elschnig A. Studien zur sympathischen ophthalmie. Graefes Arch Ophthalmol. 1910;76(3):509–546. doi:10.1007/BF01986363
- Wong VG, Anderson R, O’Brien PJ. Sympathetic ophthalmia and lymphocyte transformation. Am J Ophthalmol. 1971;72(5):960–966. doi:10.1016/0002-9394(71)91697-7
- Chan CC. Relationship between sympathetic ophthalmia, phacoanaphylatic endophthalmitis, and Vogt-Koyanagi-Harada disease. Ophthalmology. 1988;95(5):619–624. doi:10.1016/s0161-6420(88)33146-5
- Rao NA, Robin J, Hartmann D, Sweeney JA, Marak GE. The role of the penetrating wound in the development of sympathetic ophthalmia experimental observations. Arch Ophthalmol. 1983;101(1):102–104. doi:10.1001/archopht.1983.01040010104019
- Sugita S, Sagawa K, Mochizuki M, Shichijo S, Itoh K. Melanocyte lysis by cytotoxic T lymphocytes recognizing the MART-1 melanoma antigen in HLA-A2 patients with Vogt-Koyanagi-Harada disease. Int Immunol. 1996;8(5):799–803. doi:10.1093/intimm/8.5.799
- Hammer H. Cellular hypersensitivity to uveal pigment confirmed by leucocyte migration tests in sympathetic ophthalmitis and the Vogt-Koyanagi-Harada syndrome. Br J Ophthalmol. 1974;58(9):773–776. doi:10.1136/bjo.58.9.773
- Rao NA, Wong VG. Aetiology of sympathetic ophthalmitis. Trans Ophthalmol Soc U K. 1981;101((Pt 3)(3)):357–360.
- Yoon JM, Cho GE, Kang SW. A case of sympathetic ophthalmia after 23-gauge transconjunctival sutureless vitrectomy. Korean J Ophthalmol. 2015;29(3):205–207. doi:10.3341/kjo.2015.29.3.205
- Kilmartin D, Dick A, Forrester J. Sympathetic ophthalmia risk following vitrectomy: should we counsel patients? Br J Ophthalmol. 2000;84(5):448–449. doi:10.1136/bjo.84.5.448
- Tyagi M, Agarwal K, Reddy Pappuru RR, et al. Sympathetic ophthalmia after vitreoretinal surgeries: incidence, clinical presentations and outcomes of a rare disease. Semin Ophthalmol. 2019;34(3):157–162. doi:10.1080/08820538.2019.1610464
- Jennings T, Tessler HH. Twenty cases of sympathetic ophthalmia. Br J Ophthalmol. 1989;73(2):140–145. doi:10.1136/bjo.73.2.140
- Brouzas D, Koutsandrea C, Moschos M, Papadimitriou S, Ladas I, Apostolopoulos M. Massive choroidal hemorrhage after intravitreal administration of bevacizumab (Avastin) for AMD followed by controlateral sympathetic ophthalmia. Clin Ophthalmol. 2009;3:457–459. doi:10.2147/opth.s4641
- Harris D. Sympathetic ophthalmia following iridencleisis. Case report and incidence. Am J Ophthalmol. 1961;51:829–831. doi:10.1016/0002-9394(61)91821-9
- Guerriero S, Montepara A, Ciracì L, Monno R, Cinquepalmi V, Vetrugno M. A case of sympathetic ophthalmia after a severe acanthamoeba keratitis. Eye Contact Lens. 2011;37(6):374–376. doi:10.1097/ICL.0b013e3182128e93
- Buller AJ, Doris JP, Bonshek R, Brahma AK, Jones NP. Sympathetic ophthalmia following severe fungal keratitis. Eye. 2006;20(11):1306–1307. doi:10.1038/sj.eye.6702156
- Zhang Y, Zhang M-N, Jiang C-H, Yao Y. Development of sympathetic ophthalmia following globe injury. Chin Med J. 2009;122(24):2961–2966.
- Khatri A, Timalsena S, Khatri BK, et al. A rare entity: sympathetic ophthalmia presumably after blunt trauma to the phthisical eye and optical coherence tomography angiography metrics to monitor response to treatment. Clin Case Rep. 2020;8(1):149–154. doi:10.1002/ccr3.2597
- Patil VS, Surve A, Banerjee M, Kumar V. Sympathetic ophthalmia following blunt injury to phthisical eye. BMJ Case Rep. 2021;14(7):e242516. doi:10.1136/bcr-2021-242516
- Mukkamala LK, Soni N, Zarbin MA, Langer PD, Bhagat N. Posterior segment intraocular foreign bodies: a 10-year review. Oph Retina. 2017;1(4):272–277. doi:10.1016/j.oret.2017.01.007
- Shen J, Fang W, Jin X-H, Yao Y-F, Li Y-M. Sympathetic ophthalmia caused by a severe ocular chemical burn: a case report and literature review. Int J Clin Exp Med. 2015;8(2):2974–2978.
- Rahman Z, Ali S, Dutta Majumder P. Sympathetic ophthalmia following accidental burn with hot water involving the other eye. Ocul Immunol Inflamm. 2018;26(8):1225–1227. doi:10.1080/09273948.2017.1340960
- Edwards TL, McKelvie P, Walland MJ. Sympathetic ophthalmia after diode laser cyclophotocoagulation: now an issue in informed consent. Can J Ophthalmol. 2014;49(4):e102–e104. doi:10.1016/j.jcjo.2014.05.005
- Parvaresh MM, Falavarjani KG. Presumed sympathetic ophthalmia after scleral buckling surgery. Retin Cases Brief Rep. 2013;7(4):331–333. doi:10.1097/ICB.0b013e318290d70b
- Brour J, Desjardins L, Lehoang P, et al. Sympathetic ophthalmia after proton beam irradiation for choroidal melanoma. Ocul Immunol Inflamm. 2012;20(4):273–276. doi:10.3109/09273948.2012.689072
- Fries PD, Char DH, Crawford JB, Waterhouse W. Sympathetic ophthalmia complicating helium ion irradiation of a choroidal melanoma. Arch Ophthalmol. 1987;105(11):1561–1564. doi:10.1001/archopht.1987.01060110107042
- Ahmad N, Soong TK, Salvi S, Rudle PA, Rennie IG. Sympathetic ophthalmia after ruthenium plaque brachytherapy. Br J Ophthalmol. 2007;91(3):399–401. doi:10.1136/bjo.2006.102384
- Tseng VL, Matoso A, Hofmann RJ. Sympathetic ophthalmia following enucleation. Graefes Arch Clin Exp Ophthalmol. 2013;251(1):393–394. doi:10.1007/s00417-011-1866-7
- Magalhães FP, Lavinsky D, Rossi LV, Barbosa L, Moraes N. Sympathetic ophthalmia after penetrating keratoplasty: a case report evaluated by spectral-domain optical coherence tomography. Retin Cases Brief Rep. 2012;6(1):11–15. doi:10.1097/ICB.0b013e3181f7f633
- Tripathy K, Mittal K, Chawla R. Sympathetic ophthalmia following a conjunctival flap procedure for corneal perforation. BMJ Case Rep. 2016;2016. doi:10.1136/bcr-2016-214344
- Soheilian M, Jabbarpourbonyadi M, Soheilian R, Peyman GA. Bilateral uveitis after phakic intraocular lens implantation and management with adalimumab. J Cataract Refract Surg. 2012;38(6):1094–1096. doi:10.1016/j.jcrs.2012.02.026
- Green WR, Maumenee AE, Sanders TE, Smith ME. Sympathetic uveitis following evisceration. Trans Am Acad Ophthalmol Otolaryngol. 1972;76(3):625–644.
- Letson HC. A case of sympathetic ophthalmia caused by basal iridectomy. Bull Kresge Eye Inst. 1954;5(2):56–58.
- Hiyama T, Harada Y, Kiuchi Y. Effective treatment of refractory sympathetic ophthalmia with glaucoma using adalimumab. Am J Ophthalmol Case Rep. 2019;14:1–4. doi:10.1016/j.ajoc.2019.01.009
- Heydon P, Hooper C, Lawlor M, Lee A. Sympathetic ophthalmia following cyclodialysis cleft repair. Clin Exp Ophthalmol. 2019;47(6):811–813. doi:10.1111/ceo.13488
- Rao N. Sympathetic ophthalmia. In: Retina: Medical Retina. St. Louis: CV Mosby; 1989:715–721.
- Gomi CF, Makdissi FF, Yamamoto JH, Olivalves E. Estudo epidemiologico das uveites [Epidemiological study of the uveitis]. Rev Med. 1997;76:101–108. Portuguese.
- Marak GE. Recent advances in sympathetic ophthalmia. Surv Ophthalmol. 1979;24(3):141–156. doi:10.1016/0039-6257(79)90018-3
- Liddy L, Stuart J. Sympathetic ophthalmia in Canada. Can J Ophthalmol. 1972;7(2):157–159.
- Holland G. [Indication and timing of the removal of an injured eye]. Klin Monbl Augenheilkd. 1964;145:732–740. German.
- Gass JD. Sympathetic ophthalmia following vitrectomy. Am J Ophthalmol. 1982;93(5):552–558. doi:10.1016/s0002-9394(14)77368-4
- Dutta Majumder P, Anthony E, George AE, Ganesh SK, Biswas J. Postsurgical sympathetic ophthalmia: retrospective analysis of a rare entity. Int Ophthalmol. 2018;38(6):2487–2493. doi:10.1007/s10792-017-0759-0
- Wang Y, Chan C-C. Gender differences in Vogt-Koyanagi-Harada disease and sympathetic ophthalmia. J Ophthalmol. 2014;2014:157803. doi:10.1155/2014/157803
- Castiblanco CP, Adelman RA. Sympathetic ophthalmia. Graefes Arch Clin Exp Ophthalmol. 2009;247(3):289–302. doi:10.1007/s00417-008-0939-8
- Schachat AP, ed. Ryan’s Retina. 6th ed. Elsevier; 2018.
- Rao NA, Wacker WB, Marak GE. Experimental allergic uveitis: clinicopathologic features associated with varying doses of S antigen. Arch Ophthalmol. 1979;97(10):1954–1958. doi:10.1001/archopht.1979.01020020402028
- Reynard M, Shulman IA, Azen SP, Minckler DS. Histocompatibility antigens in sympathetic ophthalmia. Am J Ophthalmol. 1983;95(2):216–221. doi:10.1016/0002-9394(83)90016-8
- Azen SP, Marak GE, Minckler DS, Reynard M, Shulman I. Histocompatibility antigens in sympathetic ophthalmia. Am J Ophthalmol. 1984;98(1):117–119. doi:10.1016/0002-9394(84)90203-4
- Kilmartin DJ, Wilson D, Liversidge J, et al. Immunogenetics and clinical phenotype of sympathetic ophthalmia in British and Irish patients. Br J Ophthalmol. 2001;85(3):281–286. doi:10.1136/bjo.85.3.281
- Shindo Y, Ohno S, Usui M, et al. Immunogenetic study of sympathetic ophthalmia. Tissue Antigens. 1997;49(2):111–115. doi:10.1111/j.1399-0039.1997.tb02723.x
- Tiercy J-M, Rathinam SR, Gex-Fabry M, Baglivo E. A shared HLA-DRB1 epitope in the DR beta first domain is associated with Vogt-Koyanagi-Harada syndrome in Indian patients. Mol Vis. 2010;16:353–358.
- American Academy of Ophthalmology. 2021–2022 basic and clinical science course, section 09: uveitis and ocular inflammation. American Academy of Ophthalmology; 2021. Available from: https://search.ebscohost.com/login.aspx?direct=true&scope=site&db=nlebk&db=nlabk&AN=2939258. Accessed September 23, 2021.
- Sympathetic Ophthalmia. Immunopaedia Advancing global immunology education; December 1, 2014. Available from: https://www.immunopaedia.org.za/immunology/archive/tolerance-and-inflammation/sympathetic-ophthalmia/. Accessed September 24, 2021.
- Rao NA, Xu S, Font RL. Sympathetic ophthalmia. An immunohistochemical study of epithelioid and giant cells. Ophthalmology. 1985;92(12):1660–1662. doi:10.1016/s0161-6420(85)34087-3
- Winter FC. Sympathetic uveitis; a clinical and pathologic study of the visual result. Am J Ophthalmol. 1955;39(3):340–347. doi:10.1016/0002-9394(55)91277-0
- Rao NA. Mechanisms of inflammatory response in sympathetic ophthalmia and VKH syndrome. Eye. 1997;11(Pt 2):213–216. doi:10.1038/eye.1997.54
- Rao NA. Pathology of Vogt–Koyanagi–Harada disease. Int Ophthalmol. 2007;27(2–3):81–85. doi:10.1007/s10792-006-9029-2
- Damico FM, Kiss S, Young LH. Sympathetic ophthalmia. Semin Ophthalmol. 2005;20(3):191–197. doi:10.1080/08820530500232100
- Reynard M, Riffenburgh RS, Minckler DS. Morphological variation of Dalén-Fuchs nodules in sympathetic ophthalmia. Br J Ophthalmol. 1985;69(3):197–201. doi:10.1136/bjo.69.3.197
- Chan CC, Nussenblatt RB, Fujikawa LS, et al. Sympathetic ophthalmia. Immunopathological findings. Ophthalmology. 1986;93(5):690–695. doi:10.1016/s0161-6420(86)33694-7
- Font RL, Fine BS, Messmer E, Rowsey JF. Light and electron microscopic study of Dalén-Fuchs nodules in sympathetic ophthalmia. Ophthalmology. 1983;90(1):66–75. doi:10.1016/s0161-6420(83)34595-4
- Croxatto JO, Rao NA, McLean IW, Marak GE. Atypical histopathologic features in sympathetic ophthalmia. A study of a hundred cases. Int Ophthalmol. 1982;4(3):129–135. doi:10.1007/BF00161902
- Jakobiec FA, Marboe CC, Knowles DM, et al. Human sympathetic ophthalmia. An analysis of the inflammatory infiltrate by hybridoma-monoclonal antibodies, immunochemistry, and correlative electron microscopy. Ophthalmology. 1983;90(1):76–95. doi:10.1016/s0161-6420(83)34602-9
- Shah DN, Piacentini MA, Burnier MN, McLean IW, Nussenblatt RB, Chan CC. Inflammatory cellular kinetics in sympathetic ophthalmia a study of 29 traumatized (exciting) eyes. Ocul Immunol Inflamm. 1993;1(3):255–262. doi:10.3109/09273949309085026
- Abu El-Asrar AM, Struyf S, Van den Broeck C, et al. Expression of chemokines and gelatinase B in sympathetic ophthalmia. Eye. 2007;21(5):649–657. doi:10.1038/sj.eye.6702342
- Hooks JJ, Chan CC, Detrick B. Identification of the lymphokines, interferon-gamma and interleukin-2, in inflammatory eye diseases. Invest Ophthalmol Vis Sci. 1988;29(9):1444–1451.
- Furusato E, Shen D, Cao X, et al. Inflammatory cytokine and chemokine expression in sympathetic ophthalmia: a pilot study. Histol Histopathol. 2011;26(9):1145–1151. doi:10.14670/HH-26.1145
- Boyd SR, Young S, Lightman S. Immunopathology of the noninfectious posterior and intermediate uveitides. Surv Ophthalmol. 2001;46(3):209–233. doi:10.1016/s0039-6257(01)00275-2
- Parikh JG, Saraswathy S, Rao NA. Photoreceptor oxidative damage in sympathetic ophthalmia. Am J Ophthalmol. 2008;146(6):866–875.e2. doi:10.1016/j.ajo.2008.03.026
- Balamurugan S, Das D, Hasanreisoglu M, et al. Interleukins and cytokine biomarkers in uveitis. Indian J Ophthalmol. 2020;68(9):1750–1763. doi:10.4103/ijo.IJO_564_20
- Kase S, Meghpara BB, Ishida S, Rao NA. Expression of α-crystallin in the retina of human sympathetic ophthalmia. Mol Med Rep. 2012;5(2):395–399. doi:10.3892/mmr.2011.653
- Chan CC, Matteson DM, Li Q, Whitcup SM, Nussenblatt RB. Apoptosis in patients with posterior uveitis. Arch Ophthalmol. 1997;115(12):1559–1567. doi:10.1001/archopht.1997.01100160729010
- Zhong Z, Su G, Kijlstra A, Yang P. Activation of the interleukin-23/interleukin-17 signalling pathway in autoinflammatory and autoimmune uveitis. Prog Retin Eye Res. 2021;80:100866. doi:10.1016/j.preteyeres.2020.100866
- Kaneko Y, Wu GS, Saraswathy S, Vasconcelos-Santos DV, Rao NA. Immunopathologic processes in sympathetic ophthalmia as signified by microRNA profiling. Invest Ophthalmol Vis Sci. 2012;53(7):4197–4204. doi:10.1167/iovs.12-9465
- Colyer MH, Chun DW, Bower KS, Dick JSB, Weichel ED. Perforating globe injuries during operation Iraqi freedom. Ophthalmology. 2008;115(11):2087–2093. doi:10.1016/j.ophtha.2008.05.013
- Verhoeff FH. An effective treatment for sympathetic uveitis. Trans Am Ophthalmol Soc. 1926;24:173–191.
- Easom HA. Sympathetic ophthalmia associated with malignant melanoma. Arch Ophthalmol. 1963;70(6):786–790. doi:10.1001/archopht.1963.00960050788011
- Vote BJ, Hall A, Cairns J, Buttery R. Changing trends in sympathetic ophthalmia. Clin Experiment Ophthalmol. 2004;32(5):542–545. doi:10.1111/j.1442-9071.2004.00876.x
- Ganesh SK, Narayana KM, Biswas J. Peripapillary choroidal atrophy in sympathetic ophthalmia and management with triple-agent immunosuppression. Ocul Immunol Inflamm. 2003;11(1):61–65. doi:10.1076/ocii.11.1.61.15581
- Yang P, Liu S, Zhong Z, et al. Comparison of clinical features and visual outcome between sympathetic ophthalmia and Vogt-Koyanagi-Harada disease in Chinese patients. Ophthalmology. 2019;126(9):1297–1305. doi:10.1016/j.ophtha.2019.03.049
- Comer M, Taylor C, Chen S, Martin K, Jordan K, Meyer P. Sympathetic ophthalmia associated with high frequent deafness. Br J Ophthalmol. 2001;85(4):496. doi:10.1136/bjo.85.4.496
- Saatçi AO, Ayhan Z, Ipek ŞC, Söylev Bajin M. Intravitreal aflibercept as an adjunct to systemic therapy in a case of choroidal neovascular membrane associated with sympathetic ophthalmia. Turk J Ophthalmol. 2018;48(4):209–211. doi:10.4274/tjo.09076
- Burkholder BM, Dunn JP. Multiple serous retinal detachments seen on wide-field imaging in a patient with sympathetic ophthalmia. JAMA Ophthalmol. 2014;132(10):1220. doi:10.1001/jamaophthalmol.2014.52
- Jabs DA, Dick A, Kramer M, et al. Classification criteria for sympathetic ophthalmia. Am J Ophthalmol. 2021. doi:10.1016/j.ajo.2021.03.048
- Tripathy K, Chawla R, Temkar S, et al. Phthisis bulbi-a clinicopathological perspective. Semin Ophthalmol. 2018;33(6):788–803. doi:10.1080/08820538.2018.1477966
- Mahajan S, Invernizzi A, Agrawal R, Biswas J, Rao NA, Gupta V. Multimodal imaging in sympathetic ophthalmia. Ocul Immunol Inflamm. 2017;25(2):152–159. doi:10.1080/09273948.2016.1255339
- Gupta V, Gupta A, Dogra MR. Posterior sympathetic ophthalmia: a single centre long-term study of 40 patients from North India. Eye. 2008;22(12):1459–1464. doi:10.1038/sj.eye.6702927
- Lewis ML, Gass JDM, Spencer WH. Sympathetic uveitis after trauma and vitrectomy. Arch Ophthalmol. 1978;96(2):263–267. doi:10.1001/archopht.1978.03910050131005
- Inomata H, Rao NA. Depigmented atrophic lesions in sunset glow fundi of Vogt-Koyanagi-Harada disease. Am J Ophthalmol. 2001;131(5):607–614. doi:10.1016/S0002-9394(00)00851-5
- Bernasconi O, Auer C, Zografos L, Herbort CP. Indocyanine green angiographic findings in sympathetic ophthalmia. Graefes Arch Clin Exp Ophthalmol. 1998;236(8):635–638. doi:10.1007/s004170050134
- Saatci AO, Paşa E, Söylev MF, Koçak N, Durak I, Kaynak S. Sympathetic ophthalmia and indocyanine green angiography. Arch Ophthalmol. 2004;122(10):1568–1569. doi:10.1001/archopht.122.10.1568
- Casella AMB, Farah ME, Martins MC, Hasegawa A, Oguido APMT. Sympathetic ophthalmia - histopathological correlation with fluorescein and indocyanine green angiography: case report. Arq Bras Oftalmol. 2008;71(6):886–889. doi:10.1590/s0004-27492008000600025
- Delori FC, Dorey CK, Staurenghi G, Arend O, Goger DG, Weiter JJ. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci. 1995;36(3):718–729.
- Schmitz-Valckenberg S, Holz FG, Bird AC, Spaide RF. Fundus autofluorescence imaging. Retina. 2008;28(3):385–409. doi:10.1097/IAE.0b013e318164a907
- Samy A, Lightman S, Ismetova F, Talat L, Tomkins-Netzer O. Role of autofluorescence in inflammatory/infective diseases of the retina and choroid. J Ophthalmol. 2014;2014:1–9. doi:10.1155/2014/418193
- Fleischman D, Say EAT, Wright JD, Landers MB. Multimodality diagnostic imaging in a case of sympathetic ophthalmia. Ocul Immunol Inflamm. 2012;20(4):300–302. doi:10.3109/09273948.2012.682637
- Castiblanco C, Adelman RA. Imaging for sympathetic ophthalmia. Int Ophthalmol Clin. 2012;52(4):173–181. doi:10.1097/IIO.0b013e318265d5c7
- Forster DJ. Echographic features of the Vogt-Koyanagi-Harada syndrome. Arch Ophthalmol. 1990;108(10):1421. doi:10.1001/archopht.1990.01070120069031
- Keane PA, Sadda SR. Retinal imaging in the twenty-first century. Ophthalmology. 2014;121(12):2489–2500. doi:10.1016/j.ophtha.2014.07.054
- Correnti AJ, Read RW, Kimble JA, Morris R. Imaging of Dalen–Fuchs Nodules in a likely case of sympathetic ophthalmia by fluorescein angiography and OCT. Ophthalmic Surg Lasers Imaging. 2010. doi:10.3928/15428877-20100215-56
- Mehta N, Chong J, Tsui E, et al. Presumed foveal bacillary layer detachment in a patient with toxoplasmosis chorioretinitis and pachychoroid disease. Retin Cases Brief Rep. 2021;15(4):391–398. doi:10.1097/ICB.0000000000000817
- Muakkassa NW, Witkin AJ. Spectral-domain optical coherence tomography of sympathetic ophthalmia with Dalen-Fuchs nodules. Ophthalmic Surg Lasers Imaging Retina. 2014;45(6):610–612. doi:10.3928/23258160-20141008-01
- Rogaczewska M, Iwanik K, Stopa M. Early presentation of sympathetic ophthalmia in optical coherence tomography studies: a case report. Indian J Ophthalmol. 2020;68(9):2019–2022. doi:10.4103/ijo.IJO_2184_19
- Agrawal R, Jain M, Khan R, et al. Choroidal structural changes in sympathetic ophthalmia on swept-source optical coherence tomography. Ocul Immunol Inflamm. 2019:1–6. doi:10.1080/09273948.2019.1685110
- Aggarwal K, Agarwal A, Mahajan S, et al. The role of optical coherence tomography angiography in the diagnosis and management of acute Vogt–Koyanagi–Harada disease. Ocul Immunol Inflamm. 2018;26(1):142–153. doi:10.1080/09273948.2016.1195001
- Brar M, Sharma M, Grewal SPS, Grewal DS. Treatment response in sympathetic ophthalmia as assessed by widefield OCT angiography. Ophthalmic Surg Lasers Imaging Retina. 2018;49(9):726–730. doi:10.3928/23258160-20180831-13
- Levison AL, Baynes KM, Lowder CY, Kaiser PK, Srivastava SK. Choroidal neovascularisation on optical coherence tomography angiography in punctate inner choroidopathy and multifocal choroiditis. Br J Ophthalmol. 2017;101(5):616–622. doi:10.1136/bjophthalmol-2016-308806
- Moorthy RS, Inomata H, Rao NA. Vogt-Koyanagi-Harada syndrome. Surv Ophthalmol. 1995;39(4):265–292. doi:10.1016/s0039-6257(05)80105-5
- Ohno S, Char DH, Kimura SJ, O’Connor GR. Vogt-Koyanagi-Harada syndrome. Am J Ophthalmol. 1977;83(5):735–740. doi:10.1016/0002-9394(77)90142-8
- Singh R, Gupta V, Gupta A. Pattern of uveitis in a referral eye clinic in north India. Indian J Ophthalmol. 2004;52(2):121–125.
- Wakabayashi T, Morimura Y, Miyamoto Y, Okada AA. Changing patterns of intraocular inflammatory disease in Japan. Ocul Immunol Inflamm. 2003;11(4):277–286. doi:10.1076/ocii.11.4.277.18260
- Islam SMM, Tabbara KF. Causes of uveitis at the eye center in Saudi Arabia: a retrospective review. Ophthalmic Epidemiol. 2002;9(4):239–249. doi:10.1076/opep.9.4.239.1507
- Kedhar SR, Thorne JE, Wittenberg S, Dunn JP, Jabs DA. Multifocal choroiditis with panuveitis and punctate inner choroidopathy: comparison of clinical characteristics at presentation. Retina. 2007;27(9):1174–1179. doi:10.1097/IAE.0b013e318068de72
- Dreyer RF, Gass DJ. Multifocal choroiditis and panuveitis. A syndrome that mimics ocular histoplasmosis. Arch Ophthalmol. 1984;102(12):1776–1784. doi:10.1001/archopht.1984.01040031440019
- Morgan CM, Schatz H. Recurrent multifocal choroiditis. Ophthalmology. 1986;93(9):1138–1147. doi:10.1016/s0161-6420(86)33611-x
- Haik GM, Waugh RL, Lyda W. Sympathetic ophthalmia; similarity to bilateral endophthalmitis phacoanaphylactica; new therapeutic methods. AMA Arch Ophthalmol. 1952;47(4):437–453. doi:10.1001/archopht.1952.01700030447005
- Marak GE. Phacoanaphylactic endophthalmitis. Surv Ophthalmol. 1992;36(5):325–339. doi:10.1016/0039-6257(92)90109-7
- Chan C-C. 32 cases of sympathetic ophthalmia. Arch Ophthalmol. 1995;113(5):597. doi:10.1001/archopht.1995.01100050065032
- Chawla R, Kapoor M, Mehta A, Tripathy K, Vohra R, Venkatesh P. Sympathetic ophthalmia: experience from a tertiary care center in Northern India. J Ophthalmic Vis Res. 2018;13(4):439–446. doi:10.4103/jovr.jovr_86_17
- Sethi HS, Menon V, Sharma P, Khokhar S, Tandon R. Visual outcome after intravenous dexamethasone therapy for idiopathic optic neuritis in an Indian population: a clinical case series. Indian J Ophthalmol. 2006;54(3):177–183. doi:10.4103/0301-4738.27069
- Foster CS, Kothari S, Anesi SD, et al. The ocular immunology and Uveitis Foundation preferred practice patterns of uveitis management. Surv Ophthalmol. 2016;61(1):1–17. doi:10.1016/j.survophthal.2015.07.001
- Jonas JB, Spandau UHM. Repeated intravitreal triamcinolone acetonide for chronic sympathetic ophthalmia. Acta Ophthalmol Scand. 2006;84(3):436. doi:10.1111/j.1600-0420.2005.00634.x
- Chan RVP, Seiff BD, Lincoff HA, Coleman DJ. Rapid recovery of sympathetic ophthalmia with treatment augmented by intravitreal steroids. Retina. 2006;26(2):243–247. doi:10.1097/00006982-200602000-00029
- Ozdemir H, Karacorlu M, Karacorlu S. Intravitreal triamcinolone acetonide in sympathetic ophthalmia. Graefes Arch Clin Exp Ophthalmol. 2005;243(7):734–736. doi:10.1007/s00417-004-1064-y
- Jonas JB. Intravitreal triamcinolone acetonide for treatment of sympathetic ophthalmia. Am J Ophthalmol. 2004;137(2):367–368. doi:10.1016/S0002-9394(03)00899-7
- Mahajan VB, Gehrs KM, Goldstein DA, Fischer DH, Lopez JS, Folk JC. Management of sympathetic ophthalmia with the fluocinolone acetonide implant. Ophthalmology. 2009;116(3):552–557.e1. doi:10.1016/j.ophtha.2008.10.024
- Meira J, Madeira C, Falcão-Reis F, Figueira L. Sustained control from recurring non-infectious uveitic macular edema with 0.19 mg fluocinolone acetonide intravitreal implant – a case report. Ophthalmol Ther. 2019;8(4):635–641. doi:10.1007/s40123-019-00209-w
- Wocker L, Januschowski K. [Steroid implant in treatment of sympathetic ophthalmia: intravitreal implant of dexamethasone in cystoid macular edema in the context of sympathetic ophthalmia]. Ophthalmologe. 2019;116(4):380–383. German. doi:10.1007/s00347-018-0748-3
- Mansour AM. Dexamethasone implant as sole therapy in sympathetic ophthalmia. Case Rep Ophthalmol. 2018;9(2):257–263. doi:10.1159/000488850
- Tan XL, Seen S, Dutta Majumder P, et al. Analysis of 130 cases of sympathetic ophthalmia - a retrospective multicenter case series. Ocul Immunol Inflamm. 2019;27(8):1259–1266. doi:10.1080/09273948.2018.1517894
- Sen HN. American Academy of ophthalmology. Uveitis and Ocular Inflammation; 2021. Available from: https://search.ebscohost.com/login.aspx?direct=true&scope=site&db=nlebk&db=nlabk&AN=2939258. Accessed July 23, 2021.
- Tripathy K, Dt G, Vannadil H, Palestine A, Tsui E. Immunomodulatory Therapy (IMT) for ocular inflammation; July 23, 2021. Available from: https://eyewiki.aao.org/Immunomodulatory_Therapy_(IMT)_for_Ocular_Inflammation. Accessed October 7, 2021.
- Hrarat L, Fardeau C, Lehoang P. Immunosuppressive treatment in sympathetic ophthalmia long-term visual outcome. Acta Ophthalmol. 2014;92. doi:10.1111/j.1755-3768.2014.F091.x
- Wand K, Straub M, Lohmann CP, Mayer CS. Sympathische Ophthalmie: therapie mit steroidsparendem Immunsuppressivum Azathioprin. Ophthalmologe. 2016;113(10):867–869. doi:10.1007/s00347-016-0227-7
- Wieringa WG, Armbrust W, Legger GE, Los LI. Efficacy of high-dose methotrexate in pediatric non-infectious uveitis. Ocul Immunol Inflamm. 2019;27(8):1305–1313. doi:10.1080/09273948.2018.1529800
- Lau CH, Comer M, Lightman S. Long-term efficacy of mycophenolate mofetil in the control of severe intraocular inflammation. Clin Exp Ophthalmol. 2003;31(6):487–491. doi:10.1046/j.1442-9071.2003.00704.x
- Thorne JE, Jabs DA, Qazi FA, Nguyen QD, Kempen JH, Dunn JP. Mycophenolate mofetil therapy for inflammatory eye disease. Ophthalmology. 2005;112(8):1472–1477. doi:10.1016/j.ophtha.2005.02.020
- Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130(4):492–513. doi:10.1016/s0002-9394(00)00659-0
- Kaçmaz RO, Kempen JH, Newcomb C, et al. Cyclosporine for ocular inflammatory diseases. Ophthalmology. 2010;117(3):576–584. doi:10.1016/j.ophtha.2009.08.010
- Nussenblatt RB, Palestine AG, Chan CC, Stevens G, Mellow SD, Green SB. Randomized, double-masked study of cyclosporine compared to prednisolone in the treatment of endogenous uveitis. Am J Ophthalmol. 1991;112(2):138–146. doi:10.1016/s0002-9394(14)76692-9
- Walton RC, Nussenblatt RB, Whitcup SM. Cyclosporine therapy for severe sight-threatening uveitis in children and adolescents. Ophthalmology. 1998;105(11):2028–2034. doi:10.1016/S0161-6420(98)91120-4
- Leznoff A, Shea M, Binkley KE, Rootman DS, Rabinovitch T. Cyclosporine in the treatment of nonmicrobial inflammatory ophthalmic disease. Can J Ophthalmol. 1992;27(6):302–306.
- Nussenblatt RB, Palestine AG, Chan CC. Cyclosporin A therapy in the treatment of intraocular inflammatory disease resistant to systemic corticosteroids and cytotoxic agents. Am J Ophthalmol. 1983;96(3):275–282. doi:10.1016/s0002-9394(14)77814-6
- Hakin KN, Pearson RV, Lightman SL. Sympathetic ophthalmia: visual results with modern immunosuppressive therapy. Eye. 1992;6(Pt 5):453–455. doi:10.1038/eye.1992.95
- Pujari SS, Kempen JH, Newcomb CW, et al. Cyclophosphamide for ocular inflammatory diseases. Ophthalmology. 2010;117(2):356–365. doi:10.1016/j.ophtha.2009.06.060
- Patel SS, Dodds EM, Echandi LV, et al. Long-term, drug-free remission of sympathetic ophthalmia with high-dose, short-term chlorambucil therapy. Ophthalmology. 2014;121(2):596–602. doi:10.1016/j.ophtha.2013.09.009
- Yang CS, Liu JH. Chlorambucil therapy in sympathetic ophthalmia. Am J Ophthalmol. 1995;119(4):482–488. doi:10.1016/s0002-9394(14)71235-8
- Imrie FR, Dick AD. Biologics in the treatment of uveitis. Curr Opin Ophthalmol. 2007;18(6):481–486. doi:10.1097/ICU.0b013e3282f03d42
- Lim L, Suhler EB, Smith JR. Biologic therapies for inflammatory eye disease. Clin Exp Ophthalmol. 2006;34(4):365–374. doi:10.1111/j.1442-9071.2006.01225.x
- Palexas GN, Sussman G, Welsh NH. Ocular and systemic determination of IL-1 beta and tumour necrosis factor in a patient with ocular inflammation. Scand J Immunol Suppl. 1992;11:173–175. doi:10.1111/j.1365-3083.1992.tb01645.x
- Santos Lacomba M, Marcos Martín C, Gallardo Galera JM, et al. Aqueous humor and serum tumor necrosis factor-alpha in clinical uveitis. Ophthalmic Res. 2001;33(5):251–255. doi:10.1159/000055677
- Cochrane S, Dick A. Tumour necrosis factor-alpha-targeted therapies in uveitis. In: Pleyer U, Foster C, editors. Uveitis and Immunological Disorders. Berlin: Springer; 2007:177–192.
- Fong D, Law S, Schmidt-Erfurth U. Drugs in Ophthalmology. Germany: Springer; 2006.
- LaMattina KC, Goldstein DA. Adalimumab for the treatment of uveitis. Expert Rev Clin Immunol. 2017;13(3):181–188. doi:10.1080/1744666X.2017.1288097
- Gupta SR, Phan IT, Suhler EB. Successful treatment of refractory sympathetic ophthalmia in a child with infliximab. Arch Ophthalmol. 2011;129(2):250–252. doi:10.1001/archophthalmol.2010.358
- Ramírez ED, Guijarro González JJ, Vicuña RG. Postsurgical sympathetic ophthalmia refractory to treatment: the response to infliximab. Retin Cases Brief Rep. 2012;6(2):169–171. doi:10.1097/ICB.0b013e3182235354
- Kim J-B, Jeroudi A, Angeles-Han ST, Grossniklaus HE, Yeh S. Adalimumab for pediatric sympathetic ophthalmia. JAMA Ophthalmol. 2014;132(8):1022–1024. doi:10.1001/jamaophthalmol.2014.426
- Vallet H, Seve P, Biard L, et al. Infliximab versus adalimumab in the treatment of refractory inflammatory uveitis: a Multicenter Study from the French Uveitis Network. Arthritis Rheumatol. 2016;68(6):1522–1530. doi:10.1002/art.39667
- Hiyama T, Harada Y, Kiuchi Y. Efficacy and Safety of adalimumab therapy for the treatment of non-infectious uveitis: efficacy comparison among uveitis aetiologies. Ocul Immunol Inflamm. 2021;1–8. doi:10.1080/09273948.2020.1857791
- Simonini G, Taddio A, Cattalini M, et al. Prevention of flare recurrences in childhood-refractory chronic uveitis: an open-label comparative study of adalimumab versus infliximab. Arthritis Care Res. 2011;63(4):612–618. doi:10.1002/acr.20404
- Mesquida M, Molins B, Llorenç V, et al. Twenty-four month follow-up of tocilizumab therapy for refractory uveitis-related macular edema. Retina. 2018;38(7):1361–1370. doi:10.1097/IAE.0000000000001690
- Graham BC, Pulido JS, Winters JL. Seeing is believing: a review of apheresis therapy in the treatment of ophthalmologic disease. J Clin Apher. 2018;33(3):380–392. doi:10.1002/jca.21607
- Savar A, Andreoli MT, Kloek CE, Andreoli CM. Enucleation for open globe injury. Am J Ophthalmol. 2009;147(4):595–600.e1. doi:10.1016/j.ajo.2008.10.017
- Dogra M, Samanta R, Singh P, et al. Surgical intervention in inciting eyes of patients with sympathetic ophthalmia: a case series and review of literature. Ocul Immunol Inflamm. 2019;27(7):1154–1159. doi:10.1080/09273948.2018.1497663
- Galor A, Davis JL, Flynn HW, et al. Sympathetic ophthalmia: incidence of ocular complications and vision loss in the sympathizing eye. Am J Ophthalmol. 2009;148(5):704–710.e2. doi:10.1016/j.ajo.2009.05.033
- Payal AR, Foster CS. Long-term drug-free remission and visual outcomes in sympathetic ophthalmia. Ocul Immunol Inflamm. 2017;25(2):190–195. doi:10.3109/09273948.2015.1092557
- Dutta Majumder P, Mistry S, Sridharan S, et al. Pediatric sympathetic ophthalmia: 20 years of data from a tertiary eye center in India. J Pediatr Ophthalmol Strabismus. 2020;57(3):154–158. doi:10.3928/01913913-20200219-01

