Abstract
Background
To evaluate the efficacy and safety of rituximab-based combination therapy for Waldenström macroglobulinemia (WM), we conducted this meta-analysis by pooling the rates of overall response, major response, complete response, and grade ≥3 hematological adverse events.
Methods and materials
We searched for relevant studies in the databases of PubMed, Web of Science, Embase, and the Cochrane Library. The qualitative assessment of all the included articles was conducted with reference to the Newcastle–Ottawa Scale. A random-effects model was selected to perform all pooled analyses.
Results
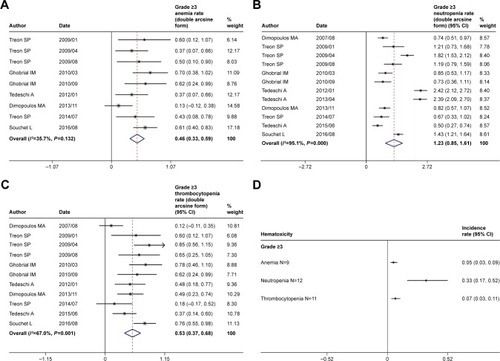
We identified altogether 22 studies with a total of 806 symptomatic WM patients enrolled. The pooled analysis indicated that the rituximab-based combination therapy achieved an overall response rate (ORR) of 84% (95% CI: 81%–87%), a major response rate (MRR) of 71% (95% CI: 66%–75%), and a complete response rate (CRR) of 7% (95% CI: 5%–10%). Rituximab plus conventional alkylating agents–containing chemotherapy (subgroup A) yielded an ORR of 86% (95% CI: 81%–89%), an MRR of 74% (95% CI: 69%–79%), and a CRR of 8% (95% CI: 4%–14%). Rituximab plus purine analog (subgroup B) resulted in an ORR of 85% (95% CI: 79%–89%), an MRR of 74% (95% CI: 66%–81%), and a CRR of 9% (95% CI: 4%–15%). Rituximab plus proteasome inhibitor (subgroup C) resulted in an ORR of 86% (95% CI: 81%–90%), an MRR of 68% (95% CI: 58%–77%), and a CRR of 7% (95% CI: 3%–11%). Rituximab plus immunomodulatory drug (subgroup D) attained relatively lower response rates, with an ORR of 67% (95% CI: 51%–81%), an MRR of 56% (95% CI: 27%–83%), and a CRR of 5% (95% CI: 1%–12%). Common grade ≥3 hematological adverse events consisted of neutropenia (33%, 95% CI: 17%–52%), thrombocytopenia (7%, 95% CI: 3%–11%), and anemia (5%, 95% CI: 3%–9%).
Conclusion
Rituximab in combination with an alkylating agent, purine analog, or proteasome inhibitor is highly effective with tolerable hematological toxicities for WM.
Keywords:
Introduction
Waldenström macroglobulinemia (WM) is a lymphoplasmacytic lymphoma (LPL) characterized by the infiltration of small B lymphocytes, lymphocytes with plasmacytoid differentiation, and plasma cells into the bone marrow and other lymphatic organs, along with a detectable serum monoclonal IgM.Citation1,Citation2 WM is a rare B-cell chronic lymphoproliferative disorder with a median survival of 5–10 years, representing ~2% of all hematological malignancies. It is more common in men and Caucasians with a median age of >60–70 years.Citation3 Indolent as it is, WM remains an incurable disease.Citation4,Citation5 Effective treatment is required for patients with symptomatic manifestations, which primarily consist of hyperviscosity, peripheral neuropathy, lymphadenopathy, hepatomegaly, splenomegaly, cytopenias, hemolytic anemia, and cryoglobulinemia.Citation6 A recurrent somatic mutation of the MYD88 gene (MYD88 Leu265Pro) has been detected iñ90% of patients with WM, which contributes to the differentiation of WM from other B-cell lymphoproliferative disorders.Citation7 CXCR4 gene somatic mutations are also found iñ40% of patients. The mutation status of MYD88 and CXCR4 genes is indicative of response to a treatment, which can serve as predictive biomarkers in personalized therapeutics.Citation8
Rituximab is a human–mouse chimeric monoclonal antibody targeting CD20 antigen, which is ubiquitously expressed on the surface of B cells. Rituximab adheres to CD20, leading to B-cell lysis mainly through antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity.Citation9 When used as a single agent, rituximab has modest anti-WM potency and increases the risk of “IgM flare” phenomenon in which most patients suffer from an obvious elevation in the serum IgM level that aggravates hyperviscosity-related complications including fatigue, blurred vision, vertigo, epistaxis, headaches, and tachypnea. Urgent plasmapheresis is required.Citation10 In comparison with rituximab monotherapy, “IgM flare” phenomenon is observed much infrequently when cytoreductive therapy with other anti-WM agents is administered before the infusion of rituximab.Citation11 Therefore, monotherapy is unsuitable, especially for patients with a high serum IgM level or a heavy tumor burden. Rituximab is the backbone of the treatment when used in combination with other agents such as proteasome inhibitors, immunomodulatory drugs, or conventional chemotherapeutic agents. However, in terms of response rate, the comparative outcomes among different combinations remain unknown. In addition, many clinical trials or retrospective studies enrolled only a small amount of patients due to the rarity of WM. We conducted this meta-analysis by pooling the response rates and hematological adverse events of different rituximab-based combinations in an attempt to provide a more comprehensive appraisal of the efficacy and safety for clinical practice.
Methods and materials
The reporting of this pooled analysis adhered to the PRISMA guidelines ().Citation12 Two investigators independently searched the databases, evaluated all potential articles, and extracted the required data (baseline characteristics and treatment outcomes) from the included articles. When confronted with discrepancies, they reached a consensus by discussion or consulting a third senior investigator, who supervised all the research procedures.
Table 1 PRISMA checklist
Literature retrieval strategy and study selection criteria
We searched for relevant studies in the databases of PubMed, Web of Science, Embase, and the Cochrane Library, with language restricted to English. The following medical subject headings terms or keywords were used in the literature retrieval: “rituximab,” “Rituxan,” “anti-CD20,” “Waldenström macroglobulinemia,” “lymphoplasmacytic lymphoma,” “lymphoplasmacytic neoplasm,” and “B cell chronic lymphoproliferative diseases.” By screening the references of all the retrieved articles, we also manually identified other potentially relevant studies to supplement our search. Multiple retrieved publications of the same study were considered as one article, and only the most recent or the most informative one was included in this meta-analysis. The latest literature search was updated on May 31, 2018.
To warrant the authenticity of this pooled analysis, all the eligible studies had to meet all the following inclusion criteria: (1) The patients were diagnosed with symptomatic WM, including relapsed or refractory, previously untreated patients; (2) the therapeutic regimen must contain rituximab; (3) treatment outcomes were presented as response rates, whether the study be a retrospective study or a prospective clinical trial; and (4) the potentially included studies must provide sufficient information about the accurate number of patients who achieved any grade response status to treatment.
Articles falling into the following categories were excluded: (1) duplicated records, experimental and basic research, clinical guidelines, reviews, commentary, case reports, conference abstracts, and studies which provided inadequate information; (2) studies in which rituximab monotherapy was the treatment schedule; (3) studies that failed to differentiate WM from LPL; and (4) studies in which the response rate to treatment was assessed according to IgM level, not by means of serum protein electrophoresis (M-spike measurement), but using other approaches, such as nephelometry.
Qualitative assessment of articles and data extraction
The qualitative assessment of all the included articles was conducted with reference to the Newcastle–Ottawa Scale (NOS) for a single-arm nonrandomized trial, a randomized controlled trial (RCT), a cohort study, or a retrospective study (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp).
The primary objective of this pooled analysis was to determine the overall response rate (ORR), which included the rate of complete response (CR: immunofixation negativity in the serum monoclonal IgM), very good partial response (VGPR: ≥90% reduction in serum IgM levels), partial response (PR: ≥50% reduction), and minor response (≥25% reduction).Citation11,Citation13 The secondary efficacy endpoints were complete response rate (CRR) and major response rate (MRR), which was defined as the sum of CR, VGPR, and PR. In terms of the safety outcome, we only discussed grade ≥3 hematological toxicity (anemia, neutropenia, and thrombocytopenia) on the basis of the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.03. Therefore, we recorded the exact number of patients who achieved CR, VGPR, PR, and minor response and suffered from grade ≥3 hematological adverse events in the predesigned table.
Statistical analysis
A random-effects model, which could provide more conservative results of treatment outcomes, was selected to perform all the pooled calculations, including ORR, MRR, CRR, and the rate of grade ≥3 hematological adverse events. The heterogeneity among studies was evaluated by means of Cochrane Q-test and was quantified using the I2 statistics. We also conducted a subgroup analysis to explore the source of heterogeneity when necessary.
Considering the rarity of WM, we estimated that many potentially included studies had a small sample size. In order to avoid the bias caused by events with very low or even zero incidence rate and the possible small sample size of some included studies, the extracted data were empirically converted into double arcsine form after Freeman–Tukey double arcsine transformation. The transformed data were then used in pooled calculations with the random-effects model. Eventually, the pooled results were transformed back to their original form and were reported.
The comparison of categorical variables between two groups was done using Pearson’s chi-squared test or Fisher’s exact test. Comparisons between multiple different subgroups were performed with partitions of Pearson’s chi-squared test by which a two-tailed P-value of less than adjusted α was considered statistically significant.
Publication bias for each synthesized calculation was evaluated using Egger’s test and Begg’s test, with P<0.05 representing that there existed a significant publication bias. All meta-analysis and publication bias tests were performed with Stata statistical software Version 12.0 (Stata Corporation, College Station, TX, USA). All the tests were two-sided with P<0.05 representing the statistical significance.
Results
Study search and characteristics
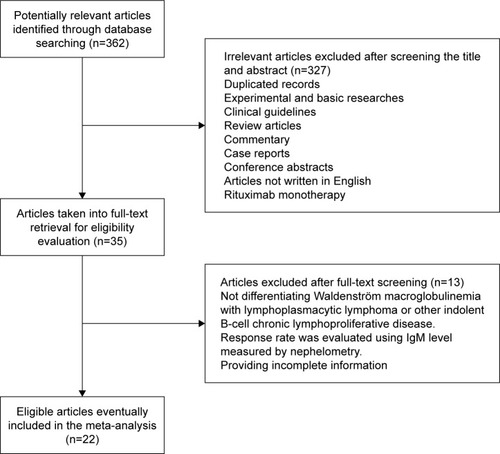
demonstrates the process by which studies were identified and selected for inclusion in our meta-analysis. We identified 362 potentially relevant articles through our initial database search. After screening the titles and abstracts, 327 articles were excluded. Among the remaining 35 articles, 13 articles were eliminated after full-text reading because they failed to provide sufficient outcome data or they did not differentiate WM from LPL. Ultimately, our meta-analysis included altogether 22 studies with a total of 806 symptomatic WM patients enrolled, among which 15 articles were single-arm phase II clinical trials, six articles were retrospective studies, and one article was a phase III RCT.Citation14–Citation35 Six of the identified studies focused on previously untreated WM patients, five of the studies enrolled pretreated patients with relapsed or refractory WM, while the rest 11 studies enrolled both. It should be noted that one included study enrolled WM patients receiving three different therapeutic regimens, and then, we considered each treatment group as an independent study to conduct the pooled analysis.Citation19
Figure 1 Selection of studies. Flow diagram demonstrating the identification and selection process of articles included in the meta-analysis.
The NOS consists of eight items, which are classified into three main factors including patient selection (four items, one star awarded for each item), comparability of the study group (one item, two stars awarded for this item), and the assessment of outcome and follow-up (three items, one star awarded for each item). The NOS score ranges from zero to nine stars, with six or more stars considered to be of high quality. With respect to the sole RCT, we merely evaluated the experimental group (rituximab-based group) with reference to the NOS. All the studies in our meta-analysis obtained at least seven stars, indicating that the quality of the included original studies was fully guaranteed. summarizes the study characteristics.
Table 2 Patient characteristics and treatment outcomes of the included studies
ORR, MRR, and CRR
To explore the source of heterogeneity, we further divided the included studies into four broad subgroups according to different therapeutic regimens. Conventional alkylating agents–containing chemotherapy group includes the cyclophosphamide + doxorubicin + vincristine + prednisone regimen, cyclophosphamide + vincristine + prednisone regimen, cyclophosphamide + dexamethasone regimen, cyclophosphamide + prednisone regimen, and bendamustine. Regimens that contain fludarabine or cladribine are categorized into purine analog group. Regimens that contain bortezomib or carfilzomib are classified into proteasome inhibitor group. Regimens that contain thalidomide or lenalidomide fall into immunomodulatory drug group.
As previously described, the extracted data were transformed into double arcsine form to be synthesized, and – demonstrate the pooled rates. Then, the pooled rates of overall response, major response, and CR were transformed back to their original form as demonstrated in .
Figure 2 Pooled overall response rate (double arcsine form) of rituximab-based combinations in patients with Waldenström macroglobulinemia. The diamond indicates the estimated overall response rate and their corresponding 95% CI (double arcsine form after Freeman–Tukey transformation).
Note: Weights are from random-effects analysis.
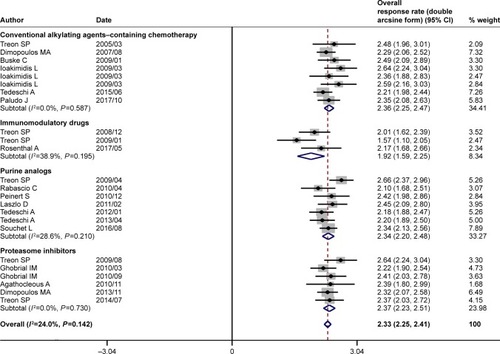
Figure 3 Pooled major response rate (double arcsine form) of rituximab-based combinations in patients with Waldenström macroglobulinemia. The diamond indicates the estimated major response rate and their corresponding 95% CI (double arcsine form after Freeman–Tukey transformation).
Note: Weights are from random-effects analysis.
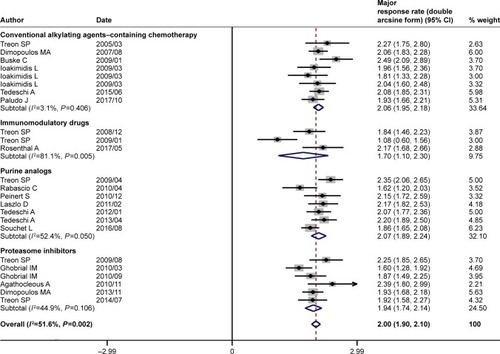
Figure 4 Pooled complete response rate (double arcsine form) of rituximab-based combinations in patients with Waldenström macroglobulinemia. The diamond indicates the estimated complete response rate and their corresponding 95% CI (double arcsine form after Freeman–Tukey transformation).
Note: Weights are from random-effects analysis.
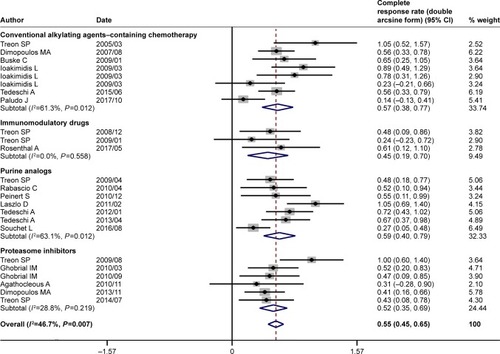
Figure 5 Summary of pooled response rate (original form) of rituximab-based combinations and different subgroups in patients with Waldenström macroglobulinemia. (A) Pooled ORR. (B) Pooled MRR. (C) Pooled CRR. (D) The percentage bar diagram for overall and subgroups A, B, C, and D for ORR, CRR, and MRR. Subgroup A represents rituximab plus conventional alkylating agents–containing chemotherapy group; subgroup B represents rituximab plus purine analog group; subgroup C represents rituximab plus proteasome inhibitor group; and subgroup D represents rituximab plus immunomodulatory drug group. *P<0.007143. The diamond indicates the estimated response rate and their corresponding 95% CI (original form).
Note: Weights are from random-effects analysis.
Abbreviations: CRR, complete response rate; MRR, major response rate; ORR, overall response rate.
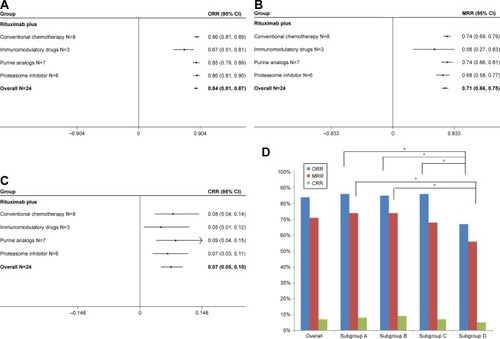
The pooled analysis indicated that rituximab-based combination therapy achieved an ORR of 84% (95% CI: 81%–87%), an MRR of 71% (95% CI: 66%–75%), and a CRR of 7% (95% CI: 5%–10%). The subgroup analysis indicated the response outcomes of rituximab plus agents with different mechanisms of action. Rituximab plus conventional alkylating agents–containing chemotherapy (subgroup A) yielded an ORR of 86% (95% CI: 81%–89%), an MRR of 74% (95% CI: 69%–79%) and a CRR of 8% (95% CI: 4%–14%). Rituximab plus purine analog (subgroup B) resulted in an ORR of 85% (95% CI: 79%–89%), an MRR of 74% (95% CI: 66%–81%), and a CRR of 9% (95% CI: 4%–15%). Rituximab plus proteasome inhibitor (subgroup C) resulted in an ORR of 86% (95% CI: 81%–90%), an MRR of 68% (95% CI: 58%–77%), and a CRR of 7% (95% CI: 3%–11%). Rituximab plus immunomodulatory drug (subgroup D) attained relatively lower response rates, with an ORR of 67% (95% CI: 51%–81%), an MRR of 56% (95% CI: 27%–83%), and a CRR of 5% (95% CI: 1%–12%). As demonstrated in , there existed no statistically significant differences in ORR, MRR, and CRR between subgroups A and B, subgroups A and C, and subgroups B and C. The response outcomes derived from subgroup A have statistical difference to those of subgroup D in ORR (86% vs 67%, P=0.001) and MRR (74% vs 56%, P=0.002). Likewise, the response outcomes derived from subgroup B have statistical difference to those of subgroup D in ORR (85% vs 67%, P=0.002) and MRR (74% vs 56%, P=0.006). The ORR was statistically different between subgroups C and D (86% vs 67%, P=0.001). All the four subgroups resulted in a roughly similar CRR.
Table 3 Comparison between different subgroups of ORR, MRR, and CRR
Hematological toxicities
We also performed the pooled analysis to explore the hematological adverse events of rituximab-based combination therapy in WM patients. Given the rare occurrence or even zero event of high-grade toxicities, Freeman–Tukey double arcsine transformation was also required for pooling hematological adverse events, as demonstrated in . Then, the pooled results were transformed back to their original form as summarized in . The most frequent grade ≥3 hematological adverse events consisted primarily of neutropenia (33%, 95% CI: 17%–52%), thrombocytopenia (7%, 95% CI: 3%–11%), and anemia (5%, 95% CI: 3%–9%).
Figure 6 Pooled rates of grade ≥3 hematological adverse events of rituximab-based combinations. Rates (double arcsine form after Freeman–Tukey transformation) of (A) anemia, (B) neutropenia, (C) thrombocytopenia, and (D) summary of grade ≥3 anemia, neutropenia, and thrombocytopenia (original form).
Note: Weights are from random-effects analysis.
Publication bias
As indicated in , there was no evident publication bias detected by Egger’s test and Begg’s test for all the meta-analysis outcomes.
Table 4 Publication bias assessment of the pooled results
Discussion
Several clinical trials examined the efficacy of rituximab monotherapy for WM, including standard rituximab mono-therapy (four weekly rituximab infusions) and an extended one (extra four weekly infusions at weeks 12–16 after standard therapy). Standard rituximab administration yielded an ORR of 40% and an MRR of 30%. The extended regimen produced an ORR of 60% and an MRR of 40%. VGPR and CR were scarcely observed in both the schedules. These studies indicated that rituximab monotherapy exhibited a moderate effect against WM.Citation36–Citation38 Rituximab monotherapy often resulted in the much more frequent occurrence of serum IgM flare, the exacerbation of symptomatic hyperviscosity, and an increased risk of IgM-related morbidities.Citation10,Citation39,Citation40 Therefore, an increasing number of studies focusing on the addition of agents with different mechanisms of action to rituximab have been conducted in an attempt to explore more effective and better-tolerated combination regimens.
To the best of our knowledge, this pooled analysis is the first one to explore the efficacy and safety of rituximab-based combination therapy in patients with symptomatic WM. We have every reason to believe that our pooled analysis can provide a comprehensive perspective and facilitate individual observation of each single study with a relatively small sample size. As previously indicated in and , rituximab-based combinations (with alkylating agents, purine analogs, proteasome inhibitors, and immunomodulatory drugs) produced an encouraging pooled ORR of 84% and an MRR of 71%, acting as the mainstay for the treatment of WM. Probably due to the indolent nature of WM, all the four different subgroups achieved low CRRs, with no statistically significant differences among each other (all P>0.05). Rituximab combined with immunomodulatory drug revealed an evidently lower ORR as compared with rituximab plus alkylating agent–containing chemotherapy (67% vs 86%, P=0.001), rituximab plus purine analog (67% vs 85%, P=0.002), and rituximab plus proteasome inhibitor (67% vs 86%, P=0.001). Meanwhile, rituximab combined with immunomodulatory drug also yielded a lower MRR as compared with rituximab plus alkylating agent–containing chemotherapy (56% vs 74%, P=0.002) and rituximab plus purine analog (56% vs 74%, P=0.006). However, the differences in ORR and MRR among the rest of the three different subgroups were statistically insignificant (all P>0.05). The result mentioned above is consistent with the fact that rituximab combined with thalidomide or lenalidomide was not recommended anymore and had been deleted from National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Waldenström’s Macroglobulinemia/Lymphoplasmacytic Lymphoma (Version 1.2018).
As for toxicities, we only pooled the rates of commonly observed grade ≥3 hematological adverse events including neutropenia (33%), thrombocytopenia (7%), and anemia (5%). We did not take nonhematological toxicity into consideration in that most of the included studies reported only a minor proportion of patients suffering from various grade ≥3 nonhematological adverse events. We did not also analyze infusion-related reactions caused by rituximab. Infusion-related reactions of rituximab were commonly observed during the first infusion, which were primarily restricted to grade ≤2 adverse events including transient dyspnea and hypertension, angioedema, bronchospasm, cough, pyrexia, chills, rash, and vomiting.Citation41 When a patient suffers from infusion-related reactions, the infusion should be slowed down or temporarily suspended. Concurrent administration of antihistamines and corticosteroids also serves as a good therapeutic or prophylactic approach.Citation42,Citation43 Besides, the number of patients who were intolerant to rituximab mainly due to infusion-related reactions was very small in most of the included studies. Ofatumumab, a fully human monoclonal anti-CD20 antibody, may be a successful substitute for rituximab to mitigate severe infusion-related reactions. IgM flare phenomenon is also commonly observed with the use of ofatumumab. Therefore, serum IgM monitoring is still required.Citation44–Citation46
Although only a small subset of patients achieved CR or VGPR in almost all the included studies, WM follows a protracted and indolent course with a median survival of 10 years. It is not uncommon to acquire significant symptomatic amelioration even with a minor response.Citation47 In other words, mere minor response may be associated with encouraging clinical benefit. Therefore, higher priority should be accorded to disease or symptom control for the treatment of WM, in which patients do not suffer from severe disease-related symptoms and “symptom-free survival” duration may prolong and last for several years despite the presence of bone marrow involvement and/or high serum IgM concentration.Citation48,Citation49 According to individual patient characteristics and clinical manifestations, therapeutic regimens that are oriented toward a sustained disease control and the prevention of end-organ damage with minimal toxicity rather than a hematological CR are strongly recommended strategies for the initial treatment of WM. The therapeutic algorithm is summarized as follows.Citation5,Citation11,Citation50–Citation54 Rituximab + bendamustine (BR) or rituximab + cyclophosphamide + dexamethasone (RCD) are primary choices for patients with WM-induced cytopenias or moderate to severe organomegaly or bulky lymphadenopathy. For patients with severe symptomatic hyperviscosity, cryoglobulinemia, or cold agglutinemia, preemptive plasmapheresis before drug administration is required, especially for those with serum IgM level of at least 4,000 mg/dL to evade IgM flare. Then, the administration of weekly subcutaneous bortezomib as a single agent is preferred before rituximab infusion (bortezomib + rituximab + dexamethasone), and thus serum IgM level may decrease instantly. Fludarabine + cyclophosphamide + rituximab regimen is also effective but of high myelotoxicity. For patients with paraprotein-related neuropathy, plasmapheresis is also needed but should not be employed as a permanent modality. RCD or FR or BR is effective, and combination regimens that contain neurotoxic agents such as bortezomib, thalidomide, and vincristine should be avoided. Carfilzomib, a second-generation proteasome inhibitor, which functions by binding irreversibly to active sites of 20S proteasome, is of low neurotoxicity. The combination of carfilzomib, rituximab, and dexamethasone (CaRD) in previously untreated patients achieved an ORR of 87% with no grade ≥3 neuropathy observed. Importantly, the response status was not influenced by MYD88 and CXCR4 gene mutations.Citation31 CaRD and bortezomib + rituximab + dexamethasone regimens are also preferably recommended in WM patients with IgM-associated light-chain amyloidosis and renal dysfunction. Bing–Neel syndrome is a rare WM complication featuring the involvement of lymphoplasmacytic cells in the central nervous system, for which an intrathecal injection is required. Combinations that contain fludarabine or bendamustine may play a critical role in Bing–Neel syndrome treatment due to their strong blood–brain barrier permeability.Citation55,Citation56
In some combination regimens, rituximab infusion is postponed until the second cycle so as to bring the effect of cytotoxic therapy into full play, thus decreasing IgM level and minimizing the risk of rituximab-induced hyperviscosity syndrome. Although the RCD regimen was effective with minimal myelotoxicity, the median time to response was 4.1 months, indicating that RCD regimen was unsuitable for a rapid disease control.Citation15,Citation57 Besides, the response to rituximab may vary among individuals, probably due to the influence exerted by FCGR3A gene polymorphism.Citation58 For young patients who are eligible for autologous stem cell transplantation, the administration of purine analogs should be avoided as an initial therapy in that such kind of agents have a strong myelosuppressive activity and are toxic to stem cells, thus impeding the harvest of stem cells. Of course, it is a good alternative that stem cells should be collected before fludarabine administration. The use of fludarabine has also close correlation with severe cytopenias, autoimmune hemolytic anemia, and an increased risk of secondary malignancies such as myelodysplastic syndrome or acute myeloid leukemia,Citation59 while rituximab in combination with cladribine yielded an ORR of nearly 90% with more modest toxicity as compared with fludarabine.Citation60 It is noteworthy that plasmapheresis alone, acting as an urgent measure taken to decrease high circulating IgM rapidly, is far from efficacious for a long-term disease control and must be followed by cytoreductive therapies that aim at killing the IgM-producing lymphoplasmacytic cells.Citation61
Ibrutinib, a kind of Bruton’s tyrosine kinase (BTK) inhibitor, abrogates the abnormal activation of nuclear factor-κB signaling pathway by disrupting the interactions between the mutated MYD88 (Leu265Pro) protein and BTK.Citation62 A phase III clinical trial evaluated the efficacy and safety of ibrutinib in patients who are refractory to rituximab-based combination therapy. Of 31 patients who were evaluated, 28 (90%) patients achieved overall response and 22 (71%) patients obtained major response. Common grade 3 or worse hematological toxicity included neutropenia in 4 (13%) patients, anemia in 2 (6%), and thrombocytopenia in 2 (6%) patients.Citation63 It is well established that recurrent somatic mutations of the MYD88 gene (MYD88 Leu265Pro) and CXCR4 gene (CXCR4 WHIM) were observed in ~90% and 40% of WM patients, respectively. Ibrutinib monotherapy was highly effective in patients with an MYD88 mutation. While, CXCR4WHIM mutations potentiate resistance to ibrutinib.Citation7,Citation64 The emergence of BTK inhibitors, such as ibrutinib, zanubrutinib, and acalabrutinib, may provide more promising options for WM treatment. However, the cost of ibrutinib is prohibitive. The efficacy has to be weighed against the cost. Besides, clinical data of such agents in combination with other drugs are inadequate.
There existed some drawbacks in our meta-analysis. First, we were unable to pool progression-free survival (PFS) and overall survival (OS) outcomes despite the fact that PFS and OS might be more indicative of the actual clinical benefit. The reasons are as follows: Different researchers defined PFS and explained PFS outcomes in quite distinct ways, including median PFS, median duration of response, and time to progression. Besides, the duration of follow-up varied among the included studies, and PFS or OS rates were reported at different follow-up times. Some included studies did not even report PFS or OS outcomes probably due to the indolence of WM. Second, the pooled calculations of all the rates were based on the published data instead of individual patient data, and the patient baseline characteristics were broadly divergent among studies. Therefore, response rates could not be synthesized according to the subgroups of age, gender, previous therapy, MYD88 and CXCR4 gene mutation status, serum IgM level, bone marrow involvement, clinical manifestations, and other risk stratification factors. Third, many included original studies did not differentiate newly diagnosed WM patients who had no prior therapies from patients with relapsed or refractory WM. Therefore, our meta-analysis failed to make a clear distinction. Fourth, the agents, dosage, cycles, and routes of administration within the same subgroup were not utterly consistent. Last, information bias was inevitable because six retrospective studies were also included in our meta-analysis.
Conclusion
In summary, our meta-analysis indicated that rituximab-based chemoimmunotherapy is highly effective with tolerable toxicity, serving as the backbone of both the initial and salvage treatment in a relatively economical manner. More importantly, we should choose the most suitable combination regimen in accordance with the individual patient’s clinical features and related comorbidities.
Acknowledgments
We extend our sincere gratitude to all the librarians of Fourth Military Medical University for their generous assistance in searching literature. All authors received no funding from any organization, institute, company, or person.
Data availability statement
Our meta-analysis overviewed and extracted data from the previously published articles, all of which are cited in the manuscript and can be found online. The processed data are available from the corresponding author upon request.
Disclosure
All authors report no conflicts of interest in this work.
References
- ManasanchEEKristinssonSYLandgrenOEtiology of Waldenstrom macroglobulinemia: genetic factors and immune-related conditionsClin Lymphoma Myeloma Leuk201313219419710.1016/j.clml.2013.02.00223473950
- OwenRGTreonSPAl-KatibAClinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s macroglobulinemiaSemin Oncol200330211011510.1053/sonc.2003.5008212720118
- SwerdlowSHCampoEPileriSAThe 2016 revision of the World Health Organization classification of lymphoid neoplasmsBlood2016127202375239010.1182/blood-2016-01-64356926980727
- VijayAGertzMAWaldenstrom macroglobulinemiaBlood2007109125096510310.1182/blood-2006-11-05501217303694
- LeblondVKastritisEAdvaniRTreatment recommendations from the Eighth International Workshop on Waldenstrom’s macro-globulinemiaBlood2016128101321132810.1182/blood-2016-04-71123427432877
- BuskeCLeblondVHow to manage Waldenstrom’s macroglobulinemiaLeukemia201327476277210.1038/leu.2013.3623385376
- TreonSPXuLYangGMYD88 L265P somatic mutation in Waldenstrom’s macroglobulinemiaN Engl J Med2012367982683310.1056/NEJMoa120071022931316
- TreonSPCaoYXuLYangGLiuXHunterZRSomatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenstrom macroglobulinemiaBlood2014123182791279610.1182/blood-2014-01-55090524553177
- SallesGBarrettMFoàRRituximab in B-cell hematologic malignancies: a review of 20 years of clinical experienceAdv Ther201734102232227310.1007/s12325-017-0612-x28983798
- GhobrialIMFonsecaRGreippPRInitial immunoglobulin M ‘flare’ after rituximab therapy in patients diagnosed with Waldenstrom macroglobulinemia: an Eastern Cooperative Oncology Group StudyCancer2004101112593259810.1002/cncr.2065815493038
- GertzMAWaldenstrom macroglobulinemia: 2017 update on diagnosis, risk stratification, and managementAm J Hematol201792220921710.1002/ajh.2455728094456
- ShamseerLMoherDClarkeMPreferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanationBMJ2015350g764710.1136/bmj.g764725555855
- OwenRGKyleRAStoneMJResponse assessment in Waldenstrom macroglobulinaemia: update from the VIth International WorkshopBr J Haematol2013160217117610.1111/bjh.1210223150997
- TreonSPHunterZBarnaganARCHOP plus rituximab therapy in Waldenstrom’s macroglobulinemiaClin Lymphoma20055427327715794864
- DimopoulosMAAnagnostopoulosAKyrtsonisMCPrimary treatment of Waldenstrom macroglobulinemia with dexamethasone, rituximab, and cyclophosphamideJ Clin Oncol200725223344334910.1200/JCO.2007.10.992617577016
- TreonSPSoumeraiJDBranaganARThalidomide and rituximab in Waldenstrom macroglobulinemiaBlood2008112124452445710.1182/blood-2008-04-15085418713945
- BuskeCHosterEDreylingMThe addition of rituximab to front-line therapy with CHOP (R-CHOP) results in a higher response rate and longer time to treatment failure in patients with lymphoplasmacytic lymphoma: results of a randomized trial of the German Low-Grade Lymphoma Study Group (GLSG)Leukemia200923115316110.1038/leu.2008.26118818699
- TreonSPSoumeraiJDBranaganARLenalidomide and rituximab in Waldenstrom’s macroglobulinemiaClin Cancer Res200915135536010.1158/1078-0432.CCR-08-086219118065
- IoakimidisLPattersonCJHunterZRComparative outcomes following CP-R, CVP-R, and CHOP-R in Waldenstrom’s macroglobu-linemiaClin Lymphoma Myeloma200991626610.3816/CLM.2009.n.01619362976
- TreonSPBranaganARIoakimidisLLong-term outcomes to fludarabine and rituximab in Waldenstrom macroglobulinemiaBlood2009113163673367810.1182/blood-2008-09-17732919015393
- TreonSPIoakimidisLSoumeraiJDPrimary therapy of Waldenstrom macroglobulinemia with bortezomib, dexamethasone, and rituximab: WMCTG clinical trial 05-180J Clin Oncol200927233830383510.1200/JCO.2008.20.467719506160
- GhobrialIMHongFPadmanabhanSPhase II trial of weekly bortezomib in combination with rituximab in relapsed or relapsed and refractory Waldenstrom macroglobulinemiaJ Clin Oncol20102881422142810.1200/JCO.2009.25.323720142586
- RabascioCLaszloDAndreolaGExpression of the human concentrative nucleotide transporter 1 (hCNT1) gene correlates with clinical response in patients affected by Waldenstrom’s macroglobu-linemia (WM) and small lymphocytic lymphoma (SLL) undergoing a combination treatment with 2-chloro-2′-deoxyadenosine (2-CdA) and RituximabLeuk Res201034445445710.1016/j.leukres.2009.07.00219647871
- GhobrialIMXieWPadmanabhanSPhase II trial of weekly bortezomib in combination with rituximab in untreated patients with Waldenstrom macroglobulinemiaAm J Hematol201085967067410.1002/ajh.2178820652865
- AgathocleousARohatinerARuleSWeekly versus twice weekly bortezomib given in conjunction with rituximab, in patients with recurrent follicular lymphoma, mantle cell lymphoma and Waldenstrom macroglobulinaemiaBr J Haematol2010151434635310.1111/j.1365-2141.2010.08340.x20880120
- PeinertSTamCSPrinceHMFludarabine based combinations are highly effective as first-line or salvage treatment in patients with Waldenstrom macroglobulinemiaLeuk Lymphoma201051122188219710.3109/10428194.2010.52432620939696
- LaszloDAndreolaGRigacciLRituximab and subcutaneous 2-chloro-2′-deoxyadenosine as therapy in untreated and relapsed Waldenstrom’s macroglobulinemiaClin Lymphoma Myeloma Leuk201111113013210.3816/CLML.2011.n.02921454213
- TedeschiABenevoloGVarettoniMFludarabine plus cyclophosphamide and rituximab in Waldenstrom macroglobulinemia: an effective but myelosuppressive regimen to be offered to patients with advanced diseaseCancer2012118243444310.1002/cncr.2630321732338
- TedeschiARicciFGoldanigaMCFludarabine, cyclophosphamide, and rituximab in salvage therapy of Waldenstrom’s macroglobulinemiaClin Lymphoma Myeloma Leuk201313223123410.1016/j.clml.2013.02.01123490992
- DimopoulosMAGarcia-SanzRGavriatopoulouMPrimary therapy of Waldenstrom macroglobulinemia (WM) with weekly bortezomib, low-dose dexamethasone, and rituximab (BDR): long-term results of a phase 2 study of the European Myeloma Network (EMN)Blood2013122193276328210.1182/blood-2013-05-50386224004667
- TreonSPTripsasCKMeidKCarfilzomib, rituximab, and dexamethasone (CaRD) treatment offers a neuropathy-sparing approach for treating Waldenstrom’s macroglobulinemiaBlood2014124450351010.1182/blood-2014-03-56627324859363
- TedeschiAPicardiPFerreroSBendamustine and rituximab combination is safe and effective as salvage regimen in Waldenstrom macroglobulinemiaLeuk Lymphoma20155692637264210.3109/10428194.2015.101271425651423
- SouchetLLevyVOuzegdouhMEfficacy and long-term toxicity of the rituximab-fludarabine-cyclophosphamide combination therapy in Waldenstrom’s macroglobulinemiaAm J Hematol201691878278610.1002/ajh.2440527135784
- RosenthalADueckACA phase 2 study of lenalidomide, rituximab, cyclophosphamide, and dexamethasone (LR-CD) for untreated low-grade non-Hodgkin lymphoma requiring therapyAm J Hematol201792546747210.1002/ajh.2469328230270
- PaludoJAbeykoonJPDexamethasone, rituximab and cyclophosphamide for relapsed and/or refractory and treatment-naive patients with Waldenstrom macroglobulinemiaBr J Haematol201717919810510.1111/bjh.1482628786474
- DimopoulosMAZervasCZomasATreatment of Waldenstrom’s macroglobulinemia with rituximabJ Clin Oncol20022092327233310.1200/JCO.2002.09.03911981004
- TreonSPEmmanouilidesCKimbyEExtended rituximab therapy in Waldenstrom’s macroglobulinemiaAnn Oncol200516113213810.1093/annonc/mdi02215598950
- GertzMARueMBloodEKaminerLSVesoleDHGreippPRMulticenter phase 2 trial of rituximab for Waldenstrom macroglobulinemia (WM): an Eastern Cooperative Oncology Group Study (E3A98)Leuk Lymphoma200445102047205510.1080/1042819041000171404315370249
- TreonSPBranaganARHunterZSantosDTournhilacOAndersonKCParadoxical increases in serum IgM and viscosity levels following rituximab in Waldenstrom’s macroglobulinemiaAnn Oncol200415101481148310.1093/annonc/mdh40315367407
- CastilloJJKananSMeidKManningRHunterZRTreonSPRituximab intolerance in patients with Waldenstrom macroglobulinaemiaBr J Haematol2016174464564810.1111/bjh.1379426523929
- PatelSVKhanDAAdverse reactions to biologic therapyImmunol Allergy Clin North Am201737239741210.1016/j.iac.2017.01.01228366484
- HongJKimJYAhnHKBone marrow involvement is predictive of infusion-related reaction during rituximab administration in patients with B cell lymphomaSupport Care Cancer20132141145115210.1007/s00520-012-1639-923111943
- LangDProuseJBarryFEvaluation of the safety and feasibility of rapid rituximab infusionAsia Pac J Clin Oncol201281717510.1111/j.1743-7563.2011.01487.x22369446
- BuskeCEradatHADiRienzoCGOfatumumab: another way to target CD20 in Waldenstrom’s macroglobulinaemiaLancet Haematol201741e4e510.1016/S2352-3026(16)30163-627914970
- FurmanRRKastritisEKyrtsonisMCOnce-weekly ofatumumab in untreated or relapsed Waldenstrom’s macroglobulinaemia: an open-label, single-arm, phase 2 studyLancet Haematol201741e24e3410.1016/S2352-3026(16)30166-127914971
- GavriatopoulouMKastritisEKyrtsonisMCPhase 2 study of ofatumumab, fludarabine and cyclophosphamide in relapsed/refractory Waldenstrom’s macroglobulinemiaLeuk Lymphoma20175861506150810.1080/10428194.2016.123354127724158
- KastritisEKyrtsonisMCMorelPCompeting risk survival analysis in patients with symptomatic Waldenstrom macroglobulinemia: the impact of disease unrelated mortality and of rituximab-based primary therapyHaematologica201510011e446e44910.3324/haematol.2015.12414926294729
- KastritisEDimopoulosMADisease control should be the goal of therapy for WM patientsBlood Adv20171252483248510.1182/bloodadvances.201700564529296898
- DhodapkarMVHoeringAGertzMALong-term survival in Waldenstrom macroglobulinemia: 10-year follow-up of Southwest Oncology Group-directed intergroup trial S9003Blood2009113479379610.1182/blood-2008-07-17208018931340
- DimopoulosMAKastritisEOwenRGTreatment recommendations for patients with Waldenstrom macroglobulinemia (WM) and related disorders: IWWM-7 consensusBlood201412491404141110.1182/blood-2014-03-56513525027391
- BenevoloGNicolosiMSantambrogioEVitoloUCurrent options to manage Waldenstrom’s macroglobulinemiaExpert Rev Hematol201710763764710.1080/17474086.2017.133959628592170
- YunSJohnsonACOkoloONWaldenstrom macroglobulinemia: review of pathogenesis and managementClin Lymphoma Myeloma Leuk201717525226210.1016/j.clml.2017.02.02828366781
- GavriatopoulouMTerposEKastritisEDimopoulosMACurrent treatment options and investigational drugs for Waldenstrom’s macroglobulinemiaExpert Opin Investig Drugs201726219720510.1080/13543784.2017.1275561
- KastritisEDimopoulosMACurrent therapy guidelines for Waldenstrom’s macroglobulinaemiaBest Pract Res Clin Haematol201629219420510.1016/j.beha.2016.08.02327825466
- AbdallahAOAtrashSMuzaffarJSuccessful treatment of Bing-Neel syndrome using intrathecal chemotherapy and systemic combination chemotherapy followed by BEAM auto-transplant: a case report and review of literatureClin Lymphoma Myeloma Leuk201313450250610.1016/j.clml.2013.03.00223747080
- SimonLFitsioriALemalRBing-Neel syndrome, a rare complication of Waldenstrom macroglobulinemia: analysis of 44 cases and review of the literature. A study on behalf of the French Innovative Leukemia Organization (FILO)Haematologica2015100121587159410.3324/haematol.2015.13374426385211
- KastritisEGavriatopoulouMKyrtsonisMCDexamethasone, rituximab, and cyclophosphamide as primary treatment of Waldenstrom macroglobulinemia: final analysis of a phase 2 studyBlood2015126111392139410.1182/blood-2015-05-64742026359434
- TreonSPYangGHanzisCAttainment of complete/very good partial response following rituximab-based therapy is an important determinant to progression-free survival, and is impacted by polymorphisms in FCGR3A in Waldenstrom macroglobulinaemiaBr J Haematol2011154222322810.1111/j.1365-2141.2011.08726.x21564078
- BenjaminiOJainPTrinhLSecond cancers in patients with chronic lymphocytic leukemia who received frontline fludarabine, cyclophosphamide and rituximab therapy: distribution and clinical outcomesLeuk Lymphoma20155661643165010.3109/10428194.2014.95720325308294
- LaszloDAndreolaGRigacciLRituximab and subcutaneous 2-chloro-2′-deoxyadenosine combination treatment for patients with Waldenstrom macroglobulinemia: clinical and biologic results of a phase II multicenter studyJ Clin Oncol201028132233223810.1200/JCO.2009.23.631520368573
- StoneMJBogenSARole of plasmapheresis in Waldenstrom’s macroglobulinemiaClin Lymphoma Myeloma Leuk201313223824010.1016/j.clml.2013.02.01323522642
- PalSSDammeijerFHendriksRWRole of Bruton’s tyrosine kinase in B cells and malignanciesMol Cancer20181715710.1186/s12943-018-0779-z29455639
- DimopoulosMATrotmanJTedeschiAIbrutinib for patients with rituximab-refractory Waldenstrom’s macroglobulinaemia (iNNOVATE): an open-label substudy of an international, multicentre, phase 3 trialLancet Oncol201718224125010.1016/S1470-2045(16)30632-527956157
- TreonSPTripsasCKMeidKIbrutinib in previously treated Waldenstrom’s macroglobulinemiaN Engl J Med2015372151430144010.1056/NEJMoa150154825853747
- MoherDLiberatiATetzlaffJAltmanDGThe PRISMA GroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementPLoS Med200967e100009710.1371/journal.pmed100009719621072

