Abstract
Human breast cancer is one of the most frequent cancer diseases and causes of death among female population worldwide. It appears at a high incidence and has a high malignancy, mortality, recurrence rate and poor prognosis. Caveolin-1 (Cav1) is the main component of caveolae and participates in various biological events. More and more experimental studies have shown that Cav1 plays a critical role in the progression of breast cancer including cell proliferation, apoptosis, autophagy, invasion, migration and breast cancer metastasis. Besides, Cav1 has been found to be involved in chemotherapeutics and radiotherapy resistance, which are still the principal problems encountered in clinical breast cancer treatment. In addition, stromal Cav1 may be a potential indicator for breast cancer patients’ prognosis. In the current review, we cover the state-of-the-art study, development and progress on Cav1 and breast cancer, altogether describing the role of Cav1 in breast cancer progression and application in clinical treatment, in the hope of providing a basis for further research and promoting CAV1 gene as a potential target to diagnose and treat aggressive breast cancers.
Introduction
Breast cancer is a common malignant disease among female population worldwide and is characterized by the highest cancer incidence, high recurrence rate, morbidity, mortality and poor prognosis.Citation1–Citation4 Breast cancer tumorigenesis is a multi-step process including proliferation, apoptosis, autophagy, invasion, migration, metastasis and drug resistance. According to pathological characteristics, human breast cancer can be divided into non-invasive breast cancer (eg, Paget disease of the breast),Citation5 invasive breast carcinoma of special type (eg, invasive apocrine carcinoma, invasive micropapillary carcinoma),Citation6,Citation7 invasive breast carcinoma of no special type (eg, invasive lobular carcinoma, invasive ductal carcinoma),Citation8–Citation10 inflammatory breast cancer (IBC) and so on. Among them, invasive breast carcinoma of no special type is the most common type and IBC patients often show a poor prognosis. According to molecular type [estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 (HER-2)], human breast cancer is divided into luminal A (ER+, PR+, HER-2−), luminal B (ER+, PR+, HER-2+), normal breast-like, HER-2 over-expressing, and basal-like breast cancer.Citation11 Triple-negative breast cancer (TNBC) is a subtype of basal-like breast cancer that is characterized by the absence of expression of ER, PR and HER2.Citation12–Citation14 With high proliferative capacity and recurrence rate, poor differentiation with large tumor size and lack of recognized biomarker, TNBC is a clinical challenge for targeted therapy.Citation15–Citation17 Canine mammary tumor (CMT) is considered a suitable model for human breast cancer, owing to it showing a similar tumor microenvironment (TME), carcinogens and cancer risk factors.Citation18,Citation19 Cancer-associated fibroblasts (CAFs) play a key role in cancer initiation and progression.Citation20 Although significant advances in surgery, chemotherapy and radiotherapy have greatly improved the prognosis of breast cancer patients,Citation21 breast cancer still remains one of the most common causes of female death. Therefore, it is necessary to summarize the latest molecular mechanisms related to breast cancer progression.
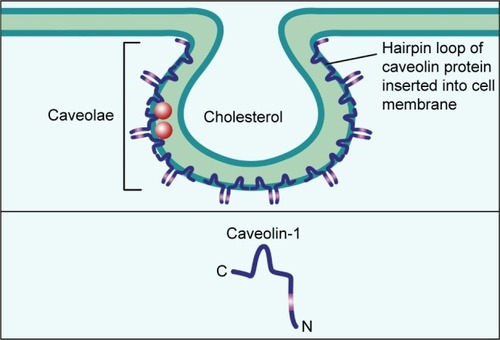
Caveolae are 50–100 nm Ω-shaped,Citation22,Citation23 cholesterol-enriched, rigid membrane microdomains that are composed of scaffold proteins named caveolins, including caveolin-1, caveolin-2 and caveolin-3 (); caveolin-1 and caveolin-2 are expressed in all human tissues, while caveolin-3 is expressed in muscles.Citation24–Citation28 Caveolin-1 (Cav1) is a 21–22 kDa integral membrane protein and the coding gene of CAV1 is located in the D7S522 locus in the q31.1 region of human chromosome 7 and consists of three exons.Citation29 Further, Cav1 can participate in various events including endocytosis, signal transduction, membrane trafficking, cholesterol homeostasis, lipid transport and storage, cell cycle, proliferation, apoptosis, cancer cell invasion, migration and metastasis.Citation30–Citation38 In normal mammary parenchymal cells’ carcinogenic process, Cav1 can act both as tumor suppressor and promoter depending on the subtypes and stages of cancers.Citation39–Citation41 In addition, recent studies have shown that caveolae integrity is associated with cancer cell survival, apoptosis and migration and metastasis;Citation42–Citation45 so we consider Cav1 in caveolae may play a necessary role in the breast cancer development.
Figure 1 The structure of caveolae.
Notes: Caveolae are 50–100 nm Ω-shaped, cholesterol-enriched, rigid membrane microdomains that are composed of scaffold proteins named caveolins. The most important constituent protein is Caveolin-1.
In order to define the interaction between Cav1 and breast cancer, in this review, we cover the state-of-the-art study, development and progress on Cav1 and breast cancer, altogether describing the role of Cav1 in breast cancer progression, including cell proliferation, apoptosis, autophagy, invasion, migration and breast cancer metastasis. Moreover, the application of Cav1 in breast cancer clinical treatment is also clarified, such as chemotherapeutics resistance, radiotherapy resistance and diagnosis, in the hope of promoting the clinical application of Cav1.
Cav1 and breast cancer cell proliferation
Cav1-induced changes in the expression and activation of ion channels and receptors on the cell membrane may play an important role in breast cancer cell proliferation. Cav1 can act as a tumor suppressor in MCF-7 cells, the downregulation of Cav1 can promote the proliferation by increasing membrane expression and function of large conductance Ca2+-activated potassium (BKCa) channel whose encoding gene contributes to malignancy, thus accelerating the process of carcinogenesis.Citation46 Contrarily, parenchymal Cav1 can also act as a tumor promoter by promoting EGFR binding to the kinase domain of caveolin-binding motif, thereby potentially activating EGFR-mediated mitosis initiation.Citation47 HER2 overexpression and excessive HER2 signaling were observed in 25% of breast cancer patients with poor prognosis;Citation48 so Alawin et al allowed γ-tocotrienol to accumulate within the caveolae microdomain, which lead to caveolae disruption, subsequent interference with HER2 dimerization in caveolae microdomain, phos-phorylation (activation) and mitogenic signaling transduction in SKBR3 and BT474 human breast cancer cells.Citation49
Cav1 can decrease G0/G1 phase cell cycle arrest and increase the S phase cell number by activating the extracellular signal-regulated kinase (ERK) 1/2 pathway and increasing the expression of cell cycle-associated proteins (cyclin D1 and β-catenin) in BT474 cells.Citation50 On the contrary, Cav1 acts as an antiproliferative factor in MDA-MB-231 and MCF-7 cells through promoting cell cycle arrest in the G2/M phase, which was accomplished by upregulation of p21, p27 and cyclin B1 and downregulation of cyclin D2, and this anti-proliferative effect was enhanced with the cooperation of docetaxel (DTX).Citation51 The completely opposite effect of Cav1 on cell proliferation may be due to the difference of used cell lines in two experiments, and more importantly, breast cancer cells were treated with DTX in Kang et al’s study.
The malignant features of cancer cells can not only affect tumor development but also the interaction between neoplastic cells and the TME can act as a significant factor in the process of breast cancer progression,Citation52 and Cav1 plays a multifunctional role in this process. High oxidative stress is usually observed in the stroma of human breast cancers and it can induce stromal catabolism,Citation53 stromal Cav1 transportation from cancer-associated stroma to breast cancer cells is low and this leads to a proliferative effect.Citation54 Bisphenol A (BPA) is commonly used as an analog of estrogen to mimic estro-genic effects,Citation55–Citation57 Xu et al found that under a hypoxic TME, BPA in MDA-MB-231 cells could trigger G-protein estrogen receptor competitive binding to Cav1, leading to the release of heat shock protein 90 to stabilize and activate hypoxia inducible factor-1 alpha, which upregulated the expression of vascular endothelial growth factor (VEGF), thus inducing proliferative effects.Citation52,Citation58 In addition to a hypoxic TME, the effect of CAFs is another factor which influences the occurrence and progression of breast cancers. Reduced expression levels of Cav1 mRNA and Cav1 in CAFs trigger the expression and secretion of stromal cell-derived factor-1, EGF and fibroblast-specific protein-1 (FSP-1), and all of them can accelerate breast cancer cell proliferation rate.Citation59
As mentioned above, we demonstrate that Cav1 can affect breast cancer cell proliferation rate by 1) influencing the expression and activation of ion channels and receptors on the cell membrane, such as BKCa channel, EGFR and HER2; 2) regulating cell cycle arrest and affecting mitosis process and 3) mediating the interaction between the TME (hypoxia conditions and CAFs) and breast cancer cells.
Cav1 and breast cancer cell autophagy and apoptosis
Apoptosis is a complex biological process with the formation of apoptotic bodies and morphological and biochemical changes in the nucleus,Citation60,Citation61 and both the intrinsic and extrinsic apoptotic pathways are typically inhibited in cancer cells through overexpression of antiapoptotic proteins and under-expression of proapoptotic proteins.Citation62 Autophagy is a process known to degrade intracellular proteins and organelles by forming various membrane structures, including autophagosomes, lysosomes and autolysosomes.Citation63 Novel types of selective autophagy have been identified, including mitophagy, pexophagy, lipophagy, ERphagy and nucleophagy, all of them facilitate the removal of damaged cellular compartments and recycle components, thereby preventing cells from apoptosis and increasing survivability.Citation64
Cav1 can regulate intrinsic and extrinsic apoptotic pathway proteins, thus affecting the process of breast cancer cell apoptosis. Owing to lipid rafts being enriched with cholesterol, methyl beta cyclodextrin (MβCD) as a cholesterol depleting agent can induce lipid raft disruption and mediate MDA-MB-231 and 468 cells apoptosis by down-regulating the mRNA and protein expression level of Cav1 and Wnt receptor LRP6, and they can synergistically affect expression of apoptosis-related protein, such as survivin, Bcl-2, Bax and caspase-3.Citation65 On the contrary, with the DTX treatment, elevated mRNA and protein expression level of Cav1 in TNBC cells induces Bcl-2 phosphorylation (inactivation) and expression of p53, Bax and cleaved poly-ADP-ribose polymerase, thus exposing MCF-7 and MDA-MB-231 cells to the apoptotic process.Citation51
Cav1-induced autophagy alternation can influence cell apoptosis. An enhanced expression level of autophagy-related proteins (Beclin-1, light chain 3-II and Atg12/5) in BT474 cells is realized by 17β-estradiol (E2)-induced Cav1 upregulation, resulting in the formation of autophagosome and inhibition of apoptosis.Citation66 Shi et al found that Cav1 deficiency and lipid raft disruption could elevate autophagy levels and inhibit apoptosis by promoting V-ATPase assembly, which could activate lysosomal function and autophagosome–lysosome fusion.Citation67
Chemotherapeutic drugs can regulate transcriptional level of CAV1, thus promoting apoptosis of breast cancer cells. Salis et al found that fluvastatin induced cytotoxic effects on MCF-7 cells through a reduction of the mRNA expression levels of CAV1 and serum and glucocorticoid-regulated kinase 1 (SGK1),Citation68 conversely, they also found metformin-induced cytotoxic effect on MCF-7 cells was realized by an enhanced mRNA expression of CAV1 while a reduction of SGK1,Citation69 and the different transcriptional regulation of CAV1 may be owing to the different chemotherapeutic drug treatments with MCF-7 cells.
Cav1 can affect the apoptotic process by regulating the expression and activation of downstream proteins. Reduced expression levels of Cav1 in CAFs lead to upregulation of tumor protein 53-induced glycolysis and apoptosis regulator (TIGAR) in the BT474 cells, which functions to limit ROS, thus preventing cells from ROS-induced apoptosis.Citation59 Docosahexaenoic acid (DHA) can facilitate caveolae internalization by sensitizing caveolae marker Cav1 to lysosomal marker LAMP-1, thereby decreasing caveolae-associated onco-protein levels via proteasomal and lysosomal pathways and decreasing HSP90 function.Citation70
As mentioned above, we demonstrate that the interaction between Cav1 and breast cancer cell apoptosis can be summarized as follows: 1) Cav1 can regulate intrinsic and extrinsic apoptotic pathway proteins, such as Bcl-2, Bax and caspase-3; 2) Cav1-induced autophagy alternation can influence cell apoptosis; 3) chemotherapeutic drugs can regulate transcriptional level of CAV1 promoting apoptosis; and 4) Cav1 can affect apoptosis process by regulating the expression and activation of downstream proteins, such as TIGAR, ROS and onco-proteins.
Cav1 and breast cancer cell invasion and migration
It is clear that epithelial to mesenchymal transition (EMT) plays a critical role in cancer progression and metastasis, and Cav1 is implicated in various aspects of EMT, thus driving breast cancer progression.Citation71–Citation73 The downregulation of epithelial markers (eg, E-cadherin and γ-catenin), upregulation of mesenchymal markers (eg, vimentin, fibronectin and N-cadherin) and transcription factors (eg, Snail and Slug) together with the acquisition of increased invasion, migration and stem-like properties are key features of the EMT program.Citation74–Citation76 Matrix metalloproteinases (MMPs) are major extracellular enzymes with the capacity of degrading and remodeling extracellular matrix (ECM) proteins and basement membranes, resulting in local invasion,Citation77–Citation79 thus promoting cancer initiation, progression, invasion, migration and metastasis.Citation80
During hyperglycemia-induced matrix-specific EMT, inhibition of fatty acid synthase/ERα signaling leads to a dramatic upregulation of Cav1 mRNA and protein, thereby enhancing Slug mRNA levels and promoting invasion capacity in MCF-7 and T47D cells.Citation81 Fucose-containing fraction of Ling-Zhi (FFLZ) inhibits the Cav-1/Smad7/Smurf2-dependent ubiquitin-mediated transforming growth factor-β receptor (TGFR) degradation and abolishes TGFR signaling pathways, thereby reducing the expression of EMT markers (eg, Snail and Slug) and suppressing 4T1 and MDA-MB-231 cells migration.Citation82 In an vitro study, Cav1 and EMT-related gene expression inhibition was observed in curcuminCitation83 and pamidronateCitation84-treated MCF-10F cells.
Cav1 knockdown can inhibit BT474 cell invasion and migration capacities via downregulating the protein expression of MMP-2, MMP-9 and MMP-1.Citation50 Antarctic krill DHA enhanced the interaction between CD95 (known as Fas) and Cav1, resulting in the downregulation of MMP-2 expression through the inhibition of FAK/SRC/PI3K/AKT signaling pathway in MCF-7 cells.Citation85,Citation86 Cav1 and MMP-14 overexpression was observed in malignant and invasive CMT tissues.Citation18
Rho GTPases play a key role in the process of microtubule cytoskeleton or actin regulation and thus regulate cell adhesion and migration.Citation87 Activation of Cav1 in IBC cells resulted in increasing invasive potential via Akt1 pathway, which phosphorylates RhoC GTPase.Citation88 Yang et al found that whether upregulation or downregulation of ROS, the migration capacity of MCF-7 and MDA-MB-231 cells was obviously inhibited via reducing the interaction between Cav1 and DLC1,Citation89 which belongs to the Rho GTPase-activating protein (GAP) family.Citation90 Díaz et al reported that Cav1 can recruit p85α (a Rab5 GAP) and thus precluding p85α-mediated Rab5 inactivation, and activated Rab5 can increase Rac1 activity and enhance MDA-MB-231 cells migration and invasion.Citation91
Endocytic trafficking of integrins plays a significant role in cell adhesion and migration, which are related to cancer cell dissemination. Focal adhesion associated kinases (FAK)-induced phosphorylation of Cav1 on Tyr14 can internalize ligand-bound integrins to early endosomes; pro-metastatic protein NEDD9-dependent dephosphorylation of pTyr14-Cav1 is required for the transformation of early endosomes to late endosomes and this leads to individual MDA-MB-231 cell migration.Citation92
As mentioned above, we demonstrate that Cav1 can affect breast cancer cell invasion and migration by 1) influencing the expression level of EMT-associated markers and transcription factors; 2) affecting the expression level of MMP; 3) regulating the expression of Rho GTPases and 4) participating Cav1-dependent trafficking of integrins.
Cav1 and breast cancer metastasis
Proliferation, cell cycle arrest, cell transport, adherence, extravasation, motility, adhesion local invasion and migration are necessary preparation for breast cancer metastasis and the prognosis of metastatic breast cancer patients is still poor.Citation93,Citation94 Various steps are involved in the cancer metastasis process, including tumor cell detachment and escape, survival in the circulatory system and ultimately growth in distant organs.Citation95,Citation96 Once normal epithelial cells detach from the surrounding ECM, a form of programmed apoptosis termed anoikis will be triggered.Citation97 To survive in the circulatory and lymphatic system, circulating tumor cells (CTCs) must acquire the ability to resist anoikis, thereby facilitating secondary tumor formation in distant sites.Citation98,Citation99 Some metastatic tumor cells are less sensitive or even resistant to anoikis and can survive without attachment to ECM.Citation100
Cav1 regulates breast cancer metastasis via participating in anoikis progression. Once MDA-MB-231 cells detach from the ECM and enter into a hemodynamic environment, expression of Cav1 mRNA and protein is enhanced by the fluid-induced low shear stress and Cav1 can endow cancer cells with anoikis resistance via inactivating caspase-8.Citation101 Similarly, Cav1 endows anoikis resistance to MDA-MB-231 cells via activation of PI3K/AKT and MEK/ERK survival signaling pathways and ITGB1–FAK signaling.Citation102
Membrane type 4 matrix metalloproteinase (MT4-MMP) co-localizes with Cav1 at the cell surface,Citation103 and Cdc42-mediated MT4-MMP internalization and recycling can promote breast cancer metastasis.Citation104 Enhanced VEGF-A/VEGFR1 activity upon Cav1 loss in metastasis-associated macrophages drives the downstream expression of MMP9 and colony-stimulating factor 1, altogether facilitating angiogenesis and metastatic growth.Citation105 Macrophage migration inhibitory factor-induced Cav1 phosphorylation contributes to HMGB1 secretion from the cytoplasm to the ECM, thereby activating TLR4 signaling and promoting breast cancer metastasis.Citation106
Alevizos et al found that breast cancer nodal metastasis correlates with extended methylation of Cav1 and CXCR4 in tumors and lymph nodes.Citation107 A negative correlation between Cav1 expression and metastatic potential was observed in the breast cancer cell lines.Citation108 Genomic and expression profiling reveal Cav1 is upregulated in bone marrow disseminated breast cancer cells relative to CTCs.Citation109
As mentioned above, we demonstrate that the interaction between Cav1 and breast cancer metastasis can be summarized as the following: 1) Cav1 can regulate breast cancer metastasis via participating in anoikis progression; 2) Cav1 regulating the expression and transport of metastasis-associated proteins, such as MT4-MMP, MMP9 and TLR4 and 3) the expression levels and methylation of Cav1 are associated with metastatic breast cancer.
Cav1 and chemotherapeutics and radiotherapy resistance
Drug resistance is regarded as one of the most important factors influencing the prognosis of cancer patients.Citation110 Thirty percent early breast cancer patients develop metastatic disease with the majority of these being resistant to current chemotherapies.Citation111 Cav1 was a potential target for preventing cancer radiation and drug resistance and improving clinical outcomes.Citation112,Citation113
With the capacity of mediating chemically cytostatic drugs efflux, breast cancer resistance proteins (BCRP) play an important role in clinical breast cancer drug resistance. ATP-binding cassette subfamily G member 2 (ABCG2) is one of the BCRPs. An elevated Cav1 mRNA and protein expression level was observed in TNBC stem cells, altogether enhancing expression level of ABCG2 via downregulating the Cav1-related β-catenin proteasomal degradation pathway and upregulating intracellular β-catenin accumulation.Citation114 Similarly, Herzog et al also found that knockdown of Cav1 decreased activity of ABCG2 and sensitized drug resistant breast cancer cells to chemotherapeutics.Citation115
Trastuzumab emtansine (T-DM1) is an antibody drug conjugate (ADC) that has been approved by the US Food and Drug Administration to treat HER-2-positive metastatic breast cancer.Citation116,Citation117 The trastuzumab in T-DM1 can bind to HER-2 receptors, followed by internalization of T-DM1 into cells and release of emtansine, resulting in cell toxicity.Citation118 Recent studies demonstrated that Cav1 can co-localize with trastuzumab to mediate T-DM1 internalization and enhance drug toxicity.Citation119 The elevated Cav1 expression could mediate endocytosis and promote the internalization of T-DM1 into HER-2-positive cancer cells.Citation120 Chung et al reported that Cav1 in BT-474 cells enhanced drug sensitivity by promoting T-DM1 internalization.Citation121 It is worth mentioning that mRNA profiling reveals that low Cav1 expression seems to sensitize BT474 cells to T-DM1.Citation122 And, in addition to internalization, Cav1 can mediate N87-TM cells’ resistance to T-DM1 via altering endocytic ADC to the lysosome and degrading T-DM1.Citation123
Cav1 overexpression in MCF-7 and MDA-MB-231 cell lines abolished the chemosensitizing effects by inhibiting eNOS/NO/ONOO− pathway and oxidant damage.Citation124 In ER(+) breast cancer cells, the MAPK pathway induced by Src phosphorylation can further phosphorylate Cav1, which activates the ER pathway and confers acquired resistance to lapatinib.Citation125 Radiation-induced Cav1 elevation promotes EGFR nuclear translocation and activates DNA-dependent protein kinase, following DNA repair and formation of radiation resistance.Citation126
As mentioned above, we demonstrate that Cav1 can affect breast cancer cell chemotherapeutics and radiotherapy resistance by 1) affecting the expression and activity of BCRP (eg, ABCG2); 2) influencing Cav-1-mediated T-DM1 internalization; 3) regulating eNOS/NO/ONOO− pathway and ER pathway and 4) promoting repair of radiation-induced DNA damage.
Cav1 and cancer stem cell
Tumor initiation is closely related to resistance to chemotherapeutics and radiotherapy resistance, and various approaches have been hypothesized to solve this issue.Citation127 Over the past decades, few researchers have addressed the issue of reversing drug resistance.Citation128,Citation129 Cancer stem cell (CSC) is increasingly set to become a vital factor in resistance to chemotherapeutics or radiotherapy, and it has been considered as a potential pathway for tumor recurrence in a variety of cancers, which significantly influences the clinical outcomes of patients.Citation110,Citation130–Citation132 This means, if a therapeutic agent fails to kill all the CSCs, the tumor may regrow.Citation133 One of the main issues in our knowledge of CSC is a lack of discovering the regulation behind recurrence of drug resistance.Citation134–Citation136
For breast cancer, CD44+/CD24−/lin− cell population was demonstrated as meeting the characteristics of CSCs. What is more, this cell population showed enhanced invasive properties but did not translate into real metastasis.Citation137 Wang et al discovered the role of Cav1 in mediating the chemoresistance of breast CSCs via silencing Cav1 in breast cancer stem cells (BCSCs), limited self-renewal ability but inducing the differentiation process of BCSCs by downregulating the β-catenin/ABCG2 pathway. Their clinical investigation supported the results in MCF-7 and MDA-MB-231 human breast cancer cell lines, revealing that Cav1 was highly elevated in TNBC. Moreover, Cav1 silencing significantly impaired the tumorigenicity and chemoresistance of breast CSCs in in vivo models.Citation114
Yuan’s group found that epirubicin increased the activity of the human Wnt6 promoter through Cav1-dependent binding of β-catenin to the proximal Wnt6 promoter in gastric cancer, which is considered an important regulator of CSCs.Citation138 Another regulator seems related to TME. Yongsanguanchai et al suggested that rapid reversible changes of CSC-like cells within tumors may result from biological mediators found in the TME, such as nitric oxide, which was elevated in H292 and H460 cells, and could promote CSC-like phenotypes of human non-small-cell lung carcinoma via Cav1 upregulation.Citation139
In sum, current studies focusing on the relationship between therapeutic resistance and Cav1 are still restricted to a few cancer types. Besides, a more comprehensive approach is required to identify the crucial role of Cav1 in cancer relapse to identify the physiological function and molecular networks that maintain stem cell survival in response to different treatments.
Cav1 and breast cancer prognosis
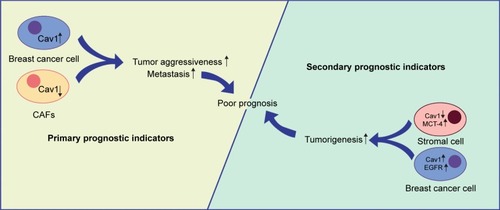
Cav1 is emerging as a potential therapeutic biomarker for breast cancer treatments.Citation40 Epithelium Cav1 and stroma Cav1 may be useful as prognostic indicators for patient treatments and assist the selection of personalized therapy.Citation141 Both the epithelial and stromal Cav1 have been detected in breast cancer patients and can be used to forecast the prognosis. Breast cancer patients with negative Cav1 expression in CAFs often show a poor outcome and low survival rate.Citation142,Citation143 Absence of Cav1 expression in CAFs allows patients with lymph node metastasis and poor prognosis.Citation7 Lack of stromal Cav1 expression is associated with a poor prognosis and worse overall survival.Citation144–Citation146 High expression level of Cav1 in invasive breast cancer cells indicates that epithelial Cav1 expression is proportional to tumor aggressiveness and poor prognosis.Citation140,Citation147 Besides, Qian et al reported that tumor (++)/stromal (−) Cav1 expression was closely associated with poor prognostic outcomes in primary human breast cancer patients.Citation148
In addition to the breast cancer cells with low stromal Cav1 expression (38.56%), Liang et al also found overexpression of cytoplasmic EGFR (53.92%) and Cav1 (44.12%), and suggested the combined stromal Cav-1/EGFR expression as an advanced prognostic marker.Citation47 It is also reported that lack of Cav1 and overexpression of monocarboxylate transporter 4 (MCT-4) in stroma show a prognostic significance to breast cancer patients.Citation149
As mentioned above, we demonstrate that stromal Cav1 expression may be a potential prognostic indicator for breast cancer patients and loss of stromal Cav1 expression often shows a poor clinical outcome. What is more, high expression levels of epithelial Cav1, cytoplasmic EGFR and stromal MCT-4 may be secondary prognostic indicators ().
Figure 2 Cav1 and breast cancer prognosis.
Notes: CAFs and epithelium Cav1 expression may be primary prognostic indicators for breast cancer patients. Loss of CAFs Cav1 expression and high expression levels of epithelium Cav1 often show a poor clinical outcome. Moreover, cytoplasmic EGFR and stromal MCT-4 may be secondary prognostic indicators.
Abbreviations: CAFs, cancer-associated fibroblasts; Cav1, caveolin-1; MCT-4, monocarboxylate transporter 4.
Conclusion and future perspective
Nowadays, breast cancer is one of the most common cancer diseases and causes of death among female population worldwide. Human breast cancer shows different pathological types, including non-invasive breast cancer, invasive breast carcinoma of special type, invasive breast carcinoma of no special type, IBC and so on. Different pathological types of breast cancer show different epidemiological characteristics and prognosis: invasive lobular carcinoma is the second most common subtype of mammary cancer to invasive ductal carcinoma,Citation150 non-invasive breast cancer is a type of breast cancer with a better prognosis, while IBC patients often show a poor prognosis. According to whether the cancer cell plasma membrane expresses ER, PR and HER2, mammary cancer can be divided into luminal A, luminal B, normal breast-like, HER-2 over-expressing and basal-like breast cancer. TNBC is a subtype of basal-like breast cancer with poor prognosis. Cav1 is the main component of caveolae, and it participates in various biological events. It has been reported that genetic changes of Cav1 might modify the risk for breast cancer,Citation151 and Cav1 acts both as a tumor suppressor as well as an oncogene, and plays a key role in breast cancer tumorigenesis.Citation152 In addition to breast cancer carcinogenic process, Cav1 is also associated with lung cancer,Citation153,Citation154 colorectal cancer,Citation155 gastric cancer,Citation156,Citation157 cervical cancer,Citation158 hepatocellular carcinoma,Citation159 pancreatic cancer,Citation160 bladder cancer,Citation161 pulmonary hypertension,Citation162–Citation164 virus infection,Citation165,Citation166 cardiovascular disease,Citation167,Citation168 diabetes mellitus,Citation169 nanomedicines endocytosisCitation170 and lipodystrophy.Citation171 Besides, it has been reported that caveo-lin-2 (Cav2) can also mediate the initiation and development of breast cancer.Citation26
We discuss the role of Cav1 in ER-positive or -negative breast cancer, and notice several contrary results. With regard to cancer cell apoptosis, Chintala et al noted that downregulation of tumor promoter Cav1 by MβCD contributed to MDA-MB-231 (ER-) apoptosis.Citation65 Their results were contradicted by the experiments of Kang et al, who showed that elevated expression level of Cav1 exposed TNBC cells (MDA-MB-231 cells) to the apoptotic process that suggested Cav1’s role as a tumor suppressor.Citation51 With regard to chemotherapeutics and radiotherapy resistance, Cav1, considered as a tumor suppressor, was phosphorylated by MAPK pathway and conferred acquired resistance to lapatinib in ER(+) breast cancer cells.Citation125 However, interestingly, this is contrary to the results of a study conducted by Zou et al in TNBC cells that demonstrated Cav1 as a tumor promoter via promoting DNA repair and formation of radiation resistance.Citation126 From the aspect of effect of Cav1 in these cases, conclusions seem totally contrary. Among the plausible explanations for these findings are different cell lines and signaling pathways, indicating whether ER expressed in different types of BC may result in diverse interaction with Cav1 and signaling pathways.
In the current review, we found that Cav1 acts both as a tumor suppressor as well as a promoter, and plays a key role in breast cancer tumorigenesis (). We also summarized the role of Cav1 in the development of breast cancer, in the hope of providing a basis for molecular targeted therapy of breast cancer ( and ). We have demonstrated the interaction between Cav1 and human breast cancer.
Table 1 The role of Cav1 in breast cancer cells tumorigenesis
Table 2 The role of Cav1 in breast cancer tissues tumorigenesis
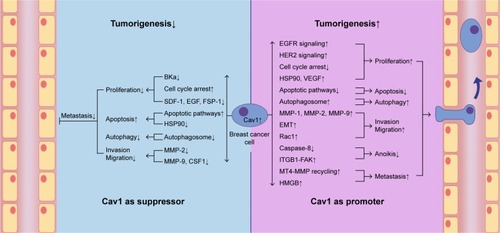
Figure 3 Cav1 acts both as a suppressor and a promoter in breast cancer cell tumorigenesis.
Notes: Cav1 can act as a suppressor in breast cancer cell carcinogenic process via suppressing breast cancer cell proliferation, autophagy, invasion and migration and promoting apoptosis. Cav1 can also act as a promoter in breast cancer cell carcinogenic process via promoting breast cancer cell proliferation, autophagy, invasion, migration and metastasis, and suppressing apoptosis and anoikis.
Abbreviations: BKCa, large conductance Ca2+-activated potassium; Cav1, caveolin-1; CSF1, colony-stimulating factor 1; EMT, epithelial to mesenchymal transition; FSP-1, fibroblast-specific protein-1; HER2, human epidermal growth factor receptor-2; HSP90, heat shock protein 90; MMP, matrix metalloproteinase; MT4-MMP, membrane type 4 matrix metalloproteinase; VEGF, vascular endothelial growth factor; SDF-1, stromal cell-derived factor-1.
Cav1 can affect breast cancer cell proliferation rate by 1) influencing the expression and activation of ion channels and receptors on the cell membrane, such as BKCa channel, EGFR and HER2; 2) regulating cell cycle arrest and affecting mitosis process; and 3) mediating the interaction between the TME (hypoxia conditions and CAFs) and breast cancer cells.
Cav1 can affect breast cancer cell apoptosis by 1) regulating intrinsic and extrinsic apoptotic pathway proteins, such as Bcl-2, Bax and caspase-3; 2) Cav1-induced autophagy alternation can influence cell apoptosis; 3) chemotherapeutic drugs can regulate transcriptional level of CAV1 and promote apoptosis; and 4) regulating the expression and activation of apoptosis-related molecules, such as TIGAR, ROS and onco-proteins.
Cav1 can affect breast cancer cell invasion and migration by 1) influencing the expression level of EMT-associated markers and transcription factors; 2) affecting the expression level of MMPs; 3) regulating the expression of Rho GTPases and 4) participating Cav1-dependent trafficking of integrins.
Cav1 can affect breast cancer metastasis by 1) participating in anoikis progression; 2) regulating the expression and transport of metastasis-associated proteins, such as MT4-MMP, MMP9 and TLR4 and 3) the expression levels and methylation of Cav1 are associated with metastatic breast cancer.
Cav1 can affect breast cancer cell chemotherapeutics and radiotherapy resistance by 1) affecting the expression and activity of BCRP (eg, ABCG2); 2) influencing Cav-1-mediated T-DM1 internalization; 3) regulating eNOS/NO/ONOO− pathway and ER pathway and 4) promoting repair of radiation-induced DNA damage.
Stromal Cav1 expression may be a potential prognostic indicator for breast cancer patients and loss of stromal Cav1 expression often show a poor clinical outcome. What is more, high expression levels of epithelial Cav1, cytoplasmic EGFR and stromal MCT-4 may be secondary prognostic indicators.
The molecular pathways that Cav1 impacts and breast cancer are interrelated. EGFR and HER2 are receptors on the breast cancer cell membrane, and Cav1 promotes breast cancer cell proliferation via EGFR or HER2-induced mitogenic signaling pathway. Besides, the regulation of cell cycle-associated proteins (eg, cyclin B1, cyclin D1, cyclin D2 and β-catenin) is a significant way that Cav1 affects the cell proliferation rate. The activation of ERK pathway not only promotes the proliferation but also endows anoikis resistance to cancer cells, ultimately promoting the progression of breast cancer. High oxidative stress promotes cell proliferation by regulating Cav1, and Cav1 can also promote cell apoptosis via activating ROS pathway and chemotherapeutics resistance via regulating oxidative damage. VEGF is another targeted molecule in Cav1-mediated breast cancer tumorigenesis. Activation of Cav1 upregulates VEGF and promotes cell proliferation, contrarily, Cav1 loss enhances VEGF-A/VEGFR1 activity and facilitates angiogenesis and metastatic growth. The regulation of intrinsic and extrinsic apoptotic pathway-related molecules (eg, survivin, Bcl-2, Bax caspase-8 and caspase-3) is an important process in Cav1-induced apoptosis, and inactivation of caspase-8 can inhibit apoptosis and endow anoikis resistance to cancer cell. MMPs (eg, MMP-1, MMP-2, MMP-9, MMP-14 and MT4-MMP) are key proteins in the EMT process that promotes breast cancer invasion, migration and metastasis. Cav1-induced MMP-2 upregulation promotes BT474 cells’ invasion and migration, while Cav1 can also downregulate the expression of MMP-2 in MCF-7 via inhibiting PI3K/AKT signaling pathway and ultimately suppressing invasion and migration. Cav1-mediated PI3K/AKT signaling pathway activation can promote anoikis resistance. To sum up, Cav1-mediated breast cancer progression-related molecular pathways are correlational, it not only shows the same or opposite effect in the same stage but also in different stages.
Caveolae are cholesterol-enriched rigid membrane microdomains that are composed of caveolins, including Cav1, Cav2 and Cav3. Therefore, the treatment of breast cancer may be achieved by targeting Cav1. First, the use of targeted drugs (eg, γ-tocotrienol and MβCD) to accumulate within the caveolae microdomain, thereby disrupting the structure of caveolae and blocking the Cav1-mediated signaling pathway, may be a means of breast cancer-targeted therapy.Citation49,Citation65,Citation67 Second, targeted drug-induced regulation of Cav1 expression level may be another targeted treatment.Citation51 Besides, targeted drugs (eg, FFLZ and antarctic krill DHA) regulate the activation of Cav1 and Cav1-mediated signaling cascades, ultimately inhibiting the progression of breast cancer.Citation82,Citation86 More importantly, targeted drugs (eg, DHA and T-DM1) can facilitate caveolae-mediated chemotherapy drug internalization by sensitizing caveolae marker Cav1, thus preventing drug resistance and improving therapeutic effect.Citation70,Citation119
Although we have summarized the relationship between Cav1 and a certain tumor progression process in this review, the effect of Cav1 on the development of breast cancer is continuous and multi-process ( and ).Citation172 Apoptosis and cell proliferation are mutually restricted; autophagy can promote cell renewal and inhibit apoptosis and invasion and migration are necessary preparations for distant metastasis. It can be seen that the occurrence and tumorigenesis of breast cancer is a continuous process, and the influence of Cav1 is multi-stage and continuous. Above all, we believe the summary of the interaction between Cav1 and breast cancer can be a basis for further research and promote CAV1 gene as a potential target to diagnose and treat aggressive breast cancer.
Disclosure
LXX received a grant from the National Natural Science Foundation of China (No 31860317). The authors report no other conflicts of interest in this work.
References
- HarbeckNGnantMBreast cancerLancet2017389100741134115027865536
- AnwarSLWahyonoAAryandonoTHaryonoSJCaveolin-1 in breast cancer: single molecule regulation of multiple key signaling pathwaysAsian Pac J Cancer Prev201516166803681226514450
- WardEMDesantisCELinCCCancer statistics: breast cancer in situCA Cancer J Clin201565648149526431342
- DesantisCEMaJGoding SauerANewmanLAJemalABreast Cancer statistics, 2017, racial disparity in mortality by stateCA Cancer J Clin201767643944828972651
- WachterDLWachterPWFaschingPACharacterization of molecular subtypes of Paget disease of the breast using immunohistochemistry and in situ hybridizationArch Pathol Lab Med2019143220621130124327
- VranicSMarchiòCCastellanoIImmunohistochemical and molecular profiling of histologically defined apocrine carcinomas of the breastHum Pathol20154691350135926208846
- RenMLiuFZhuYAbsence of caveolin-1 expression in carcinoma-associated fibroblasts of invasive micropapillary carcinoma of the breast predicts poor patient outcomeVirchows Arch2014465329129824980157
- WangCYeQCaoYDownregulation of regulator of G protein signaling 2 expression in breast invasive carcinoma of NO special type: clinicopathological associations and prognostic relevanceOncol Lett201815121322029391880
- SelviVNoriJMeattiniIRole of magnetic resonance imaging in the preoperative staging and work-up of patients affected by invasive lobular carcinoma or invasive ductolobular carcinomaBiomed Res Int2018201817
- ObeidatFNAhramMAl-KhaderAExpression of androgen receptor in invasive ductal breast carcinomas: a clinicopathological study from JordanAnn Saudi Med201838532633530284987
- SørlieTPerouCMTibshiraniRGene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implicationsProc Natl Acad Sci U S A20019819108691087411553815
- JhanJRAndrechekERTriple-negative breast cancer and the potential for targeted therapyPharmacogenomics201718171595160929095114
- AysolaKDesaiAWelchCTriple negative breast cancer – an overviewHereditary Genet20132013Suppl 2
- ChengCLThikeAATanSYChuaPJBayBHTanPHExpression of FGFR1 is an independent prognostic factor in triple-negative breast cancerBreast Cancer Res Treat201515119911125868865
- YueYAstvatsaturyanKCuiXZhangXFraassBBoseSStratification of prognosis of triple-negative breast cancer patients using combinatorial biomarkersPLoS One2016113e014966126930401
- OmidvariSHamediSHMohammadianpanahMVery late relapse in breast cancer survivors: a report of 6 casesIran J Cancer Prev20136211311725250120
- CollignonJLousbergLSchroederHJerusalemGTriple-negative breast cancer: treatment challenges and solutionsBreast Cancer201689310727284266
- EbisawaMIwanoHNishikawaMSignificance of caveolin-1 and matrix metalloproteinase 14 gene expression in canine mammary tumoursVet J2015206219119626364240
- ShinodaHLegareMEMasonGLSignificance of ERα, HER2, and CaV1 expression and molecular subtype classification to canine mammary gland tumorJ Vet Diagn Invest201426339040324760135
- EttlinJClementiEAminiPMalbonAMarkkanenEAnalysis of gene expression signatures in cancer-associated stroma from canine mammary tumours reveals molecular homology to human breast carcinomasInt J Mol Sci2017185E110128531107
- Drews-ElgerKIornsEDiasAInfiltrating S100A8+ myeloid cells promote metastatic spread of human breast cancer and predict poor clinical outcomeBreast Cancer Res Treat20141481415925270120
- ChengJPXNicholsBJCaveolae: one function or many?Trends Cell Biol201626317718926653791
- Martinez-OutschoornUESotgiaFLisantiMPCaveolae and signalling in cancerNat Rev Cancer201515422523725801618
- EcharriAdel PozoMACaveolaeCurr Biol2012224R114R11622361143
- ChamberlainLHDetergents as tools for the purification and classification of lipid raftsFEBS Lett20045591–31514986659
- TottaPGionfraFBusoneroCAcconciaFModulation of 17β-estradiol signaling on cellular proliferation by caveolin-2J Cell Physiol201623161219122526480297
- KovtunOTilluVAAriottiNPartonRGCollinsBMCavin family proteins and the assembly of caveolaeJ Cell Sci201512871269127825829513
- WangSWangNZhengYZhangJZhangFWangZCaveolin-1: an oxidative stress-related target for cancer preventionOxid Med Cell Longev20172017120
- ParatMORigginsGJCaveolin-1, caveolae, and glioblastomaNeurooncology2012146679688
- ThompsonMAPrakashYSPabelickCMThe role of caveolae in the pathophysiology of lung diseasesExpert Rev Respir Med20148111112224308657
- DuhonDBigelowRLHColemanDTThe polyphenol epigal-locatechin-3-gallate affects lipid rafts to block activation of the c-met receptor in prostate cancer cellsMol Carcinog201049873974920623641
- PikeLJGrowth factor receptors, lipid rafts and caveolae: an evolving storyBiochim Biophys Acta20051746326027315951036
- GleissmanHSegerströmLHambergMOmega-3 fatty acid supplementation delays the progression of neuroblastoma in vivoInt J Cancer201112871703171120499314
- GuptaRToufailyCAnnabiBCaveolin and cavin family members: dual roles in cancerBiochimie2014107Pt B18820225241255
- QuestAFLobos-GonzálezLNuñezSThe caveolin-1 connection to cell death and survivalCurr Mol Med201313226628123228128
- PeterMEHadjiAMurmannAEThe role of CD95 and CD95 ligand in cancerCell Death Differ201522454955925656654
- ChenDCheGValue of caveolin-1 in cancer progression and prognosis: emphasis on cancer-associated fibroblasts, human cancer cells and mechanism of caveolin-1 expression (review)Oncol Lett2014841409142125202343
- Al-AnsariMMAboussekhraACaffeine mediates sustained inactivation of breast cancer-associated myofibroblasts via up-regulation of tumor suppressor genesPLoS One201493e9090724595168
- PataniNMartinLAReis-FilhoJSDowsettMThe role of caveolin-1 in human breast cancerBreast Cancer Res Treat2012131111521901387
- MercierILisantiMPCaveolin-1 and breast cancer: a new clinical perspectiveAdv Exp Med Biol2012729839422411315
- FuPChenFPanQThe different functions and clinical significances of caveolin-1 in human adenocarcinoma and squamous cell carcinomaOnco Targets Ther20171081983528243118
- MollinedoFde La Iglesia-VicenteJGajateCIn vitro and in vivo selective antitumor activity of edelfosine against mantle cell lymphoma and chronic lymphocytic leukemia involving lipid raftsClin Cancer Res20101672046205420233887
- GajateCMollinedoFEdelfosine and perifosine induce selective apoptosis in multiple myeloma by recruitment of death receptors and downstream signaling molecules into lipid raftsBlood2007109271171917003375
- NaHKSurhYJThe antitumor ether lipid edelfosine (ET-18-O-CH3) induces apoptosis in H-ras transformed human breast epithelial cells: by blocking ERK1/2 and p38 mitogen-activated protein kinases as potential targetsAsia Pac J Clin Nutr200817Suppl 120420718296338
- ChantômeAPotier-CartereauMClarysseLPivotal role of the lipid raft SK3-Orai1 complex in human cancer cell migration and bone metastasesCancer Res201373154852486123774210
- DuCChenLZhangHCaveolin-1 limits the contribution of BKCa channel to MCF-7 breast cancer cell proliferation and invasionInt J Mol Sci20141511207062072225397596
- LiangYNLiuYWangLCombined caveolin-1 and epidermal growth factor receptor expression as a prognostic marker for breast cancerOncol Lett20181569271928229805656
- NahtaRYuDHungMCHortobagyiGNEstevaFJMechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancerNat Clin Pract Oncol20063526928016683005
- AlawinOAAhmedRAIbrahimBABriskiKPSylvesterPWAntiproliferative effects of γ-tocotrienol are associated with lipid raft disruption in HER2-positive human breast cancer cellsJ Nutr Biochem20162726627726507543
- WangRLiZGuoHCaveolin 1 knockdown inhibits the proliferation, migration and invasion of human breast cancer BT474 cellsMol Med Rep2014951723172824604116
- KangJParkJHLeeHJCaveolin-1 modulates docetaxel-induced cell death in breast cancer cell subtypes through different mechanismsCancer Res Treat201648271572626511813
- XuFWangXWuNBisphenol A induces proliferative effects on both breast cancer cells and vascular endothelial cells through a shared GPER-dependent pathway in hypoxiaEnviron Pollut2017231Pt 21609162028964603
- MontiDSotgiaFWhitaker-MenezesDPilot study demonstrating metabolic and anti-proliferative effects of in vivo anti-oxidant supplementation with N-acetylcysteine in breast cancerSemin Oncol201744322623229248134
- MartinsDBeçaFFSousaBBaltazarFParedesJSchmittFLoss of caveolin-1 and gain of MCT4 expression in the tumor stroma: key events in the progression from an in situ to an invasive breast carcinomaCell Cycle201312162684269023907124
- AlbanitoLLappanoRMadeoAEffects of atrazine on estrogen receptor α- and G protein-coupled receptor 30-mediated signaling and proliferation in cancer cells and cancer-associated fibroblastsEnviron Health Perspect2015123549349925616260
- LiuJJinXZhaoNYeXYingCBisphenol A promotes X-linked inhibitor of apoptosis protein-dependent angiogenesis via G protein-coupled estrogen receptor pathwayJ Appl Toxicol201535111309131725663485
- Castillo SanchezRGomezRPerez SalazarEBisphenol A induces migration through a GPER-, FAK-, Src-, and ERK2-dependent pathway in MDA-MB-231 breast cancer cellsChem Res Toxicol201629328529526914403
- SemenzaGLThe hypoxic tumor microenvironment: a driving force for breast cancer progressionBiochim Biophys Acta186320163382391
- ShiXYXiongLXXiaoLDownregulation of caveolin-1 upregulates the expression of growth factors and regulators in co-culture of fibroblasts with cancer cellsMol Med Rep201613174475226647977
- LiTKonNJiangLTumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescenceCell201214961269128322682249
- ZhangLRenXAltEChemoprevention of colorectal cancer by targeting APC-deficient cells for apoptosisNature201046472911058106120348907
- PfefferCMSinghATKApoptosis: a target for anticancer therapyInt J Mol Sci2018192E44829393886
- YuCLiWBLiuJBLuJWFengJFAutophagy: novel applications of nonsteroidal anti-inflammatory drugs for primary cancerCancer Med20187247148429282893
- DaskalakiIGkikasITavernarakisNHypoxia and selective autophagy in cancer development and therapyFront Cell Dev Biol2018610430250843
- BadanaAKChintalaMGavaraMMLipid rafts disruption induces apoptosis by attenuating expression of LRP6 and survivin in triple negative breast cancerBiomed Pharmacother20189735936829091885
- WangRHeWLiZChangWXinYHuangTCaveolin-1 functions as a key regulator of 17β-estradiol-mediated autophagy and apoptosis in BT474 breast cancer cellsInt J Mol Med201434382282725017566
- ShiYTanSHNgSCritical role of CAV1/caveolin-1 in cell stress responses in human breast cancer cells via modulation of lysosomal function and autophagyAutophagy201511576978425945613
- SalisOBedirAGultenSOkuyucuAKulcuCAlacamHCytotoxic effect of fluvastatin on MCF-7 cells possibly through a reduction of the mRNA expression levels of SGK1 and CaV1Cancer Biother Radiopharm201429936837525347557
- SalisOBedirAOzdemirTOkuyucuAAlacamHThe relationship between anticancer effect of metformin and the transcriptional regulation of certain genes (CHOP, Cav-1, HO-1, SGK-1 and PAR-4) on MCF-7 cell lineEur Rev Med Pharmacol Sci201418111602160924943970
- LeeEJYunUJKooKHDown-regulation of lipid raft-associated onco-proteins via cholesterol-dependent lipid raft internalization in docosahexaenoic acid-induced apoptosisBiochim Biophys Acta184120141190203
- GoetzJGLajoiePWisemanSMNabiIRCaveolin-1 in tumor progression: the good, the bad and the uglyCancer Metastasis Rev200827471573518506396
- BaileyKMLiuJCaveolin-1 up-regulation during epithelial to mesenchymal transition is mediated by focal adhesion kinaseJ Biol Chem200828320137141372418332144
- GaiXLuZTuKLiangZZhengXCaveolin-1 is up-regulated by Gli1 and contributes to GLI1-driven EMT in hepatocellular carcinomaPLoS One201491e8455124454730
- LamouilleSXuJDerynckRMolecular mechanisms of epithelial-mesenchymal transitionNat Rev Mol Cell Biol201415317819624556840
- CowinPWelchDRBreast cancer progression: controversies and consensus in the molecular mechanisms of metastasis and EMTJ Mammary Gland Biol Neoplasia2007122–39910218769505
- Moreno-BuenoGPortilloFCanoATranscriptional regulation of cell polarity in EMT and cancerOncogene200827556958696919029937
- HuangHMatrix metalloproteinase-9 (MMP-9) as a cancer biomarker and MMP-9 biosensors: recent advancesSensors201818103249
- WangHZhuYZhaoMmiRNA-29c suppresses lung cancer cell adhesion to extracellular matrix and metastasis by targeting integrin β1 and matrix metalloproteinase2 (MMP2)PLoS One201388e7019223936390
- PalSGangulyKKChatterjeeAExtracellular matrix protein fibronectin induces matrix metalloproteinases in human prostate adenocarcinoma cells PC-3Cell Commun Adhes201320510511424047237
- YaoQKouLTuYZhuLMMP-responsive “smart” drug delivery and tumor targetingTrends Pharmacol Sci201839876678130032745
- ZielinskaHAHollyJMPBahlAPerksCMCmPBahlAInhibition of FASN and ERα signalling during hyperglycaemia-induced matrix-specific EMT promotes breast cancer cell invasion via a caveolin-1-dependent mechanismCancer Lett201841918720229331414
- TsaoSMHsuHYFucose-containing fraction of Ling-Zhi enhances lipid rafts-dependent ubiquitination of TGFβ receptor degradation and attenuates breast cancer tumorigenesisSci Rep201663656327830743
- GallardoMCalafGMCurcumin and epithelial-mesenchymal transition in breast cancer cells transformed by low doses of radiation and estrogenInt J Oncol20164862534254227082017
- Ponce-CusiRCalafGMAntitumor activity of pamidronate in breast cancer cells transformed by low doses of α-particles and estrogen in vitroInt J Oncol20154662663266925873070
- ColeGMLimGPYangFPrevention of Alzheimer’s disease: omega-3 fatty acid and phenolic anti-oxidant interventionsNeurobiol Aging200526Suppl 113313616266772
- ZhengWLiJWangXYuanYZhangJXiuZEffects of Antarctic krill docosahexaenoic acid on MCF-7 cell migration and invasion induced by the interaction of CD95 with caveolin-1Life Sci201819227027729129771
- BraunACOlayioyeMARho regulation: DLC proteins in space and timeCell Signal20152781643165125889896
- JoglekarMElbezantiWOWeitzmanMDLehmanHLvan GolenKLCaveolin-1 mediates inflammatory breast cancer cell invasion via the Akt1 pathway and RhoC GTPaseJ Cell Biochem2015116692393325559359
- YangBZhuWZhengZFluctuation of ROS regulates proliferation and mediates inhibition of migration by reducing the interaction between DLC1 and Cav-1 in breast cancer cellsIn Vitro Cell Dev Biol Anim201753435436228130753
- KandpalRPRho GTPase activating proteins in cancer phenotypesCurr Protein Pept Sci20067435536516918449
- DíazJMendozaPOrtizRRab5 is required in metastatic cancer cells for Caveolin-1-enhanced Rac1 activation, migration and invasionJ Cell Sci2014127Pt 112401240624659799
- KozyulinaPYLoskutovYVKozyrevaVKPrometastatic NEDD9 regulates individual cell migration via caveolin-1-dependent trafficking of integrinsMol Cancer Res201513342343825319010
- ChenYOlopadeOIMYC in breast tumor progressionExpert Rev Anticancer Ther20088101689169818925859
- XiongNLiSTangKInvolvement of caveolin-1 in low shear stress-induced breast cancer cell motility and adhesion: roles of FAK/Src and ROCK/p-MLC pathwaysBiochim Biophys Acta Mol Cell Res1864201711222
- ValastyanSWeinbergRATumor metastasis: molecular insights and evolving paradigmsCell2011147227529222000009
- ChafferCLWeinbergRAA perspective on cancer cell metastasisScience201133160241559156421436443
- GilmoreAPAnoikisCell Death Differ200512Suppl 21473147716247493
- KimYNKooKHSungJYYunUJKimHAnoikis resistance: an essential prerequisite for tumor metastasisInt J Cell Biol201220124111
- SimpsonCDAnyiweKSchimmerADAnoikis resistance and tumor metastasisCancer Lett2008272217718518579285
- PaoliPGiannoniEChiarugiPAnoikis molecular pathways and its role in cancer progressionBiochim Biophys Acta183320131234813498
- LiSChenYZhangYShear stress promotes anoikis resistance of cancer cells via caveolin-1-dependent extrinsic and intrinsic apoptotic pathwaysJ Cell Physiol201923443730374330171601
- WangKZhuXChenYYinYMaTTubeimoside V sensitizes human triple negative breast cancer MDA-MB-231 cells to anoikis via regulating caveolin-1-related signaling pathwaysArch Biochem Biophys2018646101529580948
- NimriLBarakHGraeveLSchwartzBRestoration of caveolin-1 expression suppresses growth, membrane-type-4 metalloproteinase expression and metastasis-associated activities in colon cancer cellsMol Carcinog2013521185987022674854
- TruongAYipCPayeADynamics of internalization and recycling of the prometastatic membrane type 4 matrix metalloproteinase (MT4-MMP) in breast cancer cellsFEBS J2016283470472226663028
- CelusWdi ConzaGOliveiraAILoss of caveolin-1 in metastasis-associated macrophages drives lung metastatic growth through increased angiogenesisCell Rep201721102842285429212030
- LvWChenNLinYMacrophage migration inhibitory factor promotes breast cancer metastasis via activation of HMGB1/TLR4/NF kappa B axisCancer Lett2016375224525526952810
- AlevizosLKatakiADerventziABreast cancer nodal metastasis correlates with tumour and lymph node methylation profiles of caveolin-1 and CXCR4Clin Exp Metastasis201431551152024590865
- KowalskaKNowakowskaMDomińskaKPiastowska-CiesielskaAWCoexpression of Cav-1, AT1-R and FoxM1 in prostate and breast cancer and normal cell lines and their influence on metastatic propertiesActa Biochim Pol201663349349927213551
- MagbanuaMJMRugoHSHauraniehLGenomic and expression profiling reveal molecular heterogeneity of disseminated tumor cells in bone marrow of early breast cancerNPJ Breast Cancer2018413130211312
- KuczynskiEASargentDJGrotheyAKerbelRSDrug rechallenge and treatment beyond progression – implications for drug resistanceNat Rev Clin Oncol2013101057158723999218
- MalliniPLennardTKirbyJMeesonAEpithelial-to-mesenchymal transition: what is the impact on breast cancer stem cells and drug resistanceCancer Treat Rev201440334134824090504
- HehlgansSCordesNCaveolin-1: an essential modulator of cancer cell radio-and chemoresistanceAm J Cancer Res20111452153021984970
- WangZLiuPChenQTargeting AMPK signaling pathway to overcome drug resistance for cancer therapyCurr Drug Targets201617885386425777274
- WangZWangNLiWCaveolin-1 mediates chemoresistance in breast cancer stem cells via β-catenin/ABCG2 signaling pathwayCarcinogenesis201435102346235625085904
- HerzogMStorchCHGutPKnockdown of caveolin-1 decreases activity of breast cancer resistance protein (BCRP/ABCG2) and increases chemotherapeutic sensitivityNaunyn Schmiedebergs Arch Pharmacol2011383111120936466
- PengLChenXAntibody-drug conjugatesBioconjug Chem20152611216926577284
- KimEGKimKMStrategies and advancement in antibody-drug conjugate optimization for targeted cancer therapeuticsBiomol Ther2015236493509
- LorussoPMWeissDGuardinoEGirishSSliwkowskiMXTrastuzumab emtansine: a unique antibody-drug conjugate in development for human epidermal growth factor receptor 2-positive cancerClin Cancer Res201117206437644722003071
- SekharSCKasaiTSatohAIdentification of caveolin-1 as a potential causative factor in the generation of trastuzumab resistance in breast cancer cellsJ Cancer20134539140123833684
- ChungYCKuoJFWeiWCChangKJChaoWTCaveolin-1 dependent endocytosis enhances the chemosensitivity of HER-2 positive breast cancer cells to trastuzumab emtansine (T-DM1)PLoS One2015107e013307226172389
- ChungYCChangCMWeiWCChangTWChangKJChaoWTMetformin-induced caveolin-1 expression promotes T-DM1 drug efficacy in breast cancer cellsSci Rep201881393029500444
- von der HeydeSWagnerSCzernyAmRNA profiling reveals determinants of trastuzumab efficiency in HER2-positive breast cancerPLoS One2015102e011781825710561
- SungMTanXLuBCaveolae-mediated endocytosis as a novel mechanism of resistance to trastuzumab emtansine (T-DM1)Mol Cancer Ther201817124325329054985
- ZhengYDaiYLiuWAstragaloside IV enhances taxol chemo-sensitivity of breast cancer via caveolin-1-targeting oxidant damageJ Cell Physiol201923444277429030146689
- LiZYangSSYinPHActivated estrogen receptor-mitogen-activated protein kinases cross talk confer acquired resistance to lapatinibThoracic Cancer20156669570326557906
- ZouMLiYXiaSKnockdown of caveolin-1 sensitizes human basal-like triple-negative breast cancer cells to radiationCell Physiol Biochem201744277879129169152
- MalikBNieDCancer stem cells and resistance to chemo and radio therapyFront Biosci2012421422149
- SzakácsGPatersonJKLudwigJABooth-GentheCGottesmanMMTargeting multidrug resistance in cancerNat Rev Drug Discov20065321923416518375
- TrumppAWiestlerODMechanisms of disease: cancer stem cells – targeting the evil twinNat Clin Pract Oncol20085633734718431377
- Maugeri-SaccàMVigneriPde MariaRCancer stem cells and chemosensitivityClin Cancer Res201117154942494721622723
- DeanMABC transporters, drug resistance, and cancer stem cellsJ Mammary Gland Biol Neoplasia20091413919224345
- DonnenbergVSMeyerEMDonnenbergADMeasurement of multiple drug resistance transporter activity in putative cancer stem/progenitor cellsMethods Mol Biol200956826127919582433
- HirschHAIliopoulosDTsichlisPNStruhlKMetformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remissionCancer Res200969197507751119752085
- Marie-EgyptienneDTLohseIHillRPCancer stem cells, the epithelial to mesenchymal transition (EMT) and radioresistance: potential role of hypoxiaCancer Lett20133411637223200673
- Merlos-SuárezABarrigaFMJungPThe intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapseCell Stem Cell20118551152421419747
- PeronaRLópez-AyllónBDde Castro CarpeñoJBelda-IniestaCA role for cancer stem cells in drug resistance and metastasis in non-small-cell lung cancerClin Transl Oncol201113528929321596655
- SheridanCKishimotoHFuchsRKCD44+/CD24− breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasisBreast Cancer Res200685R5917062128
- YuanGRegelILianFWNT6 is a novel target gene of caveo-lin-1 promoting chemoresistance to epirubicin in human gastric cancer cellsOncogene201332337538722370641
- YongsanguanchaiNPongrakhananonVMutiranguraARojanasakulYChanvorachotePNitric oxide induces cancer stem cell-like phenotypes in human lung cancer cellsAm J Physiol Cell Physiol20153082C89C10025411331
- EliyatkinNAktasSDinizGOzgurHHEkinZYKupeliogluAExpression of stromal caveolin-1 may be a predictor for aggressive behaviour of breast cancerPathol Oncol Res2018241596528236153
- PucciMBravatàVForteGICaveolin-1, breast cancer and ionizing radiationCancer Genomics Proteomics201512314315225977173
- WitkiewiczAKDasguptaANguyenKHStromal caveolin-1 levels predict early DCIS progression to invasive breast cancerCancer Biol Ther20098111071107919502809
- SotgiaFMartinez-OutschoornUEHowellAPestellRGPavlidesSLisantiMPCaveolin-1 and cancer metabolism in the tumor microenvironment: markers, models, and mechanismsAnnu Rev Pathol20127142346722077552
- YeongJThikeAAIkedaMCaveolin-1 expression as a prognostic marker in triple negative breast cancers of Asian womenJ Clin Pathol201871216116728735300
- LiXSunJHuSExpression of caveolin-1 in breast cancer stroma as a potential prognostic biomarker of survival and progression: a meta-analysisWien Klin Wochenschr201712915–1655856328364168
- MaXLiuLNieWPrognostic role of caveolin in breast cancer: a meta-analysisBreast201322446246923639584
- ElsheikhSEGreenARRakhaEACaveolin 1 and caveolin 2 are associated with breast cancer basal-like and triple-negative immunophenotypeBr J Cancer200899232733418612310
- QianNUenoTKawaguchi-SakitaNPrognostic significance of tumor/stromal caveolin-1 expression in breast cancer patientsCancer Sci201110281590159621585620
- JensenDHTherkildsenMHDabelsteenEA reverse Warburg metabolism in oral squamous cell carcinoma is not dependent upon myofibroblastsJ Oral Pathol Med201544971472125420473
- TasdemirNBossartEALiZComprehensive phenotypic characterization of human invasive lobular carcinoma cell lines in 2D and 3D culturesCancer Res201878216209622230228172
- FardZTNafisiNThe relationship between 6 polymorphisms of caveolin-1 gene and the risk of breast cancerClin Breast Cancer2018185e893e89829525429
- DebMSenguptaDKarSElucidation of caveolin 1 both as a tumor suppressor and metastasis promoter in light of epigenetic modulatorsTumour Biol20143512120311204725192721
- ChanvorachotePChunhachaPCaveolin-1 regulates endothelial adhesion of lung cancer cells via reactive oxygen species-dependent mechanismPLoS One201382e5746623460862
- WuJdiDZhaoCClinical significance of Gli-1 and caveolin-1 expression in the human small cell lung cancerAsian Pac J Cancer Prev201819240140629479989
- ZhaoZHanFHYangSBHuaLXJhWZhanWHLoss of stromal caveolin-1 expression in colorectal cancer predicts poor survivalWorld J Gastroenterol20152141140114725632186
- WangYSongYCheXCaveolin-1 enhances RANKL-induced gastric cancer cell migrationOncol Rep20184031287129630015970
- LiangXChenWShiHPTBP3 contributes to the metastasis of gastric cancer by mediating CaV1 alternative splicingCell Death Dis20189556929752441
- ZhangTWangTCaiPSclareol inhibits cell proliferation and sensitizes cells to the antiproliferative effect of bortezomib via upregulating the tumor suppressor caveolin-1 in cervical cancer cellsMol Med Rep20171563566357428440485
- TakedaMSakaguchiTHiraideTRole of caveolin-1 in hepatocellular carcinoma arising from non-alcoholic fatty liver diseaseCancer Sci201810982401241129896915
- DemirciNSDoganMErdemGUIs plasma caveolin-1 level a prognostic biomarker in metastatic pancreatic cancer?Saudi J Gastroenterol201723318318928611342
- ZhouWHeLDaiYZhangYWangJLiuBMicroRNA-124 inhibits cell proliferation, invasion and migration by targeting CaV1 in bladder cancerExp Ther Med20181642811282030214503
- Garcia-RivasGJerjes-SánchezCRodriguezDGarcia-PelaezJTrevinoVA systematic review of genetic mutations in pulmonary arterial hypertensionBMC Med Genet20171818228768485
- KobayashiHKabataRKinoshitaHRare variants in RNF213, a susceptibility gene for moyamoya disease, are found in patients with pulmonary hypertension and aggravate hypoxia-induced pulmonary hypertension in micePulm Circ201883204589401877815
- GirerdBLauEMontaniDHumbertMGenetics of pulmonary hypertension in the clinicCurr Opin Pulm Med201723538639128661905
- MahdyMMEl-EkiabyNMHashishRMmiR-29a promotes lipid droplet and triglyceride formation in HCV infection by inducing expression of SREBP-1c and CaV1J Clin Transl Hepatol20164429329928097097
- SomritMWatthammawutAChotwiwatthanakunCWeerach-atyanukulWThe key molecular events during Macrobrachium rosenbergii nodavirus (MrNV) infection and replication in Sf9 insect cellsVirus Res20162231927327530
- HuZLiangMSoongTAlternative splicing of L-type Cav1.2 calcium channels: implications in cardiovascular diseasesGenes2017812344
- ChenSWangXWangJGenomic variant in CaV1 increases susceptibility to coronary artery disease and myocardial infarctionAtherosclerosis201624614815626775120
- TorellaDIaconettiCTaralloRmiRNA regulation of the hyperproliferative phenotype of vascular smooth muscle cells in diabetesDiabetes201867122554256830257973
- XiangSSaremMShahSShastriVPLiposomal treatment of cancer cells modulates uptake pathway of polymeric nanoparticles by altering membrane stiffnessSmall20181414e170424529460335
- HanBCopelandCAKawanoYCharacterization of a caveolin-1 mutation associated with both pulmonary arterial hypertension and congenital generalized lipodystrophyTraffic201617121297131227717241
- BadanaAChintalaMVarikutiGLipid raft integrity is required for survival of triple negative breast cancer cellsJ Breast Cancer201619437238428053625

