Abstract
Escitalopram (escitalopram oxalate; Cipralex®, Lexapro®) is a selective serotonin reuptake inhibitor (SSRI) used for the treatment of major depressive disorder (MDD) and anxiety disorder. This drug exerts a highly selective, potent, and dose-dependent inhibitory effect on the human serotonin transport. By inhibiting the reuptake of serotonin into presynaptic nerve endings, this drug enhances the activity of serotonin in the central nervous system. Escitalopram also has allosteric activity. Moreover, the possibility of interacting with other drugs is considered low. This review covers randomized, controlled studies that enrolled adult patients with MDD to evaluate the efficacy of escitalopram based on the Montgomery–Asberg Depression Rating Scale and the Hamilton Depression Rating Scale. The results showed that escitalopram was superior to placebo, and nearly equal or superior to other SSRIs (eg, citalopram, paroxetine, fluoxetine, sertraline) and serotonin-noradrenaline reuptake inhibitors (eg, duloxetine, sustained-release venlafaxine). In addition, with long-term administration, escitalopram has shown a preventive effect on MDD relapse and recurrence. Escitalopram also showed favorable tolerability, and associated adverse events were generally mild and temporary. Discontinuation symptoms were milder with escitalopram than with paroxetine. In view of the patient acceptability of escitalopram, based on both a meta-analysis and a pooled analysis, this drug was more favorable than other new antidepressants. The findings indicate that escitalopram achieved high continuity in antidepressant drug therapy.
Introduction
Escitalopram (escitalopram oxalate; Cipralex® [H Lundbeck A/S, Copenhagen, Denmark], Lexapro® [Forest Laboratories, Inc, St Louis, MO]) is a selective serotonin reuptake inhibitor (SSRI) that selectively binds to the human serotonin transporter (SERT). This activity inhibits serotonin (5-HT) reuptake and increases the amount of serotonin in synaptic clefts, which results in antidepressant action.
Racemic citalopram (RS-citalopram), an SSRI widely used in patients with major depressive disorder (MDD), possesses both an active S-enantiomer and clinically inactive R-enantiomer.Citation1,Citation2 Escitalopram was produced by isolating the active S-enantiomer from RS-citalopram. The structural formula of escitalopram is shown in . In vitro and in vivo studies have shown that escitalopram inhibits the serotonin transporter protein more potently than citalopram.Citation2–Citation4 For example, in vivo electrophysiological data indicated that escitalopram was four times more potent than citalopram in reducing the firing activity of presumed serotonergic neurons in the dorsal raphe nucleus of rat brain.Citation5 In November 2011, escitalopram was approved in 100 countries in Europe, North America, and other regions. Escitalopram is indicated for generalized anxiety disorder, social anxiety disorder, obsessive-compulsive disorder, panic disorder, premenstrual dysphoric disorder, and MDD.Citation6
Pharmacological profile
Pharmacodynamic profile
Escitalopram has a highly selective, dose-dependent, inhibitory effect on SERT. Its antidepressant action arises from its inhibition of serotonin reuptake into presynaptic nerve ending, which enhances serotonin activity in the central nervous system.Citation1,Citation7 Radioligand binding assays revealed that escitalopram showed particularly high selectivity for SERT compared to citalopram and several other SSRIs.Citation7–Citation9 Escitalopram is “the most typical SSRI” of the SSRI agents, because it has virtually no binding affinity for other transporters.Citation7,Citation9
Escitalopram binds to two different sites of SERTs: the high-affinity binding site (primary site) of SERT, which controls serotonin reuptake in nerve endings; and the low-affinity binding site (allosteric site), which induces structural changes in SERT. The latter (allosteric action) is thought to stabilize and prolong binding of escitalopram to the primary site.Citation3,Citation10–Citation12
Pharmacokinetic profile
The half-life of receptor occupancy for escitalopram was calculated to be approximately 130 hours, much longer than the half-life of the plasma concentration, which was approximately 30 hours.Citation13 shows the binding occupancy of escitalopram on cerebral SERTs relative to its concentration changes in plasma. An allosteric action may be involved in this prolonged occupancy. Escitalopram is metabolized in the liver, mainly by cytochrome P-450 (CYP) 2C19 and also by CYP3A4 and CYP2D6. Escitalopram inhibits liver metabolic enzymes, but primarily only CYP2D6,Citation14 with minimal inhibition of the other enzymes; the IC50 for CYP2D6 was higher than its effective blood concentration. In this regard, its interactions with other drugs would presumably be minimal.
Figure 2 Escitalopram showed 5-HT transporter occupancy that outlived its plasma concentration.
Copyright © 2007, Springer-Verlag. Adapted with permission from Klein N, Sacher J, Geiss-Granadia T, et al. Higher serotonin transporter occupancy after multiple dose administration of escitalopram compared to citalopram: an [123I]ADAM SPECT study. Psychopharmacology (Berl). 2007;191(2):333–339.Citation13
![Figure 2 Escitalopram showed 5-HT transporter occupancy that outlived its plasma concentration.Copyright © 2007, Springer-Verlag. Adapted with permission from Klein N, Sacher J, Geiss-Granadia T, et al. Higher serotonin transporter occupancy after multiple dose administration of escitalopram compared to citalopram: an [123I]ADAM SPECT study. Psychopharmacology (Berl). 2007;191(2):333–339.Citation13](/cms/asset/2fa62c3a-bc1f-434f-b788-ddd986d744c7/dppa_a_22495_f0002_c.jpg)
Clinical efficacy
Comparison with placebo
In a placebo-controlled study,Citation15 patients with MDD received escitalopram at a dose of 10 mg/day, and a control group was given placebo. After 8 weeks of therapy, the total Montgomery–Asberg Depression Rating Scale (MADRS) score changed by −16.3 in the escitalopram group and −13.6 in the placebo group. Thus, escitalopram had significantly greater efficacy than placebo. The total MADRS score of the escitalopram group began to show significant improvement compared to that of the placebo group by the second week of therapy. This demonstrated its fast-acting property. In addition, the remission rate (the percentage of patients with a total MADRS score of 12 or less) was significantly higher in the escitalopram group than in the placebo group. Thus, the initial therapeutic dose (10 mg/day) was demonstrated to be effective. Likewise, in other studies,Citation15,Citation16 escitalopram 10 or 20 mg/day was more effective than placebo in the treatment of MDD. Reduction in MADRS scores, the primary endpoint, were greater with escitalopram than with placebo at the firstCitation16 or secondCitation15 week and were maintained throughout treatment. Furthermore, Clinical Global Impression-Improvement (CGI-I) and Clinical Global Impression-Severity (CGI-S) scores were reported,Citation15 and support the MADRS score findings: escitalopram produced significant lower CGI-I scores from week 1 and CGI-S scores from week 3 than placebo, and this continued throughout treatment.
Comparison with SSRIs
Six randomized, double-blind, controlled studiesCitation16–Citation21 compared escitalopram and citalopram. Escitalopram was administered to patients with MDD for 4–8 weeks at 10–20 mg/day. All six studiesCitation16–Citation21 showed that the efficacy of escitalopram was equivalent to or greater than that of citalopram. Details of these studies follow.
In the study by Burke et alCitation16 (n = 491; randomly assigned to placebo, escitalopram, 10 mg/day, 20 mg/day, or citalopram, 40 mg/day), escitalopram (10 mg/day) was at least as effective as citalopram at endpoint. In the study by Lepola et al,Citation17 by week 8, significantly more patients had responded to treatment with escitalopram (n = 155) than with citalopram (n = 160). In the study by Lalit et al,Citation18 response rates at the end of 2 weeks were 58% for escitalopram (10 mg/day) (n = 69) and 49% for citalopram (20 mg/day) (n = 74). Response rates at the end of 4 weeks were 90% for escitalopram (10–20 mg/day) and 86% for citalopram (20–40 mg/day). The remission rates at the end of 4 weeks were 74% for escitalopram and 65% for citalopram. Additionally, there were fewer dropouts and less requirement for dose escalation with escitalopram than with citalopram. In the study of Moore et al,Citation19 MADRS scores decreased more in the escitalopram (n = 138) than in the citalopram (n = 142) arm. There were more treatment responders with escitalopram (76.1%) than with citalopram (61.3%), and adjusted remitter rates were 56.1% and 43.6%, respectively.
In the study by Yevtushenko et alCitation21 (n = 322; randomly assigned to escitalopram, 10 mg/day or citalopram, 10–20 mg/day), at study end, the mean change from baseline in MADRS total score was significantly greater in the escitalopram arm than in the 10 and 20 mg/day citalopram arms. Changes in the CGI-S and CGI-I scores and the rates of response and remission were significantly greater in the escitalopram group compared with those in the citalopram 10 and 20 mg/day groups. On the other hand, in the study by Ou et alCitation20 (n = 240, randomly assigned to escitalopram, 10–20 mg/day or citalopram, 20–40 mg/day), no significant differences were found between the two groups.
The meta-analysis of Montgomery et al,Citation22 comparing escitalopram and citalopram, supported these controlled studies: escitalopram was significantly more effective than citalopram in overall treatment effect, with an estimated mean treatment difference of 1.7 points at week 8 on the MADRS and in responder rate (8.3 percentage points) and remitter rate (17.6 percentage points) analyses, corresponding to number-needed-to-treat (NNT) values of 11.9 for response and 5.7 for remission. The overall odds ratios were 1.44 for response and 1.86 for remission, in favor of escitalopram. However, TrkuljaCitation23 reported that MADRS reduction was greater with escitalopram, but 95% confidence intervals (CIs) around the mean difference were entirely or largely below two scale points (minimally important difference) and CI around the effect size (ES) was below 0.32 (“small”) at all time points. Risk of response was higher with escitalopram at week 8 (relative risk, 1.14; 95% CI, 1.04 to 1.26) but NNT was 14 (95% CI, 7 to 111). All 95% CIs around the mean difference and ES of CGI-S reduction at week 8 were below 0.32 points and the limit of “small,” respectively. The report concluded that the claims about clinically relevant superiority of escitalopram over citalopram in short- to medium-term treatment of MDD are not supported by evidence.
A long-term, double-blind, controlled study compared paroxetine to escitalopram given for 24 weeks to patients with severe depression.Citation24 In that study, escitalopram at 20 mg/day showed better efficacy than paroxetine at 40 mg/day. The total MADRS score changed by −25.2 in patients given escitalopram and by −23.1 in those given paroxetine. Thus, the outcome was significantly better for the escitalopram group, with an intergroup difference of 2.12. Furthermore, the total Hamilton Depression Rating Scale (HAM-D17) score changed by −16.9 and −15.0 in the two groups, respectively; again, significantly better outcomes were shown for the escitalopram group than for the paroxetine group. In addition, the remission rate (percentage of patients with a total MADRS score of 12 or lower) was significantly higher (75.0%) in the escitalopram group than in the paroxetine group (66.8%). On the other hand, another studyCitation25 that compared variable doses of escitalopram (10–20 mg/day) and paroxetine (20–40 mg/day) revealed equivalent efficacy in the two groups.
In Japan, the superiority of escitalopram to placebo and its noninferiority to paroxetine (20–40 mg/day) were documented in a double-blind, parallel-group studyCitation26–Citation28 that compared escitalopram (10 mg/day and 20 mg/day for 8 weeks) to placebo or paroxetine in patients with MDD. shows the changes in the total MADRS scores. Based on the P-values, significant improvement was found in both escitalopram groups compared to the placebo group. Furthermore, based on the difference between the combined escitalopram groups and the paroxetine group, the upper limit of the 95% CI was below the noninferiority threshold limit (3.2); this demonstrated the noninferiority of escitalopram to paroxetine. In addition, previous studies have shown that the efficacy of escitalopram was equivalent to that of either fluoxetine or sertraline.Citation29,Citation30
Table 1 Comparison of changes in total MADRS scores at 8 weeks (last observation carried forward) among patients with MDD treated with escitalopram, paroxetine, or placebo
Comparison with SNRIs
In a double-blind, controlled studyCitation31 of escitalopram (10–20 mg/day) versus duloxetine (60 mg/day) for 8 weeks, the changes in the total MADRS scores were −18.0 ± 9.4 and −15.9 ± 10.3, respectively. This result showed that escitalopram was significantly superior to duloxetine. In another long-term, double-blind, controlled studyCitation32 of escitalopram (20 mg/day) versus duloxetine (60 mg/day) for 24 weeks, the total MADRS score improved significantly to a greater extent in the escitalopram group than in the duloxetine group at 1 week. This trend persisted until the 16th week. Escitalopram has also shown equivalent or superior efficacy to that of sustained-release venlafaxine (venlafaxine SR).Citation33,Citation34
Long-term administration study
In addition to the two long-term, double-blind studies with paroxetineCitation24 and duloxetine,Citation32 two other long-term studies with escitalopram were carried out in Japan, which involved patients of different age groups. The first studyCitation35 involved patients 20–64 years of age (under 65), and the second studyCitation36 involved older patients of at least 65 years of age (65 and older). Both studies examined open-label, 52-week administrations of variable doses in outpatients. The remission rate (percentage of patients with a total MADRS score of 10 or lower) increased over the administration period; after 52 weeks, the remission rate was about 70% in both groups. Patients that reached remission by the eighth week were followed, and 20 of 23 of patients in the under-65 age group maintained remission until the end of study. In the 65-and-older age group, the five patients that reached remission by the eighth week also maintained remission.
Relapse and recurrence prevention study
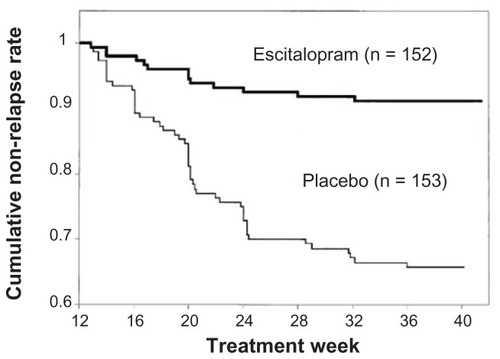
An MDD relapse prevention studyCitation37 was carried out in another group of patients aged 65 and older. Escitalopram was administered at a dose of 10 or 20 mg/day for 12 weeks. Patients that reached remission (a total MADRS score of 12 or lower) were allocated to receive either escitalopram at 10 or 20 mg/day or placebo. The two groups were followed to determine the relapse rate. The cumulative non-relapse rate remained high in the escitalopram group but decreased over time in the placebo group (). At the end of study, relapses were observed in only 9% of the escitalopram group and 33% of the placebo group; thus, the relapse rate was significantly lower in the escitalopram group.
Figure 3 Changes in the cumulative non-relapse rate.
An MDD recurrence prevention studyCitation38 examined recurrences after 16 weeks of continuous therapy with escitalopram. Patients given escitalopram at a fixed dose of 10 or 20 mg/day were compared to controls given placebo for 52 weeks of maintenance therapy. MDD recurrence was 27% in the escitalopram group – significantly lower than the 65% observed in the placebo group.
Tolerability
Patients with MDD generally exhibited favorable tolerance to escitalopram, regardless of whether they received short-term or long-term therapy. Adverse events were typically mild and temporary.Citation39 The most frequent adverse events that occurred during escitalopram therapy included insomnia, nausea, excessive sweating, fatigue/somnolence, dysspermatism, and decreased libido.Citation40
Comparison with SSRIs or SNRIs
Escitalopram was compared to other SSRIs or SNRIs in a meta-analysis of patient data from 16 double-blind, controlled studies.Citation41 When attention was focused on adverse events that occurred at a frequency of 5% or more, escitalopram showed significantly lower frequencies of diarrhea, dry mouth, and the presence of more than one adverse event compared to the other SSRIs. Escitalopram was also associated with significantly lower frequencies of nausea, insomnia, dry mouth, vertigo, excessive sweating, constipation, and vomiting than the SNRIs.
Discontinuation symptoms
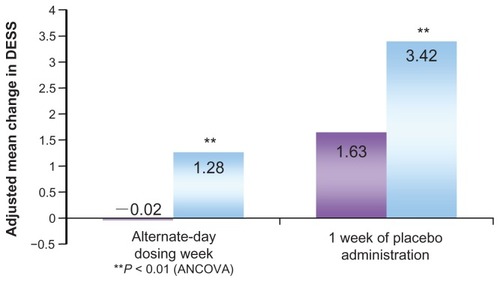
Discontinuation symptoms typically occur at the end of treatment with antidepressant drugs. A detailed studyCitation26 compared discontinuation symptoms in patients with MDD during the post-therapy observation period after 27 weeks of therapy with escitalopram (20 mg/day) or paroxetine (40 mg/day). Discontinuation symptoms were evaluated in terms of the Discontinuation Emergent Signs and Symptoms (DESS) score. During the observation period, the drug doses were gradually decreased over 1–3 weeks, followed by 1 week of alternate-day dosing and, subsequently, 1–3 weeks of placebo. The escitalopram group exhibited smaller changes in the total DESS score and significantly less frequent discontinuation symptoms compared to the paroxetine group, both at the end of alternate-day dosing and after 1 week of placebo administration ().
Figure 4 Discontinuation Emergent Signs and Symptoms (DESS 47) scores in the post-therapy observation period.
Copyright © 2006, Lippincott Williams & Wilkins. Adapted with permission from Baldwin DS, Cooper JA, Huusom AK, Hindmarch I. A double-blind, randomized, parallel-group, flexible-dose study to evaluate the tolerability, efficacy and effects of treatment discontinuation with escitalopram and paroxetine in patients with major depressive disorder. Int Clin Psychopharmacol. 2006;21(3):159–169.Citation25
Suicidality
Suicidality was studied in a detailed meta-analysisCitation42 conducted on data from 34 placebo-controlled studies on SSRIs. The analysis included >40,000 patients, approximately 2600 of whom had been treated with escitalopram. They found one instance of suicide, which occurred 6 days after treatment cessation. Another analysis of placebo-controlled studiesCitation43 specifically included patients with MDD or anxiety disorders that used escitalopram. They reported no suicides during the first 2 weeks of treatment or during the entire period of escitalopram (,24 weeks), but one suicide occurred in the placebo group. Furthermore, there was no indication of increased risk of nonfatal self-harm or suicidal thoughts among patients that received escitalopram compared with those that received placebo.Citation43 Rather, escitalopram reduced the MADRS item-10 (“suicidal thought”) or HAM-D item-3 (“suicidal thought”) scores to a significantly greater extent than placebo.Citation16,Citation43,Citation44 For an estimated >12 million patients with MDD and/or anxiety disorders treated with escitalopram, pharmacovigilance information revealed a suicide rate of 1.8 per 1 million patients; this rate was similar to that in patients treated with citalopram (2 per 1 million) and considerably lower than that in patients treated with tricyclic antidepressants (12 per 1 million) or monoamine oxidase inhibitors (MAOIs) (14 per 1 million).Citation43
Sexual dysfunction
A small, retrospective studyCitation45 (n = 47) indicated that two-thirds of patients with SSRI/SNRI-induced sexual dysfunction reported mild or marked improvements after switching to a regimen with escitalopram. However, several reports have suggested that escitalopram may be associated with increased sexual dysfunction in both men and women compared to bupropion or sertraline.Citation46,Citation47
QT prolongation
In a clinical trial in Japan,Citation28 the QT interval in heart rate was examined with Fridericia’s correction formula (QTcF = QT/cubic root of relative risk). They found no difference in the QTcF values between patients that received escitalopram (10 mg/day) and those that received placebo. However, QTcF was significantly prolonged in patients treated with escitalopram (20 mg/day) compared to that of patients treated with placebo; nevertheless, no clinically problematic adverse events related to QT prolongation were observed. The trial report argued that caution was required in administering escitalopram to aged individuals, patients with liver dysfunction, patients with defective CYP2C19 activity, or patients that received other drugs that conferred a risk of QT prolongation.Citation28
Overdosage
In a retrospective analysisCitation48 of 28 patients that underwent a supratherapeutic ingestion of escitalopram (5–300 mg), only one patient reported adverse events. That patient was admitted to a hospital for persistent lethargy, but the outcome was good. However, when escitalopram is taken at high doses or in poly-substance ingestions, CNS depression may occur. Patients (n = 13) that had taken escitalopram (mean dosage 126 mg) as a coingestant in poly-substance ingestions exhibited CNS depression (54%), cardiovascular effects (54%), and ECG changes (23%).Citation49 In one case report,Citation50 after an overdose of escitalopram (100–200 mg), a 38-year-old man exhibited severe, prolonged serotonin syndrome and elevated serum escitalopram concentration.
Patient acceptability
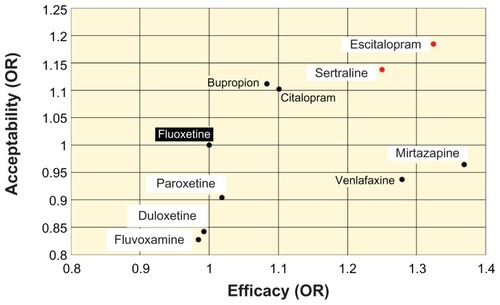
Another meta-analysisCitation51 reported on the efficacy and patient acceptability of 12 new antidepressant drugs. In that meta-analysis, patient acceptability was defined as the persistence observed in taking a drug during an 8-week therapy. Among those 12 drugs, escitalopram was associated with the highest rate of patient acceptability. The result of that meta-analysis was illustrated for family physicians using fluoxetine as the standard ().Citation52
Figure 5 Efficacy and patient acceptability of new antidepressant drugs.
Abbreviation: OR, odds ratio.
The rates of discontinuing therapy were analyzed among pooled data from double-blind, controlled studies of escitalopram versus paroxetineCitation53 or duloxetine.Citation54 The pooled data for paroxetine was derived from two studiesCitation24,Citation25 that treated patients for 24 and 27 weeks, respectively. The discontinuation rate at the end of the study period was significantly lower for patients on escitalopram (16.8%) than for those on paroxetine (27.9%). When the reason for discontinuing therapy was restricted to adverse events, the discontinuation rates remained significantly lower for escitalopram (6.6%) than for paroxetine (11.7%).
The pooled data for duloxetine were derived from two studiesCitation31,Citation32 that treated patients for 8 and 24 weeks, respectively. The discontinuation rate at the end of the study period was significantly lower for escitalopram (12.9%) than for duloxetine (24.6%). When the reason for discontinuing therapy was restricted to adverse events, the discontinuation rates remained significantly lower for escitalopram (4.6%) than for duloxetine (12.7%). Thus, escitalopram was associated with high therapy continuity.
MDD has a relatively high likelihood of recurrence. Thus, high therapy continuity with escitalopram represents an advantage for patients with this disease. There may be several reasons for the high therapy continuity of escitalopram. First, it has high efficacy and good tolerability, as shown in the clinical studies discussed previously. Thus, dropouts from escitalopram therapy due to insufficient efficacy or adverse events appeared to be limited. Furthermore, the demonstrated efficacy of escitalopram at an initial dose of 10 mgCitation15 could be detected in the early therapeutic phase by patients.Citation15,Citation32 It was speculated that early signs of improvement most likely led to increased adherence, which, in turn, led to prevention of relapseCitation37 and recurrence.Citation38
The fact that escitalopram demonstrated preventive effects on relapseCitation37 and recurrenceCitation38 represented major benefit to patients that desire to be reintegrated into society. For instance, for a company employee that wants to return to work, escitalopram may facilitate the return-to-work program, and, thus, the patient would expect to return to work smoothly.
Conclusion
This review provided an overview of escitalopram, focusing on its efficacy, tolerability, and patient acceptability in the management of MDD. In terms of efficacy, escitalopram was superior to placebo, and equal to or better than paroxetine or other SSRIs and SNRIs. In addition, escitalopram exerted a stable antidepressive action. Escitalopram had high tolerability, because adverse events related to escitalopram therapy were generally mild and temporary. Moreover, discontinuous symptoms were apparently milder than those related to paroxetine therapy.
The meta and pooled analyses showed high patient acceptability of escitalopram, which indicated that patients found it easy to continue this antidepressant therapy. Therefore, escitalopram can be regarded as an antidepressant drug associated with high therapy continuity, and the high efficacy of escitalopram is, in part, based on improved adherence due to high tolerability. In addition, the high therapy continuity of escitalopram can be expected to prevent relapses and recurrences. Comparison with placebo demonstrated that escitalopram had preventive effects on both relapse and recurrence of MDD.
A review by Murdock and KeamCitation55 discussed the positioning of escitalopram in the management of MDD. Preliminary studies have suggested that escitalopram was as effective as other SSRIs and venlafaxine XR (venlafaxine hydrochloride extended-release); furthermore, escitalopram may provide the advantage of cost-effectiveness and cost-utility. However, additional longer-term, comparative studies that evaluate specific efficacy, tolerability, health-related quality of life, and economic indices would be needed to determine definitively the position of escitalopram relative to other SSRIs and venlafaxine in the treatment of MDD. Nevertheless, available clinical and pharmacoeconomical data indicate that escitalopram is an effective first line option in the management of patients with MDD.
Because MDD recurs readily, it is important to select antidepressant drugs that allow high therapy continuity for pharmacological treatments. The effects of escitalopram highlighted in this review indicate that it is an antidepressant drug appropriate for first-line therapy.
Disclosure
The authors report no conflicts of interest in this work.
References
- WaughJGoaKLEscitalopram: a review of its use in the management of major depressive and anxiety disordersCNS Drugs200317534336212665392
- SanchezCBergqvistPBBrennumLTEscitalopram, the S-(+)-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with potent effects in animal models predictive of antidepressant and anxiolytic activitiesPsychopharmacology (Berl)2003167435336212719960
- ChenFLarsenMBSanchezCWiborgOThe S-enantiomer of R,S-citalopram, increases inhibitor binding to the human serotonin transporter by an allosteric mechanism. Comparison with other serotonin transporter inhibitorsEur Neuropsychopharmacol200515219319815695064
- MorkAKreilgaardMSanchezCThe R-enantiomer of citalopram counteracts escitalopram-induced increase in extracellular 5-HT in the frontal cortex of freely moving ratsNeuropharmacology200345216717312842122
- El MansariMSanchezCChouvetGRenaudBHaddjeriNEffects of acute and long-term administration of escitalopram and citalopram on serotonin neurotransmission: an in vivo electrophysiological study in rat brainNeuropsychopharmacology20053071269127715702136
- KimuraMEscitalopram oxalateJ Jpn Soc Hosp Pharm201248371375
- MurdochDKeamSJEscitalopram: a review of its use in the management of major depressive disorderDrugs200565162379240416266205
- OwensMJKnightDLNemeroffCBSecond-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetineBiol Psychiatry200150534535011543737
- DhillonSScottLJPloskerGLEscitalopram: a review of its use in the management of anxiety disordersCNS Drugs200620976379016953656
- ChenFLarsenMBNeubauerHASanchezCPlengePWiborgOCharacterization of an allosteric citalopram-binding site at the serotonin transporterJ Neurochem2005921212815606893
- NeubauerHAHansenCGWiborgODissection of an allosteric mechanism on the serotonin transporter: a cross-species studyMol Pharmacol20066941242125016434615
- SanchezCBogesoKPEbertBReinesEHBraestrupCEscitalopram versus citalopram: the surprising role of the R-enantiomerPsychopharmacology (Berl)2004174216317615160261
- KleinNSacherJGeiss-GranadiaTHigher serotonin transporter occupancy after multiple dose administration of escitalopram compared to citalopram: an [123I]ADAM SPECT studyPsychopharmacology (Berl)2007191233333917235610
- SpinaESantoroVD’ArrigoCClinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an updateClin Ther20083071206122718691982
- WadeAMichael LemmingOBang HedegaardKEscitalopram 10 mg/day is effective and well tolerated in a placebo-controlled study in depression in primary careInt Clin Psychopharmacol20021739510211981349
- BurkeWJGergelIBoseAFixed-dose trial of the single isomer SSRI escitalopram in depressed outpatientsJ Clin Psychiatry200263433133612000207
- LepolaUMLoftHReinesEHEscitalopram 10–20 mg/day) is effective and well tolerated in a placebo-controlled study in depression in primary careInt Clin Psychopharmacol200318421121712817155
- LalitVAppayaPMHegdeRPEscitalopram versus citalopram and sertraline: a double-blind controlled, multi-centric trial in Indian patients with unipolar major depressionIndian J Psychiatry200446433334121206792
- MooreNVerdouxHFantinoBProspective, multicentre, randomized, double-blind study of the efficacy of escitalopram versus citalopram in outpatient treatment of major depressive disorderInt Clin Psychopharmacol200520313113715812262
- OuJJXunGLWuRREfficacy and safety of escitalopram versus citalopram in major depressive disorder: a 6-week, multicenter, randomized, double-blind, flexible-dose studyPsychopharmacology (Berl)20112132–363964620340011
- YevtushenkoVYBelousAIYevtushenkoYGGusininSEBuzikOJAgibalovaTVEfficacy and tolerability of escitalopram versus citalopram in major depressive disorder: a 6-week, multicenter, prospective, randomized, double-blind, active-controlled study in adult outpatientsClin Ther200729112319233218158074
- MontgomerySHansenTKasperSEfficacy of escitalopram compared to citalopram: a meta-analysisInt J Neuropsychopharmacol201114226126820875220
- TrkuljaVIs escitalopram really relevantly superior to citalopram in treatment of major depressive disorder? A meta-analysis of head-to-head randomized trialsCroat Med J2010511617320162747
- BoulengerJPHuusomAKFloreaIBaekdalTSarchiaponeMA comparative study of the efficacy of long-term treatment with escitalopram and paroxetine in severely depressed patientsCurr Med Res Opin20062271331134116834832
- BaldwinDSCooperJAHuusomAKHindmarchIA double-blind, randomized, parallel-group, flexible-dose study to evaluate the tolerability, efficacy and effects of treatment discontinuation with escitalopram and paroxetine in patients with major depressive disorderInt Clin Psychopharmacol200621315916916528138
- HirayasuYA dose-response and non-inferiority study evaluating the efficacy and safety of escitalopram in patients with major depressive disorder: a placebo- and paroxetine-controlled, double-blind, comparative studyJpn J Clin Psychopharmacol201114883899
- Mochida Pharmacoceutical COL. Interview Form LEXAPRO Tab. 10 mg3rd ed Available from: http://di.mt-pharma.co.jp/file/if/f_lex.pdf2012Accessed November 15, 2012 Japanese
- Deliberation Result Report of LEXAPRO Tab. 10 mgPharmaceutical Food Station Examination Management Section, Ministry of Health, Labour and WelfareJapan2011 Available from: http://www.info.pmda.go.jp/shinyaku/P201100076/79000500_22300AMX00517_A100_1.pdf2011Accessed November 29, 2012 Japanese
- MaoPXTangYLJiangFEscitalopram in major depressive disorder: a multicenter, randomized, double-blind, fixed-dose, parallel trial in a Chinese populationDepress Anxiety2008251465417149753
- VenturaDArmstrongEPSkrepnekGHHaim ErderMEscitalopram versus sertraline in the treatment of major depressive disorder: a randomized clinical trialCurr Med Res Opin200723224525017288677
- KhanABoseAAlexopoulosGSGommollCLiDGandhiCDouble-blind comparison of escitalopram and duloxetine in the acute treatment of major depressive disorderClin Drug Investig2007277481492
- WadeAGembertKFloreaIA comparative study of the efficacy of acute and continuation treatment with escitalopram versus duloxetine in patients with major depressive disorderCurr Med Res Opin20072371605161417559755
- BielskiRJVenturaDChangCCA double-blind comparison of escitalopram and venlafaxine extended release in the treatment of major depressive disorderJ Clin Psychiatry20046591190119615367045
- MontgomerySAHuusomAKBothmerJA randomised study comparing escitalopram with venlafaxine XR in primary care patients with major depressive disorderNeuropsychobiology2004501576415179022
- HirayasuYA long-term administration study of escitalopram in patients with major depressive disordersJpn J Clin Psychopharmacol201114901912
- HirayasuYA long-term administration study of escitalopram in elderly patients with major depressive disorderJpn J Clin Psychopharmacol201114901912
- GorwoodPWeillerELemmingOKatonaCEscitalopram prevents relapse in older patients with major depressive disorderAm J Geriatr Psychiatry200715758159317586783
- KornsteinSGBoseALiDSaikaliKGGandhiCEscitalopram maintenance treatment for prevention of recurrent depression: a randomized, placebo-controlled trialJ Clin Psychiatry200667111767177517196058
- Cipralex®/Lexapro® (escitalopram)Product Monograph-Issue 8-June 2009CopenhagenH. Lundbeck A/S2009
- Lexapro® (escitalopram oxalate) tablets and oral solution [US prescribing information]St Louis, MOForest Laboratories, Inc2011 Available from: http://www.frx.com/pi/lexapro_pi.pdfAccessed May 10, 2011
- KennedySHAndersenHFThaseMEEscitalopram in the treatment of major depressive disorder: a meta-analysisCurr Med Res Opin200925116117519210149
- GunnellDSaperiaJAshbyDSelective serotonin reuptake inhibitors (SSRIs) and suicide in adults: meta-analysis of drug company data from placebo controlled, randomised controlled trials submitted to the MHRA’s safety reviewBMJ2005330748838515718537
- PedersenAGEscitalopram and suicidality in adult depression and anxietyInt Clin Psychopharmacol200520313914315812263
- BechPTanghojPCialdellaPAndersenHFPedersenAGEscitalopram dose-response revisited: an alternative psychometric approach to evaluate clinical effects of escitalopram compared to citalopram and placebo in patients with major depressionInt J Neuropsychopharmacol20047328329015320956
- AshtonAKMahmoodAIqbalFImprovements in SSRI/SNRI-induced sexual dysfunction by switching to escitalopramJ Sex Marital Ther200531325726216020143
- GersingKTaylorLMereadithCOutcome and adverse events for escitalopram and sertraline in a real-world setting [abstract no NR815] American Psychiatric AssociationAnnual Meeting 2005 New Research AbstractsAtlanta, GA20052126
- ClaytonAWightmanDModellJGEffects in MDD on sexual functioning of bupropion XL, escitalopram, and placebo in depressed patiets [abstract no NR-818] American Psychiatric AssociationAnnual Meeting 2005 New Research AbstractsAtlanta (GA)20052126
- LoVecchioFWattsDWinchellJKnightJMcDowellTOutcomes after supratherapeutic escitalopram ingestionsJ Emerg Med2006301171916434330
- SeifertSAMeissnerGKEscitalopram overdose: a case series [abstract no 72]J Toxicol200442495496
- OlsenDDartRRobinettMSevere serotonin syndrome from escitalopram overdose [abstract no 72]J Toxicol Clin Toxicol200442744745
- CiprianiAFurukawaTASalantiGComparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysisLancet2009373966574675819185342
- PatrickGCombsGGavaganTInitiating antidepressant therapy? Try these 2 drugs firstJ Fam Pract200958736536919607774
- KasperSBaldwinDSLarsson LonnSBoulengerJPSuperiority of escitalopram to paroxetine in the treatment of depressionEur Neuropsychopharmacol200919422923719185467
- LamRWAndersenHFWadeAGEscitalopram and duloxetine in the treatment of major depressive disorder: a pooled analysis of two trialsInt Clin Psychopharmacol200823418118718545055
- MurdochDKeamSJEscitalopram: a review of its use in the management of major depressive disorderDrugs2005651623802404