Abstract
New psychoactive drugs that have appeared over the last decade are typically dominated by cathinones and synthetic cannabinoids (SCs). SCs have been emerging as recreational drugs because they mimic the euphoria effect of cannabis while still being legal. Sprayed on natural herb mixtures, SCs have been primarily sold as “herbal smoking blends” or “herbal incense” under brand names like “Spice” or “K2”. Currently, SCs pure compounds are available from websites for the combination with herbal materials or for the use in e-cigarettes. For the past 5 years, an ever increasing number of compounds, representative of different chemical classes, have been promoted and now represent a large assortment of new popular drugs of abuse, which are difficult to properly identify. Their legal status varies by country with many government institutions currently pushing for their control. The in vitro binding to CB1/CB2 receptors is usually well-known and considerable differences have been found in the CB1 versus CB2 selectivity and potency within the different SCs, with several structure-activity relations being evident. Desired effects by CB1 agonist users are relaxation/recreative, however, cardiovascular, gastrointestinal, or psychiatric/neurological side effects are commonly reported. At present there is no specific antidote existing if an overdose of designer drugs was to occur, and no curative treatment has been approved by health authorities. Management of acute toxic effects is mainly symptomatic and extrapolated from experience with cannabis.
Introduction
Synthetic cannabinoids (SCs) were originally developed in the 1970’s for the research on ligands and the exploration of their pharmacological endocannabinoid pathways.Citation1 Just like the primary psychoactive component of cannabis, Δ9-tetrahydrocannabinol (Δ9-THC), and in the same manner as the main endogenous ligands, anandamide, and 2-arachydonylglycerol, SCs bind to the two subtypes of cannabinoid receptors, CB1 and CB2, with a varying degree of affinity. SCs were first reported for their use in recreational settings in December 2008 by a German firm who identified them in the smoking blends sold as herbs sprayed or mixed with one or more synthetic compounds, and were referred to as “Spice”, “Yucatan”, “Chill”, “K2”, or “Black Mamba”.Citation2
Over the following years SCs gained in popularity, especially among young people. Proposed as medicinal and legal products, newly synthetized cannabimimetics continue to emerge on the “legal highs” market as an alternative to phytocannabinoids. Among SCs, CB1 agonists mimic the effects of cannabis where consumers may feel happy and relaxed. However, unwanted serious adverse effects, such as neuropsychiatric disturbances or somatic effects of variable intensity, may occur with the use of recreational SCs.Citation3 Moreover, as result of the various forms of synaptic plasticity mediated by endocannabinoids, which allows the cannabinoid receptor to recognize multiple classes of compounds,Citation4 a large variety of distinct chemical substances are now sold over the Internet. This reinforces the public health problem linked to these new SCs that have, for the greater part, never been tested in human controlled settings. Nevertheless, as ever-increasing data on the identification or analysis methods, pharmacokinetics or pharmacodynamics, animal or human pharmacology or toxicity, and addictive potential are becoming available, this review is aimed to present updated information on SCs, useful for poisons centers, clinical toxicologists and emergency physicians.
Availability and usage demographics
SCs were first developed with the aim of exploring endogenous cannabinergic pathways and finding new therapeutics.Citation5 In the mid-1970s, Pfizer created CP 55,940, and in the 1980’s and 1990’s, other chemicals such as HU-210 and WIN 55,212 were investigated as potent pharmacotherapies.Citation6 The most important series of SCs appeared from the study by Huffman and Dong in 1994.Citation7 From this time on new classes of SCs have been developed, with numerous representatives inside every drug class.
The history on the use of SCs as drugs of abuse began approximately around 2004 when herbal mixtures, mainly known as Spice, were marketed on the Internet as a substitute to cannabis, in colorful attractive packages. Initially, these blends seemed to be made from plants traditionally used by shamans and/or other well known “phytochemistry” adepts. Four years later, in December 2008, the first reported case of SCs misuse appeared with the discovery of five compounds (JWH-018, CP 47,497 and its C6, C7, C8 analogs) in herbal blends produced by a German company.Citation2 Neither the seller nor the consumer of herbal mixtures, such as Spice, K2, or Black Mumba, can predict their content. For example, the overall range of concentrations in nine different brands investigated by Lindigkeit et al was between 3–11 mg/g of CP 47,497 C8, and 6–23 mg/g of JWH-073. One sample contained a small amount of JWH-018, ie, 2.3 mg/g, while two other samples were found to be free of SCs.Citation8 Similar findings were published in another study by Uchiyama et al based on 46 Spice products.Citation9 Concomitantly with the emergence of herbal blends, SCs quickly become available in large amounts of “pure” powder, sold especially on websites from the People’s Republic of China, with the aim of adding to tobacco for smoking.
Nowadays, new SCs constantly appear on the market,Citation10 along with new types of consumption, which could be called a third-generation of use, such as cartridges filled with SCs solution designed for using with e-cigarettes. These are called “buddha-blue”, “C-Liquid”, “Herbal e-Liquid”, or others and are discussed on drug-user forums.
From published data available from poisoning, toxicological, or epidemiological centers the use of SCs is common in the USA, varying from 1.4% based on Wohlfarth et al’s study on USA military urine specimens (n=20,017) collected from July 2011 to June 2012,Citation11 up to approximately 10% found in Palamar and Acosta’s study taken on high school seniors (n=11 863).Citation12 Similar findings were reported from studies on the USA nightlife scene (n=1740)Citation13 and on college students in Florida (n=852).Citation14 However, it has been recently shown that while lifetime prevalence has increased, the past 6 month prevalence has decreased substantially over time.Citation15 In Europe, a retrospective study based on serum samples collected in Germany in 2010, estimated a prevalence at approximately 2.8% (n=12/422),Citation16 and 4% (n=164/4080) in a youth population (18–34 year olds) from the French Health Barometer.Citation17 Considering the new attraction for SCs, studies or reports on driving impairment have started to be considered in USACitation18,Citation19 and in Europe.Citation20,Citation21
It is also important to note that SCs can be used as a therapeutic. Even if many fundamental research is conducted, only a few SCs are available for medical use, belonging to dibenzopyrane derivatives such as nabilone or dronabinol. They are mostly used for their antiemetic properties, especially in chemotherapy-induced nausea and vomiting, or for their orexigenic properties in anorexia. In addition, analgesic properties of cannabinoids are advanced in some Cannabis sativa plant extracts for adjunctive treatment of neuropathic pain in patients with multiple sclerosis. To date, several investigations are currently underway to find new therapeutic applications of SCs, for example, neuroprotective effects in neurodegenerative diseases such as Parkinson’s disease,Citation22 or inflammatory cell modulation.Citation23
Chemical structure and designation
SCs family is extremely large, including numerous substances belonging to various chemical groups and subgroups. New compounds which can belong to unknown chemical classes emerge constantly, making the inventory of existing products never ending. We have brought together approximately 120 SCs, which are the most recent and popular compounds, but we do not claim this list to be exhaustive ( and ).
Table 1 Chemical structure of cannabinoid 3-indole derivatives
Table 2 Chemical structure of other cannabinoid derivatives
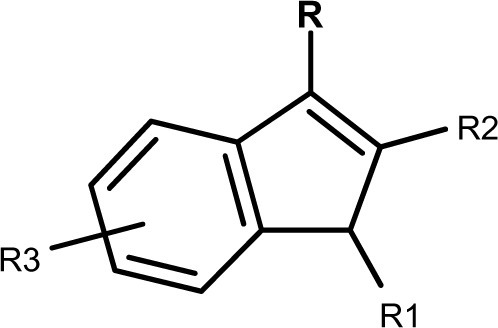
We specify trade names, radicals, formula, and CAS numbers for an easy and quicker tracking. Only a few SCs are structurally related to Δ9-THC, the others belonging to different and various chemical families. The major structural group is the indole group (, ) which includes several indole sub-groups (R): benzoyl, Naphthol, phenylacetyl, alkyl, piperazinyl, carboxylate, carboxamide, thiazolyl, and naphthylmethyl derivatives.
Besides these abundant indole derivatives, many other SCs groups have very different structural characteristics (). In spite of the diversity of products, some similarities should be noted: 1) a quite constant unsaturated and substituted five-membered ring incorporating at least one nitrogen (pyrrole), and merged to another aromatic cycle; and 2) within series, changes in substituent are often limited to the simple addition of a methyl or halogen group to a linear alkyl chain. Nevertheless, more marked modifications are possible, making the classification of such compounds very difficult. Some of these compounds are chiral, and can exist in two stereoisomer forms. SCs are usually referred to by usual trade names such as JWH-XXX (John W Huffmann), CP-XX, XXX (Charles Pfizer), HU-XXX (Hebrew University), AM-XXXX (Alexandros Makriyannis), and many others. Note that within a trade name family, several chemical classes may be represented. For example, JWH-XXX series includes Naphthol indoles, phenylacetyl indoles, naphthylmethyl indoles, naphthylmethyl indene, and Naphthol pyrroles.
Current and developing analytical methods for detection
Immunochemistry
As a general rule, immunoassay screening methodologies used to detect cannabis fail to detect SCs, but some specific enzyme-linked immunosorbent assays or homogenous enzyme immunoassays have been recently designed to detect the use of common SCs in urine, such as JWH-018, JWH-250, UR-144, and others.Citation24–Citation26
Gas chromatography-mass spectrometry
Several gas liquid chromatography (LC) connected to electron-impact mass spectrometry (GC-MS) methods have been developed to rapidly identify and quantify SCs in herbal and powder materials.Citation27–Citation31 In our own experience we used a DB-5 fused-silica capillary column (30 m×0.25 mm×0.25 μm). Oven temperature was increased from 100°C (hold time: 1 minute) by 8°C/min up to 280°C (26.5 minutes), with helium flow at 1 mL/min.
A standard method to screen new psychoactive substances was to have a total runtime of 60 minutes, with the retention times of SCs ranging between 21.9 and 48.0 minutes. The rank order (retention time) of ten analyzed SCs was as follows from the lowest rank to the highest rank: UR-144; JWH-250; HU210; RCS-4; JWH-073; JWH-018; JWH-019; AM-2201; JWH-122; JWH-081; and JWH-200. The selected mass spectrometry (MS) data that we collected in the literature, and from our own data bank are summarized in , and identify several SCs, some are among the most notorious. Consistent with the large variety of chemical structures, mass spectra under electron impact conditions differ largely from one SC to another, with some discrepancies between reported spectra in terms of ion intensity. An intense molecular ion (M+) is frequently present and fragmentations are commonly observed on both sides of the carbonyl group: for example, SCs bearing an n-pentyl moiety show an intense ion at m/z =214 corresponding to n-pentyl indoloyl,Citation27 and those bearing a 5-fluoropentyl moiety show an intense ion at m/z =232 (fluoropentyl indoloyl). The mass-spectral fragmentation pattern of SCs also includes formations of different immonium ions at m/z =144 (indoloyl moiety), 155 (Naphthol moiety), or 127 (Naphthol moiety).Citation32 GC-MS methods have been developed to determine the composition of Spice herbal mixtures but appear inadequate to measure concentrations in the user’s biological specimen.
Table 3 Gas chromatography mass spectrometry (GC-MS) identification of synthetic cannabinoids
LC-MS
Different LC-MS/MS screening or semi-quantitative and quantitative methods have been published and identify and quantify SCs in various specimens – urine, serum, blood, oral fluid, or hair.Citation33–Citation40 A classic or ultra-performing reverse phase C18 is typically used for the chromatographic separation prior to analysis by LC-tandem MS using electrospray positive ionization multiple reaction monitoring mode.Citation33–Citation40 Multiple reaction monitoring transitions for SCs are presented in . For all substances, the dominant Q1 ions were the protonated molecular ions ([M+H]+), and at least two product ions of acceptable abundance can be obtained. Ions C11H7O+ (m/z =155.0) and C10H7+ (m/z =127.0) can be referred to as common characteristic ions. Simultaneous determinations and quantifications of SCs metabolites have also been successfully applied to urine specimens.Citation37,Citation41 In order to complete targeted MS/MS screening, ultra-high performance LC coupled with high resolution quadrupole time-of-flight MS high-sensitivity methods have also been developed to directly screen SCs in specimens such as in urine or hair.Citation42–Citation45 Real time MS was employed to detect the SCs protonated molecules [M+H]+ directly on herbal matrices, without extraction or sample preparation.Citation46 The limits of quantification for the assay of SCs in urine, using an electrospray ionization source in positive polarity, are approximately 0.1–1 μg/L, with upper limits of linearity at 50–100 μg/L.Citation37 Accordingly, LC-MS-MS is the only method that presents sufficient sensibility to quantify SCs in biological fluids.
Table 4 Liquid chromatography – tandem mass spectrometry identification of synthetic cannabinoids
Extraction procedures for quantification in herbal and biological samples
Herbal material has to be crushed and stirred or sonicated in methanol or ethanol before chromatographic analysis. Human urine is the favorite biological matrix explored to detect SCs consumption. Biological samples may be enzymatically hydrolyzed before pretreatment. Solid-phase extraction or simple precipitation is next used for oral fluid or urine samples.Citation33,Citation37,Citation47 Basic liquid-liquid extraction after deproteinization with acetonitrile followed by a concentration step is generally preferred to prepare blood samples.Citation34,Citation38 Liquid-liquid extraction is preceded by an incubation with NaOH at 95°C for finely cut hair samples.
Experimental pharmacology and neuropharmacology
By acting as retrograde messengers at various synapses, endocannabinoids (anandamide and 2-arachidonoylglycerol) have a neuromodulatory role in motor activity, pain perception, feeding, emotional state, learning and memory, and reward behaviors.Citation48,Citation49 They also influence cardiovascular and immune systems and control progenitor cell proliferation.Citation48 Endocannabinoids bind to CB1 receptors mainly located at the terminals of central and peripheral neurons and to CB2 receptors principally expressed in immune and hematopoietic cells both within and outside the central nervous system. CB1 receptors are often localized presynaptically where their stimulation usually inhibits neurotransmitter release and accounts for most of the neurobehavioral effects, while CB2 receptors are mainly implicated in the immunomodulatory effects. Yet, CB1 receptors are also present at much lower concentrations in peripheral tissues and CB2 in neurons and microglia.
In vitro
The complex molecular architecture of the cannabinoid receptors allow for a single receptor to recognize multiple classes of compounds.Citation50 SCs which present a large variety of chemical structures like that previously shown in and , bind to the two types of cannabinoid receptors with a varying degree of affinity. These CB1 and CB2 receptor affinities of SCs have been determined in displacement assays using tritiated cannabinoid receptor ligands and membranes obtained from brain (CB1-rich), spleen (CB2-rich), or using culture cells transfected with CB1 or CB2 receptors.Citation51 Ki values of SCs collected from literature are grouped in .Citation7,Citation52–Citation64 The majority of compounds used as drug of abuse have Ki in the range 1 to 10 nM or 10 to 100 nM for both CB1 and CB2 receptors. Some synthetic compounds bind more strongly to CB1 receptor than Δ9-THC. JWH-210 from the Naphthoylindole family, acts as a potent cannabinoid agonist with both the CB1 and CB2 receptors, with Ki values of 0.46 nM at CB1 and 0.69 nM at CB2.Citation52 At the opposite end, JWH-071 binds with the central CB1 receptor (Ki =1,340 nM) and the peripheral CB2 receptor (Ki =2,940 nM) at a micromolar level.Citation53 The majority of SCs are unspecific CB1/CB2 ligands (), with only a few are CB2 selective such as AM-1221 (Ki =0.28 nM at CB2 vs 52.3 nM at the CB1)Citation54 and A-836,339 (Ki =0.64 nM at CB2 vs 270 nM at CB1).Citation57
Table 5 Affinities of much of synthetic cannabinoids
CB1 and CB2 receptors are G protein-coupled receptors. Two in vitro assays using cell membranes or cultured cells that express CB1 or CB2 receptors are commonly employed to determine the SCs agonist property; the [Citation35S] GTPγS assay that measures cannabinoid receptor agonist-stimulated binding to G proteins, and the cyclic adenosine monophosphate assay that quantitatively determine the drug-induced production of this important second messenger.Citation51
As a general rule, the agonists show little selectivity between the CB1 and CB2 receptors, while the antagonist compounds are highly selective.Citation65
In vivo
SCs that target CB1 and/or CB2 receptors may be classified in CB1/CB2 agonists, CB2 selective agonists, peripherally restricted CB1/CB2 agonists, CB1/CB2 antagonists, and inverse agonists.Citation66 Additionally, chiral centers can exist in many SCs and stereoisomer forms may differ in their pharmacological potencies.Citation67 All may have an interest as recreational or medicinal drugs. In vivo in mice, activation of CB1 receptors produces a “tetrad” of dose-dependent effects, including suppression of locomotor activity, hypo thermia, immobility in the ring test, and antinociception in the tail-flick or hot-plate test.Citation66 This cannabinoid tetrad is extremely useful in the characterization of the biological activity of SCs but the development of CB1, CB2, or CB1/CB2 knockout mice provided additional methods to test the SCs specificities. In addition, agonistic binding to CB1 receptors resulted in behavioral effects including euphoria, elevation or anxiety, or alteration of memory.Citation68 In agreement with in vitro studies, in vivo studies show SCs pharmacological effects commonly more potent than Δ9-THC. Similarly, SCs perfectly substituted for Δ9-THC in discrimination studies.Citation3 Activation of CB2 receptors results in release of immuno-modulating agents and reduction of inflammatory induced pain. Accordingly, our purpose primarily focused on specific or unspecific CB1 agonists.
Many studies in mice suggest a major regulatory role of cannabinoid signaling in pregnancy with multiple sites and stages of pregnancy potential targets of SCs, including preim-plantation embryo development, oviductal embryo transport, implantation, placentation, and parturition.Citation69
Studies on animals have proved that cannabinoids have an addictive potential, involving reward system and CB1 receptors. Δ9-THC activates the brain reward circuit by stimulating the mesolimbic dopamine system.Citation70,Citation71 It has been previously demonstrated that high doses of natural and SCs produced conditioned place aversion,Citation72,Citation73 whereas lower doses induced conditioned place preference.Citation74,Citation75 Furthermore, microinjections in the ventral tegmental area and nucleus accumbens produced conditioned place preference, an effect that was blocked by SR141716A (ie, rimonabant), a CB1 antagonist. Recently, Cha et al evaluated the psychological dependency potential of JWH-073, JWH-081, and JWH-210 using conditioned place preference, with significant dose-dependent increases for the SCs administered groups.Citation76 It must be noticed that SCs show a positive self-administration effect when the catheter was inserted directly into the ventral tegmental area,Citation77 but failed to induce any effect by the venous route.Citation57 Drug discrimination tests can establish a potential of abuse by comparing a substance to a well-known head file drug for its abuse potential. In such studies, CP47,497 generalizes for Δ9-THC in the rat,Citation78 JWH-018 and JWH-073 in the monkey,Citation79 and JWH-200, JWH-203, JWH-250, AM-2201, and CP 47,497-C8-homologue in mice, with interesting differences in the duration of the effect.Citation80 HU-210 generalizes for BAY 59-3074, a partial CB1/CB2 agonist in rat, and shows no effect when the CB1-antagonist is administrated.Citation81
Human use of SCs
Modalities of administration
The herbal mixtures that are sprayed with SCs and are proposed as legal alternatives to marijuana are often smoked by users.Citation2 Apart from herbal smoking blends, some consumers prefer homemade mixtures, using some “purified” powders of SCs sold on websites, solved in alcohol and spayed on herbals. With the recent development of electronic nicotine delivery system (e-cigarettes) as a new alternative for tobacco withdrawal, e-liquids containing SCs have recently appeared as a new trend and also as a more discreet way of consumption.
Ingestion of SCs is not often reported. One study detailed the cardiovascular effects on a man who had drunk SCs that was mixed with alcohol.Citation82 A case series, including eleven users that ingested brownies laced with SCs, in the manner of the well-known “space-cake” consumption of cannabis, has been recently published.Citation83 Other marginal routes of administration have been reported or have been discussed on user forum’s such as nasal insufflation, either vaporized or not. MAM-2201 was recently quantified in the serum of a 20-year-old subject that snorted a powder sold as “Synthacaine”.Citation84 Administration by injection could theoretically produce the typical effects of SCs, but experimental data on mice suggest that differences can exist between inhalation and injection, especially in catalepsy and perhaps discriminative effects.Citation85 Nevertheless, the injection route does not seem to be user’s preference, partly due to the low water solubility of a number of SCs, leading to use some adjuvants.Citation86
Dose used varied a lot between products, reported in experimental findings on SCs CB1 affinity. They fluctuate from several mg for the lead JWH-018, to several μg for the more potent HU-210. JWH-018 and JWH-073 were measured in a herbal incense product at concentrations of 17 mg/g and 22 mg/g respectively.Citation87
Recreational effects
In humans, the recreational use of SCs generates psychoactive effects similar to cannabis, such as relaxation, calmness, euphoria, hilarity, lowering of inhibitions, disorientation, and an altered perception. Effects begin after only a few minutes from inhalation, and generally disappear after approximately 2–6 hours.Citation2 SCs reportedly had both a shorter duration of action and a quicker time to peak onset of effect.Citation88
Acute and chronic, physical and psychological adverse/toxic effects
After the use of SCs, it is essentially neurological and cardiovascular effects that essentially occur, with a prevalence of 61.9% and 43.5% respectively, reported from 464 cases at Texas poisoning centers over 2010.Citation89 For the 305 adolescents, the medical outcome was known or suspected to be serious in 61% of these cases. In this young population, the most frequently reported adverse clinical effects were tachycardia (41.6%), drowsiness/lethargy (24.3%), agitation/irritability (18.5%), vomiting/nausea (21.6%), and hallucinations/delusions (10.8%). Nausea, confusion, hypertension, chest pain, and dizziness/vertigo were observed in <10% of the cases.Citation90 It is important to note that seizures were observed in 3.8% of the SCs intoxication cases, whereas they are not typically seen with marijuana use.Citation91 Most of these effects lasted for <8 hours (78.4%) and 92.9% of these cases did not have a life threatening clinical effect.Citation92 Meanwhile, some evaluations on emergency medical treatments suggest that SCs potentially pose a greater risk to users’ health than natural cannabinoids.Citation93
In addition to acute SCs-induced psychosis disorders that include disorientation and hallucinations and can have life-threatening outcome, long-term effects also are to be feared. Although the long-term consequences of SC use are unclear, in patients with psychiatric disorders, new psychiatric phenomena could appear. Celofiga et al observed a marked worsening of mood and anxiety, without exacerbation of the pre-existing known psychotic symptoms.Citation94 In 2015, Van Amsterdam et al showed that psychosis-inducing risk is higher with SCs than with natural cannabis.Citation95 Hence, the psychosis outcomes associated with SCs provide additional data to the ongoing debate on cannabinoids and psychosis.Citation96
In terms of physical damage there have been several recently reported cases; a severe pulmonary syndrome in an otherwise healthy young man which was related to habitual SCs smoking;Citation97 a case of a cardiac arrest following K2 abuse; and a case of acute cerebral infarction in a 33 year old man, all close-temporally linked with the inhalation of XLR-11.Citation98,Citation99
Little is known on pregnancy/fertility impairment effects induced by SCs in humans due to limited studies, but regular cannabis consumption throughout pregnancy is statistically associated with decreases in birth weight.Citation100 This data suggests that using SCs is a potential risk factor that could impact several stages of pregnancy.Citation69
Addictive potential
In humans, tolerance to SCs has been reported in literature.Citation101 The most common symptoms observed after an acute withdrawal are agitation, tachycardia, irritability, anxiety, and mood swings. In Auckland (New Zealand), patients withdrawing from SCs required intensive support, including medication and admission to an inpatient detoxification service. Between May 2013 and May 2014, SCs users represented the third largest group of patients admitted to this unit. Due to the stronger potency of some SCs, withdrawal signs appeared more severe but did not seem to be improved by Δ9-THC.Citation102,Citation103 Furthermore, concurrently to a craving experienced by a 23 year old man, a user of “Spice gold”, substantial but reversible short-term alterations of dopamine D2/3 receptor availability were shown in a PET Scan.Citation104
Pharmacokinetics
Concentrations in biological samples
The JWH-018 and JWH-073 concentrations determined in postmortem whole blood samples varied from 0.1 to 199 μg/L and 0.1 to 68.3 μg/L respectively.Citation105 Obtained in a patient who had smoked an herbal incense containing these two SCs, the concentrations fall from 4.8 and 4.2 μg/L (measured = 19 minutes after dose administration) to 0.6 and 0.3 μg/L (measured = 107 minutes after dose administration) for JWH-018 and JWH-073 respectively.Citation87 The concentration of UR-144 in a blood sample collected on admission of a patient who was unconscious on arrival was 6.1 μg/L. The parent compound was not found in urine but metabolites were identified.Citation106 SCs quantitated in twelve SCs serum users ranged from 0.21 to 2.94 μg/L for JWH-0250, and 0.35 to 73.05 μg/L for JWH-0122.Citation16 In users driving under the influence of SCs, a very large difference was seen between the lowest (0.07, 0.08, and 0.24 μg/L, respectively) and the highest (4, 9.9, and 24.5 μg/L) blood concentrations of AM-2201, JWH-08, and APINACA.Citation107
Wide variations were also observed for the quantitative results in 23 hair samples of SCs abuse suspects: 0.4 to 38.9 pg/mg for JWH-018, 0.1 to 0.8 pg/mg for JWH-073, 1.7 to 739.01 pg/mg for AM-2201, 0.1 to 402.0 pg/mg for JWH-122, and 0.2 to 276.0 pg/mg for MAM-2201.Citation40 In two volunteers who smoked a joint prepared from different herbal incense products, the concentrations of measured SCs in neat oral fluid 5 hours later fluctuated between 0.1 and 1 μg/L for JWH-018 and JWH-210, but it was lower than 0.1 μg/L for JWH-200.Citation33
Metabolism
Several recent publications characterized the metabolism pathways of SCs in vitro or identified degradant products in animal or human blood/urine samples. They all showed that SCs are extensively metabolized. SCs parent compounds are mainly hydroxylated, dealkylated, carboxylated, glucuronate. Furthermore, hydroxylation’s take place on the aliphatic chain, the indole, the naphthalene, or the substituted aromatic rings that can be secondarily metabolized to carboxylic acids then conjugated to glucuronic acid.Citation108,Citation109 CYP3A4 has been recently demonstrated to be the major CYP enzyme responsible for the oxidative metabolism of AKB-48.Citation110 Ashino et al suggested that SCs, especially naphthoylindole derivatives, are capable of inhibiting CYP1A enzymatic activity as do the major metabolites present in marijuana, cannabinol and cannabidiol.Citation111
Elimination rate
The half-live of JWH-018 and JWH-073 calculated from the concentration data measured by Kacinko et al in a patient who had smoked an herbal incense containing these SCs, were 41 and 44 minutes respectively.Citation87 Similar results were found after an incense JWH-018 smoking experiment performed on two healthy subjects, with the calculated half-live at 43 and 34 minutes.Citation112 These data confirm the rapid decline of JWH-018 as early as the first puncture time at 5 minutes.
In another study an adult male volunteer orally ingested a 5 mg dose of pure AM-2201, and the AM-2201 serum concentrations was reported to decreased from 1.4 μg/L at approximately 1 hour to 0.7 μg/L at 5 hours after ingestion. AM-2201 was still detectable in serum 25 hours after administration. The half-life of AM-2201 was estimated to be approximately 4 hours.Citation33
After two volunteers smoked a mixture of SCs, JWH-018, JWH-019, JWH-210, JWH-251, and JWH-307 were still detectable for approximately 26 hours in oral fluid; and JWH-251, JWH-210, and JWH-307 could be detected at 37, 47, and 55 hours, respectively.Citation33
Clinical management of acute and chronic adverse effects and addiction
As for all other new psychoactive substances, there are limited reliable data to guide clinicians managing patients with toxicity due to SCs. As a consequence, management of SCs users with acute toxic effects is mainly extrapolated from experience with longer established cannabis effects.
Diagnostic tests
Routine laboratory tests in the work-up of a potentially SCs toxic patient include 1) a basic metabolic panel with blood glucose levels, serum electrolyte concentrations, liver and kidney function tests; 2) a complete blood cell count with coagulation studies; 3) cardiac markers; and 4) total creatinine kinase. The typically available urine toxicology screen will not detect SCs but could be useful to detect other possible substances ingested. If the patient presents signs and symptoms consistent with cannabis and concurrent with a natural cannabinoid screen negative, clinicians should suspect SCs use. More specific and sensitive chromatographic methods associated to MS would successful identify SCs. Electrocardiograms might be useful especially if the patient is profoundly tachycardic, and electroencephalogram might be indicated if a seizure is suspected or observed.
Clinical exam
In addition to the above mentioned laboratory tests, detailed physical and neurological exams are imperative.
The SCs which are high-affinity and high-efficacy agonists of the CB1 receptor mimic the “tetrad” of effects induced by cannabis in rodents.Citation113 The common signs of SCs use are hallucinations, agitation, irritability,Citation114 and psycho tropic disturbances and paranoia have been also described.Citation115 Myoclonia, seizure, nausea, vomiting, and hypokalemia may worsen the clinical status.Citation115 Furthermore, cardiovascular effects such tachycardia/palpitation, hypertension, chest pain, and myocardial infarction, may occur.Citation116 However, it is necessary to consider other well-known risk factors before formally linking SCs consumption to myocardial troubles.Citation117
Due to the great variability of chemical classes that could be taken while using K2, Spice, or other mixes containing SCs, unexpected toxic effects could also appear. For example, a case series of kidney injuries was collected in 2012 and was often linked, when identified, to XLR-11 and/or UR-144.Citation118 Another unexpected clinical event, more potential than observed to our knowledge, due to the monoamine oxidase inhibitor activity of SCs demonstrated as real but weak in vitro, is serotoninergic syndrome that could be observed in patients, especially when high doses of SCs are taken by users.Citation119 More recently, new physiopathological hypotheses could explain some of the undesired effects of SCs. For example, Irie et al observed that MAM-2201 is likely to suppress neurotransmitter release in CB1 receptor expressing neurons in mouse cerebellum purkinje cells, contributing to some of the symptoms of SCs intoxication including impairments in cerebellum-dependent motor coordination and motor learning.Citation120 This kind of study is only the beginning step but it reinforces the need for vigilance to detect new toxicological syndromes in cases of suspicious SCs consumption. Faced with an unexplained clinical outcome, physicians have to be attentive in taking a proper history of the patient, with toxicological analysis (identification and quantification of the active substances in patient biological fluids as well as in taken drug), even if it is sometimes difficult to obtain. Also, physicians should always keep in mind that the combination of various SCs, which may be present in available products, and the taking of other illegal substances or alcohol could lead to unforeseen toxidromes.
Treatment
Considering that absorbed compounds are rarely identified and that clinical signs may be unspecific, no directive on the management of such patients has been formally promulgated by public health agencies. In fact, appropriate supportive care, targeting manifesting signs and symptoms and addressing any complications, is the primary treatment for the acutely intoxicated patient.Citation121 Initial management should also include monitoring telemetry for arrhythmia, myocardial ischemia, serum electrolytes, and ensuring a secure airway.Citation122 No specific antidote exists for SCs exposure and no curative treatment is approved by health authorities. The most common therapeutic intervention is hydration with intravenous fluids.Citation92 Observation until the patient demonstrates clinical improvement is recommended and chemical dependency counseling or social service involvement should be considered before discharge.Citation123
The course of treatment for SCs withdrawal is not well described in the literature. In Auckland, patients appearing at a detoxification service for support with SCs withdrawal and presenting withdrawal symptoms, are managed with benzodiazepine (diazepam) and antipsychotics (quetia-pine).Citation102 Based on a preclinical studies demonstrating a bidirectional modulatory relationship between opioid and cannabinoid systems, naloxone was tested to manage the SCs cravings in a 39 year old woman seeking detoxification for SC addiction, and could appear to be a potential option.Citation124,Citation125
Legal status
SCs have a different legal status around the world. Many states globally still schedule them “substance-by-substance” as new ones appear on the market, in an endless race against the resellers.Citation10,Citation126 On the other hand, some countries, UK being the first, have adopted a generic control status that has the great advantage of eluding the “cat and mouse game” problem. However, in the specific case of cannabimimetics that cover a large number of diversified chemical classes, it postpones the problem at one level but without solving it completely. Furthermore, generic control can pose the problem of unintended status for some substances that 1) are not CB1 agonist, and 2) show a medical interest, with an obstacle in the clinical development, as it has been seen in the UK.Citation127
Conclusion
New psychoactive drugs that have appeared over the last decennia are typically dominated by cathinones and SCs. Commercialized as synthetic cannabis, SCs are sprayed onto various herbal smoking blends and traded under brand names like Spice or K2. More recently, SCs are also sold as pure substances ready to use with herbal mixtures or liquids for e-cigarettes. New compounds belonging to new chemical series are continuously emerging which generates a phenomenon that is very difficult to check. SCs pharmacologically act by binding to CB1 and/or CB2 receptors with CB1 agonists accountable for the recreational effects of SCs. The in vitro binding characteristics of SCs have been repeatedly determined, but the pharmacological properties are infrequently explored before human use. Along with unpleasant central nervous system effects, there are several physical adverse effects including kidney damage, pulmonary, gastrointestinal, and cardiovascular effects that can make consumption unsafe. Even if most of the SCs mimic marijuana effects, some SCs bind much more strongly to CB1 receptors than natural can-nabinoids, which can lead to more potent, unpredictable, or dangerous effects. Because of the multitude of compounds, a complete toxicological profile of SCs is far from being drawn and understood. In addition, these SCs are often taken in conjunction with other recreational drugs or alcohol, which makes observed effects difficult to attribute to a specific product. Despite their status being illegal in some countries, SCs continue to be prevalent drugs of abuse with collateral damage such as increased road traffic risks. Accordingly, clinicians should be aware of this developing trend as an explanation for patients presenting unexpected toxidromes. Standard routine toxicology screens, particularly targeted for cannabis detection, may not detect the presence of these compounds. More specific methods may be required for identification and quantification in biological samples, with the ever-increasing diversity of new products making the updates unrealizable for the most part of toxicological laboratories. Meanwhile, clinicians should remain attentive for the risk of increasing morbidity and mortality associated to these products, continue to collect and publish new trends about these highs, and help promote awareness in their communities.
Disclosure
The authors report no conflicts of interest in this work.
References
- DominoEFHardmanHFSeeversMHCentral nervous system actions of some synthetic tetrahydrocannabinol derivativesPharmacol Rev19712343173365003209
- AuwärterVDresenSWeinmannWMüllerMPützMFerreirósN‘Spice’ and other herbal blends: harmless incense or cannabinoid designer drugs?J Mass Spectrum2009445832837
- CastanetoMSGorelickDADesrosiersNAHartmanRLPirardSHuestisMASynthetic cannabinoids: epidemiology, pharmacodynamics, and clinical implicationsDrug Alcohol Depend2014144124125220897
- MackieKCannabinoid receptors: where they are and what they doJ Neuroendocrinol200820Suppl 1101418426493
- PertweeRGCannabinoid pharmacology: the first 66 yearsBr J Pharmacol2006147Suppl1S163S17116402100
- JohnsonMRMelvinLSThe discovery of non-classical cannabinoid analgesicsMechoulamRCannabinoids as Therapeutic AgentsCRC PressBoca Raton: Florida1986121145
- HuffmanJWDongDSynthesis and pharmacology of cannabimimetic indolesBioorg Med Chem Lett199444563566
- LindigkeitRBoehmeAEiserlohISpice : a never ending story?Forensic Sci Int20091911–3586319589652
- UchiyamaNKikura-HanajiriROgataJGodaYChemical analysis of synthetic cannabinoids as designer drugs in herbal productsForensic Sci Int20101981–3313820117892
- European Monitoring Centre for Drugs and Drug Addiction [website on the Internet]Drug profiles Available at: http://www.emcdda.europa.eu/publications/drug-profilesAccessed April 8, 2015
- WohlfarthAScheidweilerKBCastanetoMUrinary prevalence, metabolite detection rates, temporal patterns and evaluation of suitable LC-MS/MS targets to document synthetic cannabinoid intake in US military urine specimensClin Chem Lab Med201553342343425263309
- PalamarJJAcostaPSynthetic cannabinoid use in a nationally representative sample of US high school seniorsDrug Alcohol Depend201514919420225736618
- KellyBCWellsBEPawsonMLeclairAParsonsJTGolubSANovel psychoactive drug use among younger adults involved in US nightlife scenesDrug Alcohol Rev201332658859323795887
- HuXPrimackBABarnettTECookRLCollege students and use of K2: an emerging drug of abuse in young personsSubst Abuse Treat Prev Policy201161621745369
- EganKLSuerkenCKReboussinBAK2 and Spice use among a cohort of college students in southeast region of the USAAm J Drug Alcohol Abuse201541431732226030768
- JaenickeNJPogodaWPaulkeAWunderCToennesSWRetrospective analysis of synthetic cannabinoids in serum samples – epidemiology and consumption patternsForensic Sci Int2014242818725050839
- BeckFRichardJBGuignardRLe NézetOSpilkaSIllicit drugs used in France during 2014 Note 2015-01. OFDT-INPES. http://www.ofdt.fr/BDD/publications/docs/eisxfbv4.pdfAccessed April 15, 2015 French
- LouisAPetersonBLCouperFJXLR-11 and UR-144 in Washington state and state of Alaska driving casesJ Anal Toxicol201438856356825217547
- LemosNPDriving under the influence of synthetic cannabinoid receptor agonist XLR-11J Forensic Sci20145961679168325088081
- TuvSSKrabsethHKarinenROlsenKMØiestadELVindenesVPrevalence of synthetic cannabinoids in blood samples from Norwegian drivers suspected of impaired driving during a seven weeks periodAccid Anal Prev201462263124129318
- AdamowiczPLechowiczWThe Influence of Synthetic Cannabinoid UR-144 on Human Psychomotor Performance – A Case Report Demonstrating Road Traffic RisksTraffic Inj Prev Epub2015320
- MoreSVChoiDKPromising cannabinoid-based therapies for Parkinson’s disease: motor symptoms to neuroprotectionMol Neurodegener2015101725888232
- PiconeRPKendallDAMol EndocrinolMinireview: From the Bench, toward the clinic: therapeutic opportunities for cannabinoid receptor modulationMol Endocrinol201529680181325866875
- ArnstonAOfsaBLancasterDSimonJRMc MullinMLoganBValidation of a novel immunoassay for the detection of synthetic cannabinoids and metabolites in urine specimensJ Anal Toxicol201337528429023625703
- MohrAOfsaBKeilASimonJMcMullinMLoganBEnzyme-Linked Immunosorbent Assay (ELISA) for the detection of use of the synthetic cannabinoid agonists UR-144 and XLR-11 in human urineJ Anal Toxicol201438742743124908262
- BarnesAJYoungSSpinelliEMartinTMKletteKLHuestisMAEvaluation of a homogenous enzyme immunoassay for the detection of synthetic cannabinoids in urineForensic Sci Int2014241273424845968
- SimolkaKLindigkeitRSchiebelHMPapkeUErnstLBeuerleTAnalysis of synthetic cannabinoids in “spice-like” herbal highs: snapshot of the German market in summer 2011Anal Bioanal Chem2012404115717122710567
- CoxAODawRCMasonMDUse of SPME-HS-GC-MS for the analysis of herbal products containing synthetic cannabinoidsJ Anal Toxicol201236529330222582264
- MoosmannBKneiselSGirreserUBrechtVWestphalFAuwärterVSeparation and structural characterization of the synthetic cannabinoids JWH412 and 1-[(5-fluoropentyl)-1H-indol-3yl]-(4-methylnaphthalen-1-yl)methanone using GC-MS, NMR analysis and a flash chromatography systemForensic Sci Int20122201–3e17e2222264627
- ChoiHHeoSChoeSSimultaneous analysis of synthetic cannabinoids in the materials seized during drug trafficking using GC-MSAnal Bioanal Chem2013405123937394423208283
- LangerNLindigkeitRSchiebelHMErnstLBeuerleTIdentification and quantification of synthetic cannabinoids in ‘spice-like’ herbal mixtures: a snapshot of the German situation in the autumn of 2012Drug Test Anal201461–2597123723183
- KneiselSBiselPBrechtVBroeckerSMüllerMAuwärterVIdentification of the cannabimimetic AM-1220 and its azepane isomer (N-methylazepam-3yl)-3-(1-naphthoyl)indole in research chemical and several herbal mixturesForensic Toxicol201230126134
- KneiselSSpeckMMoosmannBCorneillieTMButlinNGAuwärterVLC/ESI-MS/MS method for quantification of 28 synthetic cannabinoids in neat oral fluid and its application to preliminary studies on their detection windowsAnal Bioanal Chem2013405144691470623535743
- DziadoszMWellerJPKlintscharMTeskeJScheduled multiple reaction monitoring algorithm as a way to analyze new designer drugs combined with synthetic cannabinoids in human serum with liquid chromatography-tandem mass spectrometryJ Chromatogr B Analyt Technol Biomed Life Sci20139298489
- DerungsASchwaningerAEMansellaGBingisserRKraemerTLiechtiMESymptoms, toxicities, and analytical results for a patient after smoking herbs containing the novel synthetic cannabinoid MAM-2201Forensic Toxicology2013311164171
- SalomoneALucianoCDi CorciaDGeraceEVincentiMHair analysis as a tool to evaluate the prevalence of synthetic cannabinoids in different populations of drug consumersDrug Test Anal201461–212613424115381
- ScheidweilerKBHuestisMASimultaneous quantification of 20 synthetic cannabinoids and 21 metabolites, and semi-quantification of 12 alkyl hydroxy metabolites in human urine by liquid chromatography-tandem mass spectrometryJ Chromatogr A2014132710511724418231
- MazzarinoMde la TorreXBotrèFA liquid chromatography-mass spectrometry method based on class characteristic fragmentation pathways to detect the class of indole-derivative synthetic cannabinoids in biological samplesAnal Chim Acta2014837708225000860
- SimõesSSSilvaIAjenjoACDiasMJValidation and application of an UPLC-MS/MS method for the quantification of synthetic cannabinoids in urine samples and analysis of seized materials from the Portuguese marketForensic Sci Int201424311712525127518
- KimJParkYParkMSimultaneous determination of five naphthoylindole-based synthetic cannabinoids and metabolites and their deposition in human and rat hairJ Pharm Biomed Anal201510216217525282599
- JangMShinIKimJYangWSimultaneous quantification of 37 synthetic cannabinoid metabolites in human urine by liquid chromatography-tandem mass spectrometryForensic Toxicol201510.1007/s11419-015-0265-x
- GottardoRChiariniADal PràIDirect screening of herbal blends for new synthetic cannabinoids by MALDI-TOF MSJ Mass Spectrom201247114114622282100
- SundströmMPelanderAAngererVHutterMKneiselSOjanperäIA highsensitivity ultra-high performance liquid chromatography/high-resolution timeof-flight mass spectrometry (UHPLC-HR-TOFMS) method for screening synthetic cannabinoids and other drugs of abuse in urineAnal Bioanal Chem2013405268463847423954996
- KronstrandRBrinkhagenLBirath-KarlssonCRomanMJosefssonMLCQTOF-MS as a superior strategy to immunoassay for the comprehensive analysis of synthetic cannabinoids in urineAnal Bioanal Chem2014406153599360924424965
- GottardoRSorioDMusileGScreening for synthetic cannabinoids in hair by using LC-QTOF MS: a new and powerful approach to study the penetration of these new psychoactive substances in the populationMed Sci Law2014541222723842479
- MusahRADominMAWallingMAShepardJRRapid identification of synthetic cannabinoids in herbal samples via direct analysis in real time mass spectrometryRapid Commun Mass Spectrom20122691109111422467461
- DowlingGReganLA method for CP 47, 497 a synthetic nontraditional cannabinoid in human urine using liquid chromatography tandem mass spectrometryJ Chromatogr B Analyt Technol Biomed Life Sci20118793–4253259
- CallénLMorenoEBarroso-ChineaPCannabinoid receptors CB1 and CB2 form functional heteromers in brainJ Biol Chem201228725208512086522532560
- BlankmanJLCravattBFChemical probes of endocannabinoid metabolismPharmacol Rev201365284987123512546
- AboodMEMolecular biology of cannabinoid receptors: mutational analyses of the CB receptorsReggioPHThe cannabinoids receptorsHumana PressGreensboro, NC2009203234
- PertweeRGPharmacological actions of cannabinoidsHandbook of Experimental PharmacologyedHeidelburg–Verlag2005168151
- HuffmanJWZenginGWuMJStructure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB(1) and CB(2) receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB(2) receptor agonistsBioorg Med Chem20051318911215582455
- AungMMGriffinGHuffmanJWInfluence of the N-1 alkyl chain length of cannabimimetic indoles upon CB(1) and CB(2) receptor bindingDrug Alcohol Depend200060213314010940540
- MakriyannisADengHWO patent 200128557Cannabimimetic indole derivatives Granted 2001-06-07
- HuffmanJWSzklennikPVAlmondA1-Pentyl-3-phenylacetyl-indoles, a new class of cannabimimetic indolesBioorg Med Chem Lett200515184110411316005223
- FrostJMDartMJTietjeKRIndol-3ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activityJ Med Chem201053129531519921781
- McGaraughtySChuKLDartMJYaoBBMeyerMDA CB(2) receptor agonist, A-836339, modulates wide dynamic range neuronal activity in neuropathic rats: contributions of spinal and peripheral CB(2) receptorsNeuroscience200915841652166119063946
- DengHGiffordANZvonokAMPotent cannabinergic indole analogues as radioiodinatable brain imaging agents for the CB1 cannabinoid receptorJ Med Chem200548206386639216190764
- SmithVJSynthesis and pharmacology of N-alkyl-3-(halonaphtoyl) indolesPhD dissertationClemson UniversitySouth Carolina, USA2008
- AbadjiVLinSTahaG(R)-methanandamide: a chiral novel anandamide possessing higher potency and metabolic stabilityJ Med Chem19943712188918938021930
- HuffmannJWSmithVJChenJWileyJLMartinBRStructure-activity relationships at the CB1 and CB2 receptors for 1-alkyl-3(1-naphtoyl-4 and 8-halogen substituted) indoles19th Annual Symposium on the International Cannabinoid Research SocietyJuly 7–11, 2009St Charles, Illinois, USA2
- WrobleskiSTChenPHynesJJrRational design and synthesis of an orally active indolopyridone as a novel conformationally constrained cannabinoid ligand possessing anti-inflammatory propertiesJ Med Chem200346112110211612747783
- HuffmannCannabimimetic indoles, pyrroles, and Indenes: structure-activity relationships and receptor interactionsReggioPHThe cannabinoids receptorsHumana Press20094994
- MakriyannisALaiXZLuDNovel biphenyl and biphenyl-like cannabinoidsPatent US20040087590 A1562004
- Console-BramLMarcuJAboodMECannabinoid receptors: nomenclature and pharmacological principlesProg Neuropsychopharmacol Biol Psychiatry201238141522421596
- PertweeRGThe diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarinBr J Pharmacol2008153219921517828291
- HowlettACThe cannabinoid receptorsProstaglandins Other Lipid Mediat20026869619631
- PacherPBatkaiSKunosGThe endocannabinoid system as an emerging target of pharmacotherapyPharmacol Rev200658338946216968947
- SunXDeySKSynthetic cannabinoids and potential reproductive consequenceLife Sci2014971727723827241
- Ng Cheong TonJMGerhardtGAFriedemannMThe effects of delta 9-tetrahydrocannabinol on potassium-evoked release of dopamine in the rat caudate nucleus: an in vivo electrochemical and in vivo microdialysis studyBrain Res19884511–259682855215
- FrenchEDDillonKWuXCannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigraNeuroreport1997836496529106740
- ParkerLAGilliesTTHC-induced place and taste aversions in Lewis and Sprague-Dawley ratsBehav Neurosci1995109171787734082
- McGregorISIssakidisCNPriorGAversive effects of the synthetic cannabinoid CP 55,940 in ratsPharmacol Biochem Behav19965336576648866969
- LeporeMVorelSRLowinsonJGardnerELConditioned place preference induced by delta 9-tetrahydrocannabinol: comparison with cocaine, morphine, and food rewardLife Sci19955623–24207320807776834
- BraidaD1PozziMCavalliniRSalaMConditioned place preference induced by the cannabinoid agonist CP 55,940: interaction with the opioid systemNeuroscience2001104492392611457579
- ChaHJLeeKWSongMJDependence Potential of the Synthetic Cannabinoids JWH-073, JWH-081, and JWH-210: In Vivo and In Vitro ApproachesBiomol Ther (Seoul)201422436336925143817
- ZangenASolinasMIkemotoSGoldbergSRWiseRATwo brain sites for cannabinoid rewardJ Neurosci200626184901490716672664
- WeissmanAMilneGMMelvinLSJrCannabimimetic activity from CP47,497, a derivative of 3-phenylcyclohexanolJ Pharmacol Exp Ther198222325165236290642
- GinsburgBCSchulzeDRHrubaLMcMahonLRJWH-018 and JWH-073: Δ9 -tetrahydrocannabinol-like discriminative stimulus effects in monkeysJ Pharmacol Exp Ther20123401374521965552
- GatchMBForsterMJΔ9-Tetrahydrocannabinol-like discriminative stimulus effects of compounds commonly found in K2/SpiceBehav Pharmacol201425875075725325289
- De VryJJentzschKRDiscriminative stimulus effects of the structurally novel cannabinoid CB1/CB2 receptor partial agonist BAY 59-3074 in the ratEur J Pharmacol20045051–312713315556145
- LapointJJamesLPMoranCLNelsonLSHoffmanRSMoranJHSevere toxicity following synthetic cannabinoid ingestionClin Toxicol (Phila)201149876076421970775
- ObafemiAIKleinschmidtKGotoCFoutDCluster of acute toxicity from ingestion of synthetic cannabinoid-laced browniesJ Med Toxicol Epub2015
- LonatiDBuscagliaEPapaPMAM-2201 (analytically confirmed) intoxication after “Synthacaine” consumptionAnn Emerg Med201464662963224530110
- MarshellRKearney-RamosTBrentsLKIn vivo effects of synthetic cannabinoids JWH-018 and JWH073 and phytocannabinoid Δ9-THC in mice: inhalation versus intraperitoneal injectionPharmacol Biochem Behav2014124404724857780
- BLULIGHT [forum on the Internet]Other drugs Forum Available from: http://www.bluelight.org/vb/threads/539892-Injection-of-syntheticcannabinoids-like-a-JWHAccessed May 13, 2015
- KacinkoSLXuAHomanJWMcMullinMMWarringtonDMLoganBKDevelopment and validation of a liquid chromatography-tandem mass spectrometry method for the identification and quantification of JWH-018, JWH-073, JWH-019, and JWH-250 in human whole bloodJ Anal Toxicol201135738639321871146
- WinstockARBarrattMJSynthetic Cannabis: A comparison of patterns of use and effect profile with natural Cannabis in a large global sampleDrug Alcohol Depend20131311–210611123291209
- ForresterMBKleinschmidtKSchwarzEYoungASynthetic can-nabinoid exposures reported to Texas poison centersJ Addict Dis201130435135822026527
- ForresterMBAdolescent synthetic cannabinoid exposures reported to Texas poison centersPediatr Emerg care2012281098598923023462
- de HavenonAChinBThomasKCAfraPThe secret “spice”: an undetectable toxic cause of seizureNeurohospitalist20111418218623983854
- HoyteCOJacobJMonteAAAl-JumaanMBronsteinACHeardKJA characterization of synthetic cannabinoid exposures reported to the National Poison Data System in 2010Ann Emerg Med201260443543822575211
- WinstockALynskeyMBorschmannRWaldronJRisk of emergency medical treatment following consumption of Cannabis or synthetic cannabinoids in a large global sampleJ Psychopharmacol201529669870325759401
- CelofigaAKoprivsekJKlavzJUse of synthetic cannabinoids in patients with psychotic disorders: case seriesJ Dual Diagn201410316817325392292
- Van AmsterdamJBruntTvan den BrinkWThe adverse health effects of synthetic cannabinoids with emphasis on psychosis-likeJ Psychopharmacol201529325426325586398
- SpadernaMAddyPHD’SouzaDCSpicing things up: synthetic cannabinoidsPsychopharmacol (Berl)20132284525540
- AlhadiSTiwariAVohraRGeronaRAcharyaJBilelloKHigh times, low sats: diffuse pulmonary infiltrates associated with chronic synthetic cannabinoid useJ Med Toxicol20139219920623539384
- IbrahimSAl-SaffarFWannenburgTA unique case of cardiac arrest following K2 abuseCase report Cardiologia2014120607
- TakematsuMHoffmanRSNelsonLSSchechterJMMoranJHWienerSWA case of acute cerebral ischemia following inhalation of a synthetic cannabinoidClin Toxicol (Phila)201452997397525241766
- FergussonDMHorwoodLJNorthstoneKMaternal use of cannabis and pregnancy outcomeBJOG20021091212711843371
- Every-PalmerSSynthetic cannabinoid JWH-018 and psychosis: an explorative studyDrug Alcohol Depend20111172–315215721316162
- MacfarlaneVChristieGSynthetic cannabinoid withdrawal: a new demand on detoxification servicesDrug Alcohol Rev201534214715325588420
- NaccaNVattiDSullivanRSudPSuMMarraffaJThe synthetic cannabinoid withdrawal syndromeJ Addict Med20137429629823609214
- RomingerACummingPXiongGEffects of acute detoxification of the herbal blend ‘Spice Gold’ on dopamine D2/3 receptor availability: a [18F]fallypride PET studyEur Neuropsychopharmacol201323111606161023452563
- ShanksKGDaahnTTerrelARDetection of JWH-018 and JWH-073 by UPLC-MS-MS in postmortem whole blood caseworkJ Anal Toxicol201236314515222417829
- AdamowiczPZubaDSekulaKAnalysis of UR-144 and its pyrolysis product in blood and their metabolites in urineForensic Sci Int201323332032724314536
- KarinenRTuvSSØiestadELVindenesVConcentrations of API-NACA, 5F-APINACA, UR-144 and its degradant product in blood samples from six impaired drivers compared to previous reported concentrations of other synthetic cannabinoidsForensic Sci Int20152469810325485949
- GambaroVArnoldiSBellucciSCharacterization of in vitro metabolites of JWH-018, JWH-073 and their 4-methyl derivatives, markers of the abuse of these synthetic cannabinoidsJ Chromatogr B Analyt Technol Biomed Life Sci20149576876
- Strano-RossiSAnzillottiLDragoniSMetabolism of JWH-015, JWH-098, JWH-251, and JWH-307 in silico and in vitro: a pilot study for the detection of unknown synthetic cannabinoids metabolitesAnal Bioanal Chem2014406153621363624804821
- HolmNBNielsenLMLinnetKCYP3A4 mediates oxidative metabolism of the synthetic cannabinoid AKB-48AAPS J Epub2015
- AshinoTHakukawaKItohYNumazawaSInhibitory effect of synthetic cannabinoids on CYP1A activity in mouse liver microsomesJ Toxicol Sci201439681582025374372
- TeskeJWellerJPFieguthARothämelTSchulzYTrögerHDSensitive and rapid quantification of the cannabinoid receptor agonist naphthalen-1-yl-(1-pentylindol-3yl)methanone (JWH-018) in human serum by liquid chromatography-tandem mass spectrometryJ Chromatogr B Analyt Technol Biomed Life Sci20108782726592663
- WiebelhausJMPoklisJLPoklisAVannRELichtmanAHWiseLEInhalation exposure to smoke from synthetic “marijuana” produces potent cannabimimetic effects in miceDrug Alcohol Depend2012126331632322776442
- BrewerTLCollinsMA review of clinical manifestation in adolescent and young adults after use of synthetic cannabinoidsJ Spec Pediatr Nurs201419211912624320158
- Hermanns-ClausenMKneiselSSzaboBAcute toxicity due to the confirmed consumption of synthetic cannabinoids: clinical and laboratory findingsAddiction2013108353454422971158
- MirAObafemiAYoungAKaneCMyocardial infarction associated with use of the synthetic cannabinoid K2Pediatrics20111286e1622e162722065271
- AlexandreJDebruyneDCoquerelALe BoisselierRComment on A Unique Case of Cardiac Arrest following K2 AbuseCase Rep Cardiol2015201573914925883810
- Centers for Disease Control and Prevention (CDC)Acute kidney injury associated with cannabinoid use in multiple states, 2012Morb Mortal Wkly Rep20136269398
- FisarZInhibition of monoamine oxidase activity by cannabinoidsNaunyn Schmiedebergs Arch Pharmacol2010381656357220401651
- IrieTKikura-HanajiriRUsamiMUchiyamaNGodaYSekinoYMAM-2201, a synthetic cannabinoid drug of abuse, suppresses the synaptic input to cerebellar Purkinje cells via activation of presynaptic CB1 receptorsNeuropharmacology20159547949125747605
- WeaverMFHopperJAGundersonEWDesigner drugs 2015: assessment and managementAddict Sci Clin Pract201510825928069
- HealthTSBurroughsZThompsonAJTecklenburgFWAcute intoxication caused by a synthetic cannabinoid in two adolescentsJ Pediatr Pharmacol Ther201217217718123118671
- HarrisCRBrownASynthetic cannabinoid intoxicationJ Emerg Med201344236036622989695
- RodgmanCJVerricoCDWorthyRBLewisEEInpatient detoxification from a synthetic cannabinoid and control of postdetoxification cravings with naltrexonePrim Care Companion CNS Disord2014164
- RobledoPBerrenderoFOzaitaAMaldonadoRAdvances in the field of cannabinoid-opioid cross-talkAddict Biol200813221322418482431
- Drug Enforcement Administration, Department of JusticeSchedules of controlled substances: temporary placement of three synthetic cannabinoids into schedule I. Final orderFed Regist201580205042504725730924
- KingLALegal controls on cannabimimetics: an international dilemma?Drug Test Anal201461–2808723881527
- ShevyrinVMelkozerovVNeveroAEltsovOBaranovskyAShafranYSynthetic cannabinoids as designer drugs: new representatives of indol-3carboxylates series and indazole-3-carboxylates as novel group of cannabinoids. Identification and analytical dataForensic Sci Int201424426327525305529