Abstract
Aim
The relationship between ligaments and bone is a complex and heterogeneous junction involving bone, mineralized fibro cartilage, non-mineralized fibro cartilage and ligaments. Mesenchymal stem cells (MSC) can be used in vivo to control inflammation and aid in tissue repair, according to studies. This review focused on using exosomes as an alternative to MSC, as a cell-free therapy for modulating the remodelling process.
Methods
To conduct a systematic review of the literature, the phrases “exosome” and “ligament” or “tendon” and “extracellular vesicle” and “stem cells” were used as the search keywords in PubMed (MEDLINE), OVID, the Cochrane Library, and Science Direct. From the literature, 73 studies in all were found. Six studies were included in this systematic review after full-text evaluation.
Results
Six included studies covered a range of MSC types, isolation techniques, animal models, and interventions. Biomechanical results consistently indicated the beneficial impact of conditioned media, vesicles, and exosomes on treating tendons and ligaments. Noteworthy findings were the reduction of inflammation by iMSC-IEVs, chondrocyte protection by iPSC-EVs (extracellular vesicles generated by inflammation-primed adipose-derived stem cells), osteolysis treatment using DPSC-sEVs (small extracellular vesicles derived from dental pulp stem cells), and the contribution of exosome-educated macrophages to ligament injury wound healing.
Conclusion
Exosomes may serve as a cell-free therapeutic substitute for modulating the remodelling process, particularly in ligament healing.
Introduction
Injuries to ligaments and tendons are frequent issues in orthopaedics. Therapies that can increase the effectiveness of surgical ligament and tendon restoration or hasten nonoperative recovery are required.Citation1 Fifty percent of musculoskeletal injuries are diagnosed as tendon and ligament injuries. Common sprains and strains, among other tendon and ligament ailments, frequently recover without the need for surgery. Nevertheless, the procedure is frequently sluggish and leads to the development of inferior scar tissue, which takes years to regenerate into more useful tissue. Surgical intervention is frequently necessary for more severe injuries, such as a total rupture of a tendon or ligament. When a tendon is acutely injured, the healing process starts right away. Three chronological phases are commonly recognized for this process: remodeling, proliferation, and inflammation. Despite their overlap, these phases are distinguished by different cellular mechanisms and cytokine profiles. The production of clots in injured tissue triggers the inflammatory stage of tendon repair, which starts as soon as an acute injury occurs. This stage involves the formation of clots in injured arteries, the activation of inflammatory cells, and the recruitment of fibroblasts to carry out the remaining healing steps.Citation2
Anterior Cruciate Ligament (ACL) rupture is one of the most common injuries of the knee joint, which results in instability and pain in the knee and increases the risk of knee osteoarthritis and disability.Citation3 ACL reconstruction is currently the recommended surgical therapy for ACL ruptures that result in ongoing instability of the knee joint, because ACL repair, or suturing the ACL ligament, is not always successful due to the lack of fibrin in the ACL ligament. To rebuild the ACL, autologous tissue grafts are necessary. ACL reconstruction is considered a safe and effective surgical procedure for managing unstable ACL rupture.Citation4 The healing process that integrates the ligament to the bone (ligament bone healing), as in ACL reconstruction and ACL repair, is still challenging in orthopaedics and sports medicine.Citation3
The outcome of an ACL reconstruction operation depends on how well the integration between the transplanted tendon to the bone in the created tunnel. The complicated, well-coordinated process of tendon repair leaves the mechanical and chemical of reconstructed tissue fully regenerated like the native tissue.Citation5 Tendon tissue has a limited ability to self-heal and regenerate after injury because it has an inadequate vascular supply and endures heavy loading.Citation6 One of the key determinants of the effectiveness of ACL restoration surgery is the rate of bone-tendon integration.Citation7 Additionally, it’s crucial to figure out ways to speed up bone and graft recovery so that ACL restoration patients can resume their regular activities sooner.Citation8
Following a tendon injury, suboptimal outcomes are frequently attributed to an exaggerated inflammatory response, primarily instigated by infiltrating macrophages, and concurrent insufficient regenerative activities mediated by the resident tendon cells, including tenocytes, tendon stem/progenitor cells, and epitenon cells. While inflammation plays a crucial role in initiating the healing cascade and clearing damaged tissue, an excessive inflammatory reaction can lead to detrimental consequences such as apoptosis of tendon cells, matrix degradation, and the formation of peritendinous scar tissue. These pathological processes ultimately impede the recovery of tendon structural integrity, strength and excursion.Citation9
The relationship between ligaments and bone is a complex and heterogeneous junction involving bone, mineralized fibro cartilage, non-mineralized fibro cartilage and ligaments. This structure plays an essential role in transmitting energy from one bone to another and preventing the accumulation of excess energy. Various approaches for enhancing the integration between ligaments and bones encompass mechanical stimulation, enveloping tendons with periosteum, and introducing cells, MSCs, bone marrow stroma, and growth factors to fill the interface.Citation8 Research has demonstrated that administering MSCs in vivo can control inflammation and aid in tissue healing. MSCs are frequently employed in tissue engineering because of their ability to multiply, self-renew, and differentiate into distinct tissues. Growth factors, cytokines, and extracellular vesicles are examples of paracrine substances that are important for the regenerative actions of MSCs.Citation4 Multipotent adult stem cells called bone marrow MSCs (BM-MSCs) have emerged as a crucial source of cells for tissue engineering and cell therapy.Citation8
Most cells are known to secrete extracellular vesicles (EVs), which include a range of nucleic acids, lipids, and proteins from the parental cell and are involved in cell-to-cell communication and the regulation of cell behaviour.Citation9 The particle size of EVs can be used to categorize them into small EVs (sEVs, 200nm, pelleted at 100,000g), and large EVs (IEVs, >200nm, pelleted at 10,000g).Citation10
Although almost all cell types are capable of producing EVs, EVs from various cell types and cell states transport diverse cargo molecules and have unique therapeutic potential. The latest research showed that extracellular vesicles generated by inflammation-primed adipose-derived stem cells (iEVs) could reduce inflammation in the early phase of tendon healing.Citation9
Exosomes have the greatest therapeutic potential of all the extracellular vesicles. Exosomes are small (30–150nm) membrane EVs that have been discovered to function as macromolecule transporters. These macromolecules include proteins, mRNAs, microRNAs (miRNA), lipids, and signalling cytokines. Exosomes are also an important type of intercellular messenger that can be detected by receptor cells by endocytosis.Citation4 Exosomes are widely dispersed in different body fluids and carry and transmit vital signal molecules, establishing a unique intercellular communication system. Exosomes can be secreted by almost all cell types, and they are frequently found in physiological fluids such as blood, milk, urine, tears, saliva, and ascites.Citation11
Exosomes are of widespread interest due to their function in cell biology and possible applications in therapy and diagnostics. It represents a fresh method of cell interaction and supports a variety of biological functions in both health and sickness.Citation12 The heart, liver, skin, cartilage, tendons, and ligaments are just a few of the tissues and organs that exosomes can help regenerate and repair. Exosomes have been demonstrated to have a variety of uses as naturally occurring nanoparticles throughout the past 20 years.Citation13 Exosomes can control and have an impact on cell death during tissue repair. Using iEVs from inflammation-primed adipose-derived stem cells (iASCs) can reduce inflammation and promote intrinsic tendon repair by transporting active chemicals that control macrophage and tendon cell activities, according to a study by Shen and Len.Citation9
Exosomes have been shown to have several important uses, includes their use as biomarkers, drug delivery systems, exosome therapy, and cancer vaccinations. A total of 116 experiments have been documented, of which 50% are connected to biomarker applications, 28.44% are exosome therapy trials, 5.17% are drug delivery system trials, 14.66% are exosome basic analysis trials, and roughly 1.72% are exosome vaccination trials.Citation14 We looked for additional evidence to support the possibility of exosomes in ligament healing based on the research.
Methods
Search Technique
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards, a thorough search was conducted. From January 2000 to November 2022, the search engine looked through the databases in PubMed, Science Direct, the Cochrane Library and OVID. The following terms were looked into: ”exosome” AND ”ligament” OR ”tendon” AND ”extracellular vesicle” AND ”stem cells”. The following MeSH Terms were used in the search: (exosome” [Mesh Terms]), (ligament” [Mesh Terms]), (tendon” [Mesh Terms]), (extracellular vesicle” [Mesh Terms]), and (stem cells” [Mesh Terms]. All of the references were manually searched.
Eligibility Requirements
All in vivo investigations on controlled animals that examined the function and biomechanics of tendon-ligament repair that were published in English met the inclusion criteria. Journals published in languages other than English and duplicate journals were among the exclusion criteria.
Data Collection and Selection
Using the inclusion and exclusion criteria, the titles and abstracts were scrutinized. Two writers (A.Y. and N.A.) independently evaluated the eligibility of the entire texts of all chosen papers. Using the ARRIVE (Animal Research: Reporting of in vivo Experiments) guidelines, the methodological quality of each article was evaluated. As a result, we integrate modified ARRIVE with Consolidating Reporting of Trials. The risk of bias instrument from the Systematic Review Centre for Laboratory Animal Experimentation was used to evaluate internal validity. The evaluations were all completed independently by two writers (A.Y and N.A). Any disagreements were discussed with the other authors in order to be resolved.
The following information was taken out: study design; type of animal used for in vivo studies; method used to establish animals or cells in studies included; type and specific donor of MSCs; method used to isolate CM, EVs, or exosomes; interventions; comparison; length of follow-up; primary outcome for in vivo studies and results; any significant deviations from control or baseline; and other outcomes. We investigated any quantitative outcome measures that were comparable to clinical outcome measures for in vivo investigations, with biomechanical testing serving as the main outcomes. The classification of MSC types by BM-derived, adipose tissue-derived, and induced MSCs was agreed upon by the authors. displays the data collected for in vivo study results.
Table 1 Overview of the Studies
Results
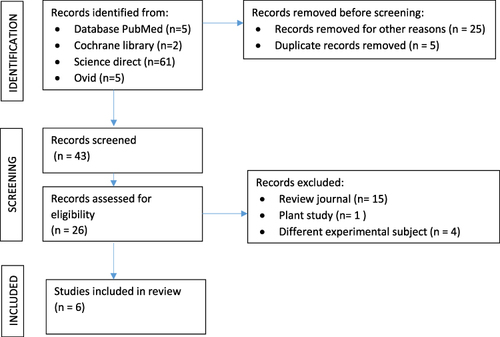
depicts a PRISMA flow diagram that summarizes the research selection procedure. From the literature, 73 studies in all were found. After the titles and abstracts were screened, 48 articles were found to be qualified for further review. Six studies were included in this systematic review after full-text evaluation.
provides a summary of the study, and provides more detail, including the study’s design, the types of MSC used and their sources, the isolation of the CM and its subcomponents, animal damage models for the in vivo investigation, and the therapies. Small animals—three rats and two mice—were used in the majority of research. In one investigation, a large animal (a rabbit) was employed. The tendon was the subject of four research, the anterior cruciate ligament (ACL), one, and the calvarial osteolysis model, the other. Collagenase injection, partial resection, total resection and inadvertent tear were used to create injury models. Two studies used BM-derived MSCs the most frequently, followed by one study using dental pulp stem cells, one study using adipose tissue, and two studies using induced pluripotent stem cells (iPSC). Three were made with rat blood, while three were made with human blood. Various techniques were used to isolate the MSCs’ conditioned medium. In two studies, the conditioned media were administered through injection. The EVs were distinguished from the CM in investigations by Chamberlain et al.Citation5 Hsueh et alCitation18, and Shen et al.Citation19 In four investigations, the control groups received injections of PBS, a study utilizing a model of mouse calvarial osteolysis.
Table 2 In vivo Study results
provides a summary of the in vivo study data, including the primary outcome measures, scores or results, a statistical significance statement, and a list of additional outcome measures. Biomechanical results were given in all investigations. The favourable effects of conditioned media, extra vesicular vesicles, and exosomes in tendon and ligament treatment were generally validated by biomechanical results. Previous research demonstrated that by controlling macrophage heterogeneity, iMSC-IEVs might reduce inflammation in tendinopathy.Citation20 As shown by Hsueh et al, iPSC-EVs protected chondrocytes by promoting cell proliferation, repressing early senescence, and preserving the homeostasis of collagen II synthesis and matrix degradation enzymes.Citation18 According to Tian et al, hypoxia-induced DPSC-sEV may be used to treat infectious or inflammatory osteolysis.Citation15 Furthermore, exosome-educated macrophages (EEMs) may offer a cutting-edge method for accelerating wound healing in skeletal injuries, according to a prior publication.Citation5
Discussion
Inflammation, proliferation, and remodelling are the three stages that the repair process for a tendon injury must go through. Inflammation and the infiltration and activation of several cell types, including macrophages, in the repairing tendon tissue mark the beginning of the healing process after a tendon injury.Citation16 Later stages of tendon healing can result in scar formation due to strong inflammatory responses. Then, tendons will exhibit changes in their histology, biochemistry, and biomechanical characteristics, making it impossible for them to regain their native strength and elasticity and making them more susceptible to break again in the event of a subsequent injury. In order to facilitate effective recovery following tendon injury, it is crucial to manage inflammation.Citation6
This study demonstrated that, compared to the control groups in the three investigations, CM was linked with considerably superior functional and biomechanical outcomes. In contrast, EV was almost always linked to significantly better functional and biomechanical outcomes than the control groups in the remaining investigations. Two investigations revealed the suppression of inflammation indicators. Induced pluripotent stem cells have been shown in several trials to lessen inflammation.Citation18,Citation20 We are aware that iMSC-IEVS reduces inflammation and pain in tendinopathy by polarizing inflammatory macrophages toward anti-inflammatory macrophages, in part through regulating the p38 MAPK signaling pathway by delivering DUSP2 and DUSP3.Citation20 M1 macrophage numbers could drop as a result of iMSC-IEVS. In addition, it raises the number of M2 macrophages in the tendon tissue of both rats and people. In addition, Hsueh et al found that EVs produced from human iPSC (iPSC-EVs) can reverse the expression of collagen I and decrease the expression of matrix metalloproteinase (MMPS-1/-3) in fibroblasts, catabolic enzymes linked to ECM degradation and OA pathogenesis.Citation18 Although iPSC cultivation is more complicated, prior studies have shown that iPSCs produce 16 times as many EVs as MSCs.
Furthermore, we are aware of the benefits of employing EEM as a cell therapy to cure tissue damage. In preclinical research, conditioned media (secretome) shows signs of improvement in the tendon and ligament healing process.Citation17 According to a different study, hypoxia preconditioning increased the therapeutic impact of DPSC-sEV on LPS-induced osteolysis by inducing the secretion of DPSC-sEV.Citation15 EEM therapy sped up tendon angiogenesis as it healed. A decreased M1/M2 macrophage ratio and an increase in endothelial cells 14 days after injury suggest that EVs extracted from MSC-conditioned medium may trigger a biological response. In the initial stages of tendon repair, they might aid tendon matrix regeneration.Citation19
According to Cui et al, BMDM exosomes play an important role in the peritendinous microenvironment where they act as vital messengers facilitating intercellular crosstalk by promoting the proliferation and migration abilities of tenocytes, fibroblasts, and pro-fibrotic activity around the repaired tendon.Citation21 Exosomes made from adipose stem cells (ADSC-Exos), among the numerous forms of regenerative medicine, provide the best chances for tendon regeneration.Citation22–24 ADSCs are potential cartilage cells with anti-inflammatory and cytoprotective properties. Exosomes down-regulate the aging traits of OA osteoblasts and facilitate the paracrine effect of ADSCs.Citation9
ACL reconstruction’s surgical prognosis may be improved by a solid combination of surgical, biological, and biomechanical augmentation, according to prior research. Additionally, it has been demonstrated that growth factors have beneficial impacts on a number of biological procedures important for accelerating ACL recovery. By promoting angiogenesis and osteogenesis of the graft in the tunnel, the administration of BM-MSCs and vascular endothelial growth factor (VEGF) greatly enhanced and promoted cell proliferation, differentiation, and matrix deposition in their study. Increases in collagen type III, graft signal intensity, and ultimate tensile strength, as well as a decrease in tunnel and bone-tunnel interface diameters, are all indicators of the cell’s progress.Citation8
Exosomes made from stem cells, for example, can be used in cell-free therapies and have advantages over using actual stem cells, including not inducing an immune response against them, lowering the risk of thromboembolism, and evaluation in line with pharmacological agents.Citation13,Citation25,Citation26 Exosomes can control and have an impact on cell death throughout the network repair process. Because SPM exosomes include proteins that can control M1/2 macrophage polarization, they are effective at enhancing the quality of tendon/ligament integration into bone.Citation27,Citation28 Exosomes have also demonstrated the ability to modify the matrix by elevating TIMP expression while lowering MMP-3 expression.Citation29
In particular, exosomes mediated intercellular communication in the methods listed below. Exosome membrane proteins can first bind to the proteins on the surface of target cells to activate signalling pathways in those cells. Second, protease in the extracellular matrix has the ability to break exosome membrane proteins. The exosome membrane can fuse directly with the target cell membrane and release non-selectively proteins. The cleaved fragments can act as ligands to bind to a receptor on the cell membrane.Citation11 Exosomes have recently attracted much more attention and have been the subject of more extensive efforts to be used as the new therapeutic tools for delivering therapeutic molecules to modify inflammation and related diseases because of their ability to transfer bioactive molecules from one cell to another and subsequently cause changes in the recipient cells. Recent research has demonstrated that exosomes may be utilized to transport miRNAs and short-interfering RNAs (siRNA) in vivo. One benefit of delivering these RNAs via exosomes is that they increase the effectiveness of RNA distribution to the target tissues by preventing RNase degradation. Numerous recent investigations have demonstrated that the release of exosomes is a tactic used by cells to reduce inflammation and avoid tissue damage, which is frequently the result of an overly aggressive inflammatory response.Citation30
In four straightforward ways, the mechanism through which exosomes promote tendon-bone healing is initially articulated by regulating the polarization of macrophages and preventing inflammatory responses. This is followed by encouraging the expression of specific cytokines, aiding in reconstructing the tendon–bone interface cell phenotype gradient. Moreover, it increases the expression of factors involved in bone metabolism, promoting osteogenesis and reducing osteolysis. Lastly, there is the promotion of the angiogenesis process. Exosomes can inhibit the adhesion of the tendon to surrounding tissues, thus fostering healing in both the tendon and tendon–bone interface. In other instances, they also increase the expression of collagen fibers and fibrocartilage. In other situations, on the other hand, exosomes might overly encourage fibrosis, which would result in tendon adhesion and scarring. This could be connected to the kind of non-coding RNA of exosomes, their source, concentration, and acting time, which is an important area for future research.Citation31
The explanations above have explained the great benefits of exosomes, but exosomes themselves are very fragile in the external environment and have fragile biological activity. Therefore, special studies are needed to optimize the benefits of exosomes.
There were several limitations to this study. First, all six studies had no report on functional outcomes. Second, using two types of animals (rats and rabbits) with different kinds of MSC and different levels of CM used in our reviewed studies will cause different outcomes. According to earlier systematic evaluations, CM was nearly always linked to noticeably superior functional and biomechanical outcomes compared to the control groups.Citation17 In vitro investigations that found that the CM group had higher cell viability, proliferation, and migration rates corroborated these findings.
In a mouse model of muscle injury, exosomes released by BMSCs encourage myogenesis in vitro and muscle regeneration. Exosomes released during myoblast differentiation into myotubes can stimulate ADSC myogenesis and enhance myofiber regeneration at the site of damage. Exosomes can be secreted by a variety of cells, so before the study, the cell type should be considered.Citation32
The development of therapeutic technology based on exosomes has made the regenerative health industry grow. Promising pre-clinical results contributed to market share expansion. However, to protect the public from health risks and damage resulting from side effects from pre-clinical test results, a strict and locally implemented regulatory scheme and good communication are needed. It is imperative to educate the general public about the dangers of unproven therapies in order to increase awareness, dispel myths, and ultimately minimize patient harm. Public discussions, increased patient data sharing from regulatory agencies, and public awareness campaigns about the dangers of unproven secretome-based treatments should all be part of this.Citation33
Conclusion
This review is still limited to preclinical studies. Advances in exosome-based therapy are essential for better results. Although further studies are required to confirm the exosome’s benefit, the results of this study suggest that exosomes can be used as an alternative to MSC as cell-free therapy, especially for modulating the remodelling process in ligament or tendon healing.
Disclosure
Prof. Dr. Nicolaas Budhiparama reports personal fees from DePuy Johnson & Johnson, personal fees from Zimmer Biomet, outside the submitted work; and sits on the editorial board of BJJ, CORR, JISAKOS, KSSR, Arthroplasty, OJSM, and Journal of Orthopaedic Surgery. The authors report no other conflicts of interest in this work.
Acknowledgments
This study received funding from Padjadjaran University, which granted the award to Nur Atik.
References
- Leong NL, Kator JL, Clemens TL, James A, Enomoto-Iwamoto M, Jiang J. Tendon and ligament healing and current approaches to tendon and ligament regeneration. J Ortho Res. 2020;38(1):7–12. doi:10.1002/jor.24475
- Wu F, Nerlich M, Docheva D. Tendon injuries: basic science and new repair proposals. EFORT Open Rev. 2017;2(7):332–342. doi:10.1302/2058-5241.2.160075
- Dagget MC, Busch K, Ferretti A, Monaco E, Bruni G, Saithna A. Percutaneous anterior cruciate ligament repair with needle arthroscopy and biological augmentation. Arthrosc Techniq. 2021;10(2):e289–e295. doi:10.1016/j.eats.2020.10.006
- Wu XD, Kang L, Tian J, et al. Exosome derived from magnetically actuated bone mesenchymal stem cells promote tendon-bone healing through the miR-21-5p/SMAD7 pathway. Mater Today Bio. 2022;15:100319. doi:10.1016/j.mtbio.2022.100319
- Chamberlain CS, Clements AE, Kink JA, et al. Extracellular vesicle-educated macrophages promote early achilles tendon healing. Stem Cells. 2019;37:682. doi:10.1002/stem.2988
- Zhang M, Liu H, Cui Q, et al. Tendon stem cell-derived exosomes regulate inflammation and promote the high-quality healing of injured tendon. Stem Cell Res Ther. 2020;11(402):1–15. doi:10.1186/s13287-020-01918-x
- Batista JP, Chahla J, Dalmau-Pastor M, Maestu R, Kunze KN, Guelfi M. Arthroscopic anterior cruciate ligament repair with and without suture augmentation: technical note. J ISAKOS. 2021;6:251–256. doi:10.1136/jisakos-2020-000508
- Setiawati R, Utomo DN, Rantam FA, Ifran NN, Budhiparama NC. Early graft tunnel healing after anterior cruciate ligament reconstruction with intratunnel injection of bone marrow mesenchymal stem cells and vascular endothelial growth factor. Orthop J Sports Med. 2017;5(6):1–8. doi:10.1177/2325967117708548
- Shen H, Lane RA. Extracellular vesicle from inflammation-primed adipose-derived stem cells enhance Achilles tendon repair by reducing inflammation and promoting intrinsic healing. BioRxiv. 2023;15(6):617–627.
- Nwachukwu BU, Patel BH, Lu Y, Allen AA, Williams RJ. Anterior cruciate ligament repair outcomes: an update systematic review of recent literature. Arthros J. 2019;2019:3023208.
- Miao C, Zhou W, Wang X, Fang J. The research progress of exosomes in osteoarthritis, with particular emphasis on the mediating roles of miRNAs and Inc RNAs. Front Pharmacol. 2021;12:685623. doi:10.3389/fphar.2021.685623
- Isola A, Chen S. Exosomes: the messengers of health and disease. Curr Neuropharmacol. 2016;15(1):157–165. doi:10.2174/1570159X14666160825160421
- Malekpour K, Hazrati A, Zahar M, et al. The potential use of mesenchymal stem cells and their derived exosomes for orthopedic diseases treatment. Stem Cell Rep. 2022;18:933–951. doi:10.1007/s12015-021-10185-z
- Rezaie J, Feghhi M, Etemadi T. A review on exosomes application in clinical trials: perspective, questions and challenges. Cell Commun Signal. 2022;20(145):1–13.
- Tian J, chen W, Xiong Y, et al. Small extracellular vesicles derived from hypoxic preconditioned dental pulp stem cells ameliorate inflammatory osteolysis by modulating macrophage polarization and osteoclastogenesis. Bioact Mater. 2022;22:326–342. doi:10.1016/j.bioactmat.2022.10.001
- Sunwoo JY, Eliasberg CD, Carballo CB, Rodeo SA. The role of the macrophage in tendinopathy and tendon healing. J Orthop Res. 2020;38(8):1666–1675. doi:10.1002/jor.24667
- Rhatomy S, Prasetyo TE, Setyawan R, et al. Prospect of stem cells conditioned medium (secretome) in ligament and tendon healing: a systematic review. Stem Cells Transl Med. 2020;9:895–902. doi:10.1002/sctm.19-0388
- Hsueh YH, Buddakosai W, Le PN, et al. Therapeutic effect of induced pluripotent stem cell-derived extracellular vesicles in an vitro and in vivo osteoarthritis model. J Orthop Translat. 2022;38:141–155. doi:10.1016/j.jot.2022.10.004
- Shen H, Yoneda S, Abu-Amer Y, Guilak F, Gelberman RH. Stem cell-derived extracellular vesicles attenuate the early inflammatory response after tendon injury and repair. J Orthop Res. 2020;38(1):117–127. doi:10.1002/jor.24406
- Ye T, Chen Z, Zhang J, et al. Large extracellular vesicles secreted by human iPSC-derived MSCs ameliorate tendinopathy via regulating macrophage heterogeneity. Bioact Mater. 2022;21:194–208. doi:10.1016/j.bioactmat.2022.08.007
- Cui H, He Y, Chen S, Zhang D, Yu Y, Fan C. Macrophage-derived miRNA-containing exosomes induce peritendinous fibrosis after tendon injury through the miR-21-5p/Smad7 pathway. Mol Ther Nucleic Acids. 2019;14:115–130.
- Trzyna A, Banaś-Ząbczyk A. Adipose-derived stem cells secretome and its potential application in “stem cell-free therapy. Biomolecule. 2021;11:878. doi:10.3390/biom11060878
- Villatoro AJ, Del M, Martín-Astorga C, Alcoholado C, Del Mar Sánchez-Martín M, Becerra J. Proteomic analysis of the secretome and exosomes of feline adipose-derived mesenchymal stem cells. Animals. 2021;11:295. doi:10.3390/ani11020295
- Fu G, Lu L, Pan Z, Fan A, Yin F. Adipose-derived stem cell exosomes facilitate rotator cuff repair by mediating tendon-derived stem cells. Regenerative Med. 2021;16(4):359–372. doi:10.2217/rme-2021-0004
- Zhuang J, Hang R, Sun R, et al. Multifunctional exosomes derived from bone marrow stem cells for fulfilled osseointegration. Front Chem. 2022;2022:1.
- Wang Y, Yu D, Liu Z, et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther. 2017;8(189). doi:10.1186/s13287-017-0632-0
- Li R, Li D, Wang H, et al. Exosomes from adipose-derived stem cells regulate M1/M2 macrophage phenotypic polarization to promote bone healing via miR-451a/MIF. Stem Cell Res Ther. 2022;13(1). doi:10.1186/s13287-022-02975-0
- Li Z, Li Q, Tong K, et al. BMSC-derived exosomes promote tendon-bone healing after anterior cruciate ligament reconstruction by regulating M1/M2 macrophage polarization in rats. Stem Cell Res Ther. 2022;13:1.
- Al Halawani A, Mithieux SM, Yeo GC, Hosseini-Beheshti E, Weiss AS. Extracellular vesicles: interplay with the extracellular matrix and modulated cell responses. Int J Mol Sci. 2022;23:3389. doi:10.3390/ijms23063389
- Noonin C, Thongboonkerd V. Exosome-inflammasome crosstalk and their roles in inflammatory responses. Theranostics. 2021;11(9):4436–4451. doi:10.7150/thno.54004
- Zou M, Wang J, Shao Z. Therapeutic potential of exosomes in tendon and tendon-bone healing: a systematic review of preclinical studies. J Funct Biomater. 2023;14(6):299. doi:10.3390/jfb14060299
- Song K, Jiang T, Pan P, Yao Y, Jiang Q. Exosomes from tendon derived stem cells promote tendon repair through miR-144-3p-regulated tenocyte proliferation and migration. Stem Cell Res Ther. 2022;13(80):1–18. doi:10.1186/s13287-022-02723-4
- Asadpour A, Yahaya BH, Bicknell K, Cottrell GS, Widera D. Uncovering the gray zone: mapping the global landscape of direct-to-consumer businesses offering interventions based on secretomes, extracellular vesicles, and exosome. Stem Cell Res Ther. 2023;14(111):1–7.