Abstract
Cryopyrin-associated periodic syndromes (CAPS) are a subgroup of the hereditary periodic fever syndromes, which are rare autoinflammatory and inherited disorders, characterized by recurrent inflammation and varying degrees of severity. CAPS are thought to be driven by excessive production of interleukin-1β (IL-1β), through over-activation of the inflammasome by gain of function mutations in the gene encoding cryopyrin (NLRP3). This conclusion is supported by the remarkable efficacy of IL-1β blockade in these conditions. Rilonacept (ArcalystTM; Regeneron) is the first us Food and Drug Administration-approved treatment for familial cold autoinflammatory syndrome and Muckle–Wells syndrome and the first in a new line of drugs designed for longer-acting IL-1 blockade. Rilonacept has been associated with a decrease in disease activity, high-sensitivity C-reactive protein (hsCRP) and serum amyloid A (SAA) in the treatment of CAPS. The clinical safety and efficacy of rilonacept in CAPS and non-CAPS populations will be summarized in this review. Rilonacept is also beneficial for patients who tolerate injections poorly, due to an extended half-life over the unapproved CAPS treatment, anakinra, requiring weekly rather than daily self-administration. Other autoinflammatory disorders may also benefit from rilonacept treatment, with clinical trials in progress for systemic onset juvenile idiopathic arthritis, gout and familial mediterranean fever.
Introduction
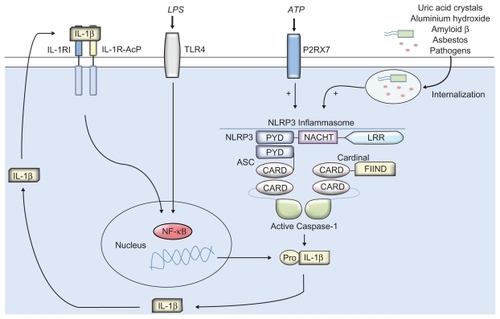
Cryopyrin-associated periodic syndromes (CAPS) are a group of rare autoinflammatory and inherited disorders with many symptoms in common, including recurrent attacks of systemic inflammation and fever, often the subject of misdiagnosis. They include familial cold autoinflammatory syndrome (FCAS), Muckle–Wells syndrome (MWS), and neonatal onset multi-system inflammatory disorder (NOMID) (also known as chronic infantile neurologic cutaneous and articular syndrome [CINCA]).Citation1–Citation4 CAPS are characterized by recurrent inflammation widely thought to be driven by an excessive production of interleukin-1 (IL-1) family member IL-1β, supported by patient response to IL-1β blocking therapies.Citation5–Citation8 IL-1β binds to the IL-1 receptor I (IL-1RI) and signal transduction is facilitated by the recruitment of IL-1R-accessory protein (IL-1RI-AcP) (). IL-1Ra is a specific receptor antagonist that is an endogenous control of IL-1 signalling. IL-1β has key roles in fever, hematopoiesis, appetite control, and bone metabolism and is known to be a key mediator of immune response,Citation9 able to regulate the production of several well documented proinflammatory mediators including cytokines, chemokines and matrix metalloproteinases.Citation10–Citation12 IL-1β has diverse signalling potential and as such is tightly controlled by several mechanisms. FCAS are MWS thought to arise from mis-sense mutations of the NLRP3 (CIAS1) gene encoding NLRP3 (cryopyrin), which can also be found in severe cases of CINCA.Citation13–Citation16 NLRP3 is a component of a multiprotein complex known as the inflammasome, which is an important regulator of immune response.Citation17,Citation18 The inflammasome is known to recognize and respond to a range of stimuli including pathogen-associated molecular patterns (PAMPs) like lipopolysaccharide from gram-negative bacteria and also endogenous stimuli termed damage-associated molecular patterns (DAMPs) such as uric acid.Citation19 NLRP3 inflammasome assembly leads to the activation of caspase-1, which in turn facilitates the maturation of IL-1β, IL-18 and IL-33 ().Citation14,Citation20 Traditional anti-inflammatory treatments for CAPS include the use of corticosteroids, disease-modifying antirheumatic drugs (DMARDS) and anti-tumor necrosis factor (anti-TNF) therapy. Anakinra was the first biologic designed for the selective blockade of IL-1 that was shown to have clinical efficacy in patients with MWS, when administered by daily subcutaneous injection.Citation8,Citation21 Response to IL-1 therapy supported the hypothesis of IL-1β dysregulation being a key mediator in CAPS. Anakinra is a human recombinant IL-1 receptor agonist (rhIL-1Ra), similar to endogenous IL-1Ra. Due to the initial success of anakinra and subsequent studies further supporting the clinical efficacy of IL-1 treatment for FCAS and MWS,Citation21–Citation23 progress has been made with the development of novel selective IL-1 biologics. One such biologic is canakinumab (ACZ885; Novartis Pharma), a fully humanized monoclonal antibody targeted against IL-1β with FDA approval for the treatment of FCAS and MWS. The drug has been able to show good tolerability and has the advantage of an extended half-life over anakinra, conferred by monoclonal therapy.Citation24 Rilonacept (ArcalystTM; Regeneron) pioneered this new generation of IL-1 targeted therapies in CAPS. The drug was the first FDA approved biologic for use in the treatment of FCAS and MWS after demonstrating remarkable clinical safety and efficacy.
Figure 1 The NLRP3 inflammasome is generally assembled upon activation by a range of pathogen-associated molecular patterns through pattern recognition receptors (PRRs) such as TLRs (eg, LPS activation of TLR4), or by interaction with components of internalized damage associated molecular pattern molecules (eg, uric acid crystals) leading to IL-1 cytokine maturation. Mutations to the NACHT domain of the NLRP3 subunit can result in spontaneous activation of the inflammasome and may lead to over-production and secretion of IL-1β, which is thought to be a causative factor in the clinical manifestations of CAPS.
Clinical manifestations of CAPS
Patients with FCAS and MWS have a significant symptom overlap and commonly include fatigue, fever, rash, inflammation of the skin, eyes, bones, joints, and meninges. Symptoms can have onset in infancy, with patients often experiencing episodes on a daily basis. There is a strong link to low temperature-induced episodes in FCAS patients, while the factors initiating symptoms in MWS are not as apparent they are thought to include cold, stress and exercise. Those with NOMID/CINCA are the most severely affected, owing to inflammation of the central nervous system. Symptoms include seizures, abnormal bone growth, ocular, visual and cognitive impairment as well as a range of musculoskeletal defects. It is estimated that the incidence of CAPS is one in a million worldwide, though symptoms have historically been misdiagnosed due to the physician unfamiliarity with these rare conditions. Patients suffer from recurrent and debilitating flares which have a dramatic impact on both personal and professional life.Citation25 Effective treatments have only just started to emerge and utilize biologics designed for IL-1 blockade. The biologic rilonacept offers significant clinical benefit for the treatment of FCAS and MWS, which will herein be the focus of this review.
Rilonacept – mode of action
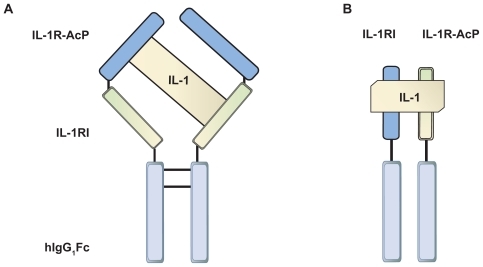
Rilonacept, a product of TRAP (Target-Related Affinity Profiling) technology developed by Regeneron pharmaceuticals, Citation26 is a dimeric fusion protein composed of the ligand binding domains of the extracellular portions of the human IL-1RI and the IL-1R-AcP linked to the human IgG1 Fc domain (). Rilonacept acts similar to the endogenous IL-1RA soluble decoy receptor by binding IL-1β but with 100 times greater affinity than native receptors. The drug is also able to bind IL-1RA and IL-1α though with lower affinity. Blockade of both IL-1α and IL-1β may be advantageous by further limiting IL-1 signalling potential. Rilonacept acts to sequester IL-1 and thereby prevents interaction with endogenous cell-surface receptors, resulting in a reduction of IL-1-mediated signal transduction events, which are known to induce inflammation.Citation26
Figure 2 A diagram to illustrate the structural differences of the endogenous IL-1 receptor complex and the designer protein rilonacept. A) Rilonacept – a dimeric protein comprising the IL-1RI chain and IL-1R-ACP chain, linked by fusion to Fc domains which confer an extended half-life. B) Endogenous membrane bound IL-1 receptor complex of IL1-RI and IL-1R-AcP. Both receptor chains are required for IL-1 binding to initiate signal transduction. Rilonacept is able to bind IL-1β with greater affinity.
Pharmacology
In adult patients aged 18 years or older, rilonacept should be administered with an initial loading dose of 320 mg, delivered by subcutaneous injection (SI) to two separate sites, followed by weekly injections of 160 mg. No dosage adjustments are required in adults. In pediatric patients from 12 to 17 years of age, an initial loading dose of rilonacept should be given at 4.4 mg/kg up to a maximum of 320 mg, followed by weekly injections of 2.2 mg/kg up to a maximum of 160 mg at one or two different sites. Rilonacept has a high therapeutic index and can be administered to a broad population. The dosage size does not affect the drugs terminal half-life, with only a relatively small effect on clearance within a broad dose range (50 μg/kg to 2000 mg). The terminal half-life in the pediatric patients (6.3 days) is slightly shorter than in the adult population (7.0 days); due to their similarity no adjustment in the dosing schedule is required. Individual studies of rilonacept found both the maximum concentration (Cmax) and area under the curve (AUC) increased proportionally with the dose administered, evaluated up to a maximum of 320 mg. The weight-adjusted Cmax was approximately 17 μg/mL in healthy patients with mean steady-state trough values, following weekly SI of 100 mg, increasing proportionally with the dose up to a maximum of 11.8 μg/mL. In CAPS patients, the mean steady-state trough levels of rilonacept ranged from 20 to 27 μg/mL, after weekly si of 160 mg for up to 48 weeks. Steady-state appeared to be reached by 6 weeks of treatment. The terminal phase elimination half-life was approximately 7 days, and was dose independent, with an absorption phase half-life of approximately 1.8 days, after SI. This figure was derived from the population pharmacokinetic estimate of the absorption rate constant (half-life = ln(2)/Ka).Citation27
Similar values were obtained for rilonacept in patients with end-stage renal disease (ESRD) on hemodialysis and CAPS patients. The terminal half-life for ESRD patients was 7.36 days compared to 8.86 days in CAPS. Clearance was also similar between the two populations 0.829 L/day (ESRD) and 0.808 (CAPS). Based on these results a dose adjustment was not required in ESRD patients as there was no evidence that renal failure slowed elimination of rilonacept. The detection of low titer anti-rilonacept antibodies in a number of patients during the course of treatment had no significant affect on pharmacokinetic parameters.Citation27
Though no study has been reported so far, limited clinical data suggest that rilonacept steady state is not affected by the patient’s sex, age (26 to 78 years) or body weight (50 to 120 kg). Since only Caucasian patients were enrolled into this study, the affect of ethnicity could not be evaluated.Citation28
Clinical studies in CAPS
Rilonacept was granted us Food and Drug Administration (FDA) approval and given ‘orphan drug’ status upon two sequential phase III clinical studies (supported by Regeneron), based on the favorable findings in an open-label pilot study to investigate the safety and efficacy of rilonacept in 5 patients with FCAS.Citation22 In the open-label pilot all patients following a baseline evaluation, received rilonacept at an initial loading dose of 300 mg by si given over 3 days. Efficacy and safety were evaluated at 6 and 10 days post treatment, patients remained off treatment unless a disease flare occurred. In this instance patients then received 300 mg of rilonacept and were maintained on 100 mg weekly doses. All patients entered the extended phase of the trial and were followed up for a period of 24 months. Four of the 5 patients who failed to go into remission, as determined by inflammatory markers (high-sensitivity CRP [hsCRP] <0.5 mg/dL and/or serum amyloid A [SAA] <10 mg/L and a daily diary score of <0.5, not achieved), were eligible for an escalated dosage in the extended phase with rilonacept administered at 160 mg per week and 320 mg per week if after 4 weeks they still failed remission criteria. All patients responded to treatment within several hours, showing signs of reduction in rash, fever and arthralgia. Significant reduction in erythrocyte sedimentation rate (ESR), hsCRP and SAA were achieved at 100 mg per week and further improvement achieved at higher dose administration (statistically significant for ESR [P < 0.05]) with no serious adverse affects. Rilonacept demonstrated a sustained suppression of IL-1β-induced inflammation and patients demonstrated good tolerability.
Rilonacept was further evaluated for safety and efficacy in two sequential phase III clinical studies (supported by Regeneron) in patients with CAPS.Citation29 Of the 47 adult patients enrolled 44 completed both studies. Each CAPS patient was found to have an NLRP3 mutation, and exhibited signs and symptoms of FCAS or MWS (44 with FCAS and 3 with MWS).
Study 1 was randomized, double-blinded and placebo controlled. Patients received an initial 320 mg dose of rilonacept followed by a weekly dose of 160 mg or placebo for 6 weeks by SI. In study 2, all subjects were given 160 mg rilonacept weekly for 9 weeks in the single-blind phase, followed by a 9 week randomized, double-blind withdrawal period in which patients were randomly assigned rilonacept at a dose of 160 mg per week or administered placebo. Efficacy was evaluated at 3-week intervals in the clinic and by using Daily Health Assessment Form (DHAF) to assess disease-related symptom severity to encompass feelings of fever/chills, joint pain, eye redness/pain and fatigue.
In study 1 of 44 patients, 24 received rilonacept and demonstrated a significant reduction in mean key symptom score in the first 24 hours of treatment compared with the 23 patients randomized to the placebo. Rilonacept was significantly better than the placebo with reduced multisymptom and single-symptom flares (P ≤ 0.0001 for each comparison) with a low mean key symptom score (P ≤ 0.0001). Improvement was also seen in patient and physician disease activity scores (P ≤ 0.0001 for each comparison) as well as decreased hsCRP (P ≤ 0.0001) and SAA (P = 0.006).
In study 2 – part A, patients randomized to rilonacept continued with treatment and maintained the benefits from the first part of the study. Patients previously on the placebo were switched to rilonacept and showed rapidly improvement in key symptoms, hsCRP and SAA. Part B, showed rilonacept to be significantly superior to the placebo in maintaining reduced multisymptom flares (P = 0.003), single symptom flares (P ≤ 0.001) and a low mean key symptom score (P = 0.0002).
Safety and tolerability
Treatment with rilonacept was well tolerated in the initial open-label pilot study of 5 FCAS patients, who all maintained 100% compliance with the treatment.Citation22 Adverse events were classified as mild or moderate, with no patient requiring discontinuation of rilonacept. The most common adverse event was respiratory tract infection that resolved over the course of the study. One patient with pre-existing basal cell carcinoma had 2 new lesions removed during the study and 1 patient with pre-existing oral ulcers indicated an increased frequency and prolonged healing of these ulcers while receiving rilonacept. Two patients had significant weight gain of 11.3 kg and 13.0 kg, respectively. No injection site reactions occurred during the course of the study. No serious adverse events occurred in these 5 patients and higher dosage levels did not correlate with an increased in adverse events.
Rilonacept was also well tolerated by patients enrolled in the subsequent phase III clinical trials.Citation28 In the phase III study 1, the most common adverse events were injection site reactions and upper respiratory tract infections. A total of 17 (74%) patients receiving rilonacept and 13 (54%) patients receiving the placebo had treatment related adverse events. The injection site reactions included erythema, swelling, pruritis, mass, bruising, inflammation, pain, edema, dermatitis, discomfort, urticaria, vesicles, warmth and hemorrhage. The next most commonly reported adverse event was upper respiratory infection. Most injection site reactions lasted for 1 to 2 days and none were assessed as severe. There were no serious adverse events reported during these studies. In study 1, infections reported by patients (treated in the winter months) administered with rilonacept compared to those on placebo were 48% and 17% respectively. In study 2 – part B, the randomized withdrawal phase (taking place in the summer months), patient reports of infection were similar between rilonacept (18%) and the placebo (22%). Rilonacept was evaluated in a range of patient populations, where infection incidence was also found to be similar with 34% of 360 patients treated with rilonacept and 27% of 179 patients treated with placebo.Citation27 The most frequent adverse events for patients treated with rilonacept in study 2 – Part B were injection site reaction, headache, arthralgia and diarrhea. In study 2 – part A, 2 patients discontinued treatment; in 1 patient this was due to pre-existing elevations in transaminase levels, later determined to be caused by hepatitis C viral infection and the another was due to worsening joint pain in two fingers. In study 2 – part B, 1 patient reported a serious adverse event presenting in worsening sciatica. All serious adverse events were judged by the investigator to be unrelated to rilonacept treatment. Where rilonacept was used in unapproved indications; one patient on chronic glucocorticoid treatment, developed a mycobacterium intracellulare infection and another 71-year-old patient acquired Streptococcus pneumoniae meningitis that resulted in death.Citation29
Further safety considerations
The open-label FCAS studyCitation22 found significant increases in red blood cell count and hemoglobin; neutrophils (−1.9 k/mm3) and platelets (67 k/mm3) may be decreased, however did not fall below reference range. CAPS patients 6 weeks after rilonacept treatment, showed an increase from baseline in mean total cholesterol (19 mg/dL), high-density lipoprotein cholesterol (2 mg/dL), low-density lipoprotein cholesterol (10 mg/dL) and triglycerides (57 mg/dL). Patients may be at increased risk of developing cardiovascular complications. CAPS patients in the open label extension who had received rilonacept for at least 6 weeks found 19 of 55 patients (35%) tested positive for anti-rilonacept antibodies, using high-sensitivity ELISA detection, on at least one occasion. Efficacy and safety appeared unaffected and their clinical significance is unclear. The upper limit for the dosage of rilonacept has not been established; intravenous infusion at doses up to 2000 mg monthly has been tolerated in a non-CAPS population for up to 6 months. One patient developed transient neutropenia (ANC < 1 × 109/L), but did not develop an associated infection.Citation28
Other considerations
Due to the immunosuppressive nature of rilonacept in sequestering IL-1α and IL-1β and concomitant signaling affects, there are a number of risk factors that have not been through rigorous study. No data concerning vaccination while on rilonacept are available and it is highly recommended for all vaccinations to be taken before treatment commences. No studies have evaluated safety in pregnant women and it is advised rilonacept should not be taken unless there are significant benefits out weighting the potential risk involved to the fetus. Cytochrome-C p450 (CYP450) levels may increase during the course of rilonacept treatment stemming from the finding that during chronic inflammation, CYP450 levels can be suppressed by high cytokine levels. These patients may be receiving modifying drugs such as warfarin, which has a narrow therapeutic index and these patients may be at increased risk of adverse events. Patients who have or develop infections, chronic infections, who are immunocompromised, those on anti-TNF- or other IL-1-blocking therapies or become hypersensitive should not take rilonacept for risk of developing severe adverse events.Citation28
Rilonacept in non-CAPS populations
Rilonacept has potential for the treatment of a number of other autoinflammatory disorders including familial Mediterranean fever (FMF),Citation30 TNF-associated periodic syndromes (TRAPS),Citation31 diabetes,Citation32 atherosclerosis,Citation33 chronic gout,Citation34 and systemic onset juvenile idiopathic arthritis (SoJIA).Citation35 Regeneron, among other pharmaceutical companies, are currently conducting clinical studies in disorders where IL-1 may play an important role (see ). The rilonacept trials where data are already available are described.
Table 1 A summary of new clinical trials evaluating rilonacept in other IL-1 related disorders as well as CAPSCitation46
Gout
Patients with gout are largely treated with nonsteroidal anti-inflammatory drugs (NSAIDs) or colchicine, although corticosteroids may also be administered where these treatments cannot be used. Urate lowering therapies, such as allopurinol, are also used in combination with prophylactic treatment to reduce flare incidence and severity.Citation36 While traditional treatments remain suboptimal, IL-1 targeted treatment has demonstrated potential. A nonrandomized, single-blind controlled pilot study was carried out to evaluate the benefit of rilonacept in chronic gouty arthritis.Citation34 All 10 patients enrolled in the study had a history of chronic gouty arthritis with inflamed joints for 4 or more weeks prior to enrolment. Placebo was given for 2 weeks before an initial loading dose of 320 mg of rilonacept was administered by subcutaneous injection. This was subsequently followed by 5-weekly injections of 160 mg of rilonacept, before patients entered a 6-week withdrawal phase. Clinical responses were evaluated by 10 point/21 increment visual analogue scale (VAS), patient and physician global assessment, joint count and hsCRP levels. Sixty percent (6 of 10) of patients reported at least a 50% improvement in self-reported VAS pain scores (P = 0.001) while 50% (5 of 10) reported at least 75% improvement (P ≤ 0.01). These improvements were not sustained in the withdrawal period. hsCRP levels fell from 0.4 mg/dL (following placebo) to 0.02 mg/dL after rilonacept treatment (P = 0.004). Treatment was well tolerated, with the most common adverse event being injection site reaction. One 50-year-old patient withdrew from treatment due to severe injection site erythema and induration, both of these adverse events were judged by the investigator to be related to study treatment. Three patients were found to have anti-rilonacept antibodies (non-neutralizing), though as efficacy and safety were apparently unaffected their clinical significance is unclear. Regeneron supported a double-blind, placebo-controlled phase II clinical study of 83 gout patients that found an 81% reduction in the number of symptom flares per patient over 12 weeks of rilonacept treatment as compared to placebo (P = 0.0011).Citation37
Systemic onset juvenile idiopathic arthritis (SoJIA)
SoJIA patients are often reliant on chronic corticosteroid treatment as the use of NSAIDs, methotrexate and anti-TNF treatment (eg, etanercept) can often prove ineffective. The risks associated with prolonged steroidal treatment include cataracts, growth retardation and vertebrae compression factures. Citation38 Cytokine blockade in SoJIA has shown potential for disease treatment by clinical and in vitro studies.Citation39,Citation40 An initial 4-week double-blind, placebo controlled study evaluated 21 active SoJIA patients (aged 5 to 20) administered with rilonacept by SI of 2.2 mg/kg/week (up to 160 mg) to 4.4 mg/kg/week (up to 320 mg). After 4 weeks American College of Rheumatology criteria (ACR) Pedi 30/50/70 were 76.2%/61.9%/33.3% respectively.Citation41 The subsequent open-label extension study of 23 SoJIA patients, evaluated the safety and efficacy of rilonacept over 24 months with rilonacept given by SI at 2.2 mg/kg/week (up to 160 mg) to 4.4 mg/kg/week (up to 320 mg). At 6 months during treatment ACR pedi 30/50/70 responses were seen in 87%/78%/61% of patients and 70%/70%/57% at 24 months. Sustained response was demonstrated in more than 50% of SoJIA patients over 24 months in both clinical and laboratory assessments, with all patients able to stop steroidal treatment by 24 months. Six patients reported serious adverse events, including macrophage activation syndrome, pulmonary fibrosis, anemia and arthritis flare, which are unlikely to be as a result of rilonacept treatment. The most common adverse events were injection site reaction and upper respiratory infection.Citation42 Differential cytokine blockade in SoJIA may be advantageous in tailoring treatment where current therapies have failed.
Managed treatment
Financial cost is often a key barrier associated with the use of biologics in disease treatment. Patients requiring life long treatment, can often be at significant financial disadvantage, an implication shown with anti-TNF biologics therapy.Citation43 A study of 36 patients prescribed anti-TNF biologics for the treatment of autoinflammatory disorders, found a correlation between the discontinuation of treatment and financial burden. Regular follow-ups over the course of 3 years found 52% of patients (19 of 36) had discontinued treatment due to financial reasons while 8% of patients (3 of 36) continued but treatment was suboptimal with spaced out regimes.Citation44 This study is representative of a global issue. Anakinra, an off label treatment with clinical efficacy in CAPS, is associated with an approximate monthly cost (based on average wholesale price) of US$1515 and rilonacept, the FDA approved treatment for CAPS, has an approximate average monthly cost of US$30,000.Citation45 Regeneron have taken measures to help manage the cost of rilonacept therapy and have set up the Arcalyst Resource Centre (ARC) program to support insured patients with reimbursement, putting physicians familiar with diagnosing and treatment of CAPS in contact with patient’s insurance providers to determine coverage, obtain and assist with prior authorization requests and appeals. They can refer patients to foundations that may assist with high co-payments, while uninsured patients may be eligible for Regeneron’s assistance program that can offer the drug free of charge to qualifying patients or for non-qualifying patients, help with finding alternative coverage.Citation45
Conclusions
IL-1β is intimately involved in the pathogenesis of CAPS. Many CAPS patients have mutations of the NALP3 gene with in vitro experiments demonstrating that mutation in this gene can lead to excessive IL-1β production through the activity of the NALP3 inflammasome. This was strongly supported by the remarkable success of the IL-1 targeted biologic anakinra. Since this initial success, new biologics designed for IL-1 blockade have been designed, developed and granted FDA approval for the treatment of CAPS. Rilonacept pioneered this new generation of CAPS treatment offering significant clinical advantages over the off-label treatment anakinra. The long term implications of treatment are unclear though the benefits may out way the risk for many. Due to the low incidence of CAPS the cost of approved treatment is presently higher than most other biologics, though a number of comprehensive programs are in place to help patient’s access treatment. The longer half-lives of the new biologics rilonacept and canakinumab, offer CAPS patients an alternative to past treatments and those who find daily injection intolerable or have trouble with compliance. The potential of selective IL-1 blockade may see rilonacept augmenting existing treatments for other autoinflammatory disorders.
Disclosures
The authors declare no conflicts of interest.
References
- NevenBCryopyrinopathies: update on pathogenesis and treatmentNat Clin Pract Rheumatol20084948148918665151
- HoffmanHMHereditary immunologic disorders caused by pyrin and cryopyrinCurr Allergy Asthma Rep20077532333017697637
- PrieurAMA chronic, infantile, neurological, cutaneous and articular (CINCA) syndrome. A specific entity analysed in 30 patientsScand J Rheumatol Suppl19876657683482735
- HoffmanHMFamilial cold autoinflammatory syndrome: phenotype and genotype of an autosomal dominant periodic feverJ Allergy Clin Immunol2001108461562011590390
- HoffmanHMMutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndromeNat Genet200129330130511687797
- HoffmanHMPrevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonistLancet200436494471779178515541451
- ThorntonBDSuccessful treatment of renal amyloidosis due to familial cold autoinflammatory syndrome using an interleukin 1 receptor antagonistAm J Kidney Dis200749347748117336710
- HawkinsPNInterleukin-1-receptor antagonist in the Muckle- Wells syndromeN Engl J Med2003348252583258412815153
- DinarelloCAImmunological and inflammatory functions of the interleukin-1 familyAnnu Rev Immunol20092751955019302047
- Kogan-SakinIProstate stromal cells produce CXCL-1, CXCL-2, CXCL-3 and IL-8 in response to epithelia-secreted IL-1Carcinogenesis200930469870519233959
- MaFYBlockade of the c-Jun amino terminal kinase prevents crescent formation and halts established anti-GBM glomerulonephritis in the ratLab Invest200989447048419188913
- TsuzakiMIL-1 beta induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cellsJ Orthop Res200321225626412568957
- AksentijevichIDe novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseasesArthritis Rheum200246123340334812483741
- ChurchLDPrimer: inflammasomes and interleukin 1beta in inflammatory disordersNat Clin Pract Rheumatol200841344218172447
- ArosteguiJIClinical and genetic heterogeneity among Spanish patients with recurrent autoinflammatory syndromes associated with the CIAS1/PYPAF1/NALP3 geneArthritis Rheum200450124045405015593220
- AgannaEAssociation of mutations in the NALP3/CIAS1/ PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and AA amyloidosisArthritis Rheum20024692445245212355493
- MartinonFThe inflammasomes: guardians of the bodyAnnu Rev Immunol20092722926519302040
- MuruveDAThe inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune responseNature2008452718310310718288107
- PetrilliVThe inflammasome: a danger sensing complex triggering innate immunityCurr Opin Immunol200719661562217977705
- ArendWPIL-1, IL-18, and IL-33 families of cytokinesImmunol Rev2008223203818613828
- RossJBUse of anakinra (Kineret) in the treatment of familial cold autoinflammatory syndrome with a 16-month follow-upJ Cutan Med Surg200812181618258152
- Goldbach-ManskyRA pilot study to evaluate the safety and efficacy of the long-acting interleukin-1 inhibitor rilonacept (Interleukin-1 Trap) in patients with familial cold autoinflammatory syndromeArthritis Rheum20085882432244218668591
- LachmannHJUse of canakinumab in the cryopyrin-associated periodic syndromeN Engl J Med2009360232416242519494217
- SavicSMcDermottMFInflammation: canakinumab for the cryopyrin-associated periodic syndromesNat Rev Rheumatol200951052953019798026
- StychBDobrovolnyDFamilial cold auto-inflammatory syndrome (FCAS): characterization of symptomatology and impact on patients’ livesCurr Med Res Opin20082461577158218423104
- EconomidesANCytokine traps: multi-component, high-affinity blockers of cytokine actionNat Med200391475212483208
- HoffmanHMRilonacept for the treatment of cryopyrin-associated periodic syndromes (CAPS)Expert Opin Biol Ther20099451953119344287
- Arcalyst (rilonacept) [prescribing information]Tarrytown, NYRegeneron2008
- HoffmanHMEfficacy and safety of rilonacept (Interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studiesArthritis Rheum20085882443245218668535
- RoldanRAnakinra: new therapeutic approach in children with Familial Mediterranean Fever resistant to colchicineJoint Bone Spine200875450450518541452
- SacreKDramatic improvement following interleukin 1beta blockade in tumor necrosis factor receptor-1-associated syndrome (TRAPS) resistant to anti-TNF-alpha therapyJ Rheumatol200835235735818260167
- PickersgillLMMandrup-PoulsenTRThe anti-interleukin-1 in type 1 diabetes action trial – background and rationaleDiabetes Metab Res Rev200925432132419405081
- ApostolakisSIL-1 cytokines in cardiovascular disease: diagnostic, prognostic and therapeutic implicationsCardiovasc Hematol Agents Med Chem20086215015818473780
- TerkeltaubRThe interleukin 1 inhibitor rilonacept in treatment of chronic gouty arthritis: results of a placebo-controlled, monosequence crossover, non-randomised, single-blind pilot studyAnn Rheum Dis200968101613161719635719
- LequerreTInterleukin-1 receptor antagonist (anakinra) treatment in patients with systemic-onset juvenile idiopathic arthritis or adult onset Still disease: preliminary experience in FranceAnn Rheum Dis200867330230817947302
- RiderTGJordanKMThe modern management of goutRheumatology (Oxford)2009
- Rilonacept [IL-1 Trap, Arcalyst; Regeneron Pharmaceuticals] has reduced the incidence of gout flares in a phase II studyInpharma200811655991
- HainesKAJuvenile idiopathic arthritis: therapies in the 21st centuryBull NYU Hosp Jt Dis200765320521117922671
- YokotaSTherapeutic efficacy of humanized recombinant antiinterleukin- 6 receptor antibody in children with systemic-onset juvenile idiopathic arthritisArthritis Rheum200552381882515751095
- PascualVRole of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockadeJ Exp Med200520191479148615851489
- LovellDJPreliminary evidence for sustained bioactivity of IL-1 Trap (rilonacept), a long acting IL-1 inhibitor, in systemic juvenile idiopathic arthritis (SJIA)Program and abstracts of the American College of Rheumatology (ACR) 71st Annual MeetingNovember 6–11 2007Boston, MassachusettsAbstract 1282
- LovellDJLong-term safety and efficacy of rilonacept in patients with systemic juvenile idiopathic arthritisProgram and abstracts of the American College of Rheumatology (ACR) Annual MeetingOctober 17–21 2009Philadelphia Presentation 2053
- FeldmannMAnti-TNF therapy: where have we got to in 2005?J Autoimmun200525Suppl262816260118
- EleishiHHThree years experience with the prescription of anti-TNF-alpha inhibitors at Dr Soliman Fakeeh hospital in Jeddah, Saudi ArabiaInt J Rheum Dis200912141920374311
- KapurSBonkMERilonacept (Arcalyst), an interleukin-1 Trap for the treatment of cryopyrin-associated periodic syndromesP T200934313814119561849
- Registered clinical studies in the United States of America and global locations URL: http://clinicaltrials.gov/ct2/results?term=rilonaceptAccessed October 12, 2009
