Abstract
The high morbidity, mortality, and health care costs associated with invasive fungal infections, especially in the critical care setting and immunocompromised host, have made it an excellent target for prophylactic, empiric, and preemptive therapy interventions principally based on early identification of risk factors. Early diagnosis and treatment are associated with a better prognosis. In the last years there have been important developments in antifungal pharmacotherapy. An approach to the new diagnosis tools in the clinical mycology laboratory and an analysis of the use new antifungal agents and its application in different clinical situations has been made. Furthermore, an attempt of developing a state of the art in each clinical scenario (critically ill, hematological, and solid organ transplant patients) has been performed, trying to choose the best strategy for each clinical situation (prophylaxis, pre-emptive, empirical, or targeted therapy). The high mortality rates in these settings make mandatory the application of early de-escalation therapy in critically ill patients with fungal infection. In addition, the possibility of antifungal combination therapy might be considered in solid organ transplant and hematological patients.
Introduction
Hospital medicine has advanced greatly in the past few decades. Patients with complex medical and surgical disorders are surviving longer due to equally complex medical and surgical interventions, which often involve “collateral damage” by avoiding normal body defensive mechanisms.
Invasive fungal infections (IFIs) in adult patients, especially in the critical care setting, solid organ transplant (SOT), and hematological patients, have become an excellent target for prophylactic, empiric, and pre-emptive therapy interventions due to their increasing incidence, high morbidity and mortality rates, and associated health care costs. Early diagnosis and treatment are associated with a better prognosis. Although at present, the number of systemic antifungal agents has increased significantly, the choice of antifungal drug must be based on the individual characteristics of the patient, clinical scenario, and the presence of hemodynamic instability. A tailored therapy (de-escalation) must also be considered in some clinical situations.
Different possible strategies based on diagnoses stage has been described as prophylactic, empiric, pre-emptive and targeted antifungal therapy (). Prophylactic treatment refers to the preventive administration of an antifungal agent to patients at risk of IFI without attributable signs and symptoms. Empiric treatment is defined as the initiation of antifungal treatment in patients at high risk of IFIs and established clinical signs and symptoms, but without microbiological documentation, whereas preemptive therapy is applied when the decision of treatment is based on early diagnostic test. Finally, targeted therapy needs a pathogen identification to be defined.
Figure 1 Different antifungal strategies for treatment in invasive fungal infections based on diagnostic stage.
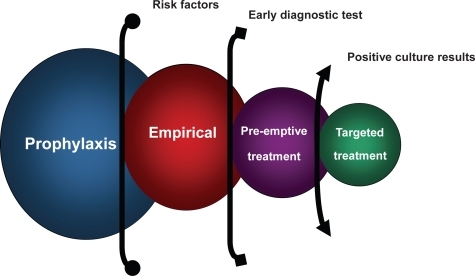
The aim of this multidisciplinary review was to analyze the best option for treating this special population of adults patients, describing the antifungal armamentarium, making an approach to the mycology laboratory diagnosis and finally developing a state of the art clinical scenario (critically ill, hematological and transplant patients) trying to choose the best strategy for each clinical situation (prophylaxis, empirical, pre-emptive, or targeted therapy).
Role of the mycology laboratory in the antifungal therapy options
Microbiological diagnosis for preventive treatment
The incidence of IFIs has steadily increased in the past two decades. These infections represent an additional difficulty in the management of immunocompromised patients and are a contributory cause of death in allogeneic hematopoietic stem cell and SOT recipients and in patients undergoing intensive chemotherapy (CitationCordonnier et al 2006).
The high mortality associated with IFIs is partly correlated to the difficulties of making an early diagnosis due to the nonspecific clinical features and the low sensitivity of microscopy, histological examination, conventional radiology and cultures of specimens obtained from at-risk patients (CitationHope et al 2005). However, improved survival can be obtained by earlier initiation of antifungal therapy. The time period between the biological onset of a fungal infection and the appearance of clinical signs and symptoms represents a window of opportunity that, if identified through prospective screening, may allow for pre-emptive therapeutic intervention. In this diagnostic area, progress could come from prospective screening strategies using new serodiagnostic assays (galactomannan and (1–3)-β-d-glucan) and/or polymerase chain reaction (PCR) techniques for the detection of fungal-specific DNA.
Thus, to improve earlier diagnosis and survival of IFIs, new nonculture-based microbiological tools should be used in conjunction with modern imaging techniques in addition to conventional microbiological, histological, and radiological procedures.
Nonculture-based microbiological tools
With the aim of improving the rapid and early diagnosis of IFIs, new microbiological nonculture-based assays have been developed in the last years, including detection of fungal galactomannan, (1–3)-β-d-glucan antigen, Candida albicans germ tube antibodies and fungal DNA.
Galactomannan detection
Galactomannan (GM) is a polysaccharide cell wall component released by the Aspergillus species during hyphal growth. The detection of galactomannan by sandwich-enzyme immunoassay (EIA), Platelia Aspergillus (Bio-Rad Laboratories, Marnes-La-Coquette, France), has been approved in Europe and the USA for use in hematopoietic stem cell transplant (HSCT) recipients. Furthermore, its detection in body fluids (mainly serum and bronchoalveolar lavage) was included in the 2002 EORTC-MSG consensus definitions of invasive aspergillosis (IA) as a microbiological criterion having the same weight as the isolation of Aspergillus spp. or the microscopic demonstration of hyphae from a nonsterile body site (CitationAscioglu et al 2002).
Using EIA, circulating galactomannan may be detected at a median of 5–8 days (range 1–27 days) before clinical signs and symptoms of IA become evident. Furthermore, the concentration of circulating galactomannan corresponds with the fungal tissue burden and may therefore be used to monitor the patient’s response to antifungal treatment (CitationMaertens et al 2001).
The results of the unique meta-analysis published of the diagnostic value of GM detection with the Platelia Aspergillus® assay have shown that for proven and probable cases of IA, the pooled (adults and pediatrics) sensitivity was only 61% whereas the overall specificity was 93%. In general, the negative predictive value and the specificity are excellent (>95%), suggesting that the assay can be used to rule out the diagnosis of IA (CitationPfeiffer et al 2006). False-positive results have been reported in adults (5.7%–14%), but among pediatric patients and neonates these are notably higher (83%) (CitationSulahian et al 2003). Reasons for false reactivity remain largely unknown, although piperacillin – tazobactam could be a cause of cross-reactivity in adults (CitationSulahian et al 2003), and cross-reacting epitopes from Bifidobacterium spp. were proposed as a cause in neonates (CitationMennink-Kersten et al 2004). In summary, the GM assay has moderate accuracy for the diagnosis of IA in immunocompromised patients. The test is more useful in patients who have hematological malignancies or who have undergone hematopoietic cell transplantation than in solid-organ transplant recipients. Nevertheless, some important issues in relation to the performance of this assay still remain unanswered, such as the impact of antifungal prophylaxis, the influence of therapy with fungal cell wall inhibitors, or the handling of false-positive and false-negative values (CitationMaertens et al 2006).
Recenty, a very interesting study has evaluated GM detection in BAL fluid in critically ill patients with signs of pneumonia. Patients were classified as having proven, probable, or possible IA. Using a cutoff index of 0.5, the sensitivity and specificity of GM detection in BAL fluid was 88% and 87%, respectively. The sensitivity of serum GM was only 42%. In 11 of 26 proven cases of IA, BAL culture and serum GM remained negative, whereas GM in BAL was positive. Following these rresults GM detection in BAL fluid could be useful in establishing or excluding the diagnosis of IA in intensive care units (ICU) (CitationMeersseman et al 2008).
Detection of (1,3)-β-d-glucan
Glucans are a cell wall component of most pathogenic fungi except Zygomycetes and Cryptococcus spp. (1–3)-β-d-glucan can be detected in serum in amounts as low as 1 pg/ml by commercial assays. One of them, Fungitell (Associates of Cape Cod Inc., East Falmout, MA), has been approved by the FDA as an adjunct for the diagnosis of IFIs in the USA, based on its evaluation in hematological patients (CitationOdabasi et al 2004). At a cut-off of 60 pg/ml, the negative predictive value of twice-weekly sampling was 100%, and sensitivity was 100% if one positive assay was considered a positive result. Furthermore, the results were not influenced by the use of prophylactic or empirical antifungals. (1,3)-β-d-glucan is a broad spectrum fungal marker and can detect invasive infections due to Aspergillus, Candida, Fusarium, Acremonium, Scedosporium, Pneumocystis jiroveci, etc., but, after a positive result, the invasive infection must be assessed using radiological and microbiological techniques. To date, overall experience with this test remains limited; furthermore, its methodological concerns (use of endotoxin-free and glucan-free glassware) and false-positive results (due to albumin, immunoglobulins, glucan-containing gauze, hemodialysis or Gram-positive bacteremia) make its use difficult in a clinical setting. However, based on the excellent negative predictive value, detection of (1–3)-β-d-glucan seems to be most useful for excluding IFIs (CitationPickering et al 2005).
Multicenter clinical trial results have demonstrated that (1,3)-β-d-glucan assay can be used in clinical specimens with a high specificity and positive predictive value for subjects with proven or probable IFI when compared with control subjects (CitationOstrosky-Zeichner et al 2005). This test appears to be useful both as a single-point assay for hospitalized patients with a possible fungal infection and as part of a surveillance strategy in high-risk patients. A cut-off value of 60 or 80 pg/mL appears to be optimal for this test. At a cutoff of 60 pg/mL, the sensitivity and specificity of the assay were 69.9% and 87.1%, respectively, with a positive predictive value (PPV) of 83.8% and a negative predictive value (NPV) of 75.1%. At a cutoff value of 80 pg/mL, the sensitivity and specificity were 64.4% and 92.4%, respectively, with a PPV of 89% and an NPV of 73%.
Detection of fungal DNA
In recent years novel molecular methods, notably the amplification of gene sequences unique to fungi by polymerase chain reaction (PCR) assays, have been developed to improve the diagnosis of life-threatening IFIs in high-risk patients. PCR offers the potential for rapid diagnosis. However, due to the absence of a standardized and validated commercial method, the routine use of PCR in the diagnosis of IFI cannot yet be recommended. Real-time techniques combined with automated DNA extraction may, however, allow standardization and reproducibility between centers, and may broaden the clinical applicability of PCR-based diagnosis in the near future (CitationMaertens et al 2006).
Antibodies against Candida albicans germ tubes
A C. albicans mannoprotein located on the germ tube cell wall surface is recognized by sera from patients with invasive candidiasis (IC). Recently, an indirect immunofluorescence assay to detect antibodies (CAGTA) against this antigen has been developed and commercialized (C. albicans IFA IgG; Vircell Laboratories, Spain) (CitationMoragues et al 2004; CitationPonton et al 1994). The test has shown an overall sensitivity of 77%–89% and a specificity of 91%–100% and has been useful in the diagnosis of IC in intravenous heroin users, bone marrow transplant recipients and hematological or intensive care patients (CitationQuindos et al 2004). Detection of CAGTA in patients with invasive infections caused by Candida spp. other than C. albicans (C. tropicalis, C. parapsilosis, C. glabrata, C. dubliniensis, C. guilliermondii, and C. krusei) may also be positive, although titers are lower than for candidiasis caused by C. albicans. In addition, the detection of CAGTA may be useful for the therapeutic monitoring of patients with IC, since the administration of antifungal therapy usually results in decreasing titers of CAGTA (CitationMoragues et al 2004).
In 2006, our group evaluated an immunofluorescence assay for CAGTA detection in a selected population of critically ill patients (CitationZaragoza et al 2006). Although there were no differences between CAGTA-positive and -negative patients in terms of age, gender, sequential organ failure assessment score, and renal and hepatic failure, the intra-ICU mortality rate was significantly lower in patients who tested positive for CAGTA (25% vs 65.2%; P = 0.025). These results imply that a strategy based on the early determination of CAGTA expression might reduce the ICU mortality rate of patients with risk factors for the development of IC. However, more studies are needed to validate this approach in the critical care setting.
Combinations of nonculture-based microbiological tools
Recent studies focusing on the combination of nonculture-based microbiological tools have demonstrated improved diagnostic accuracy when combining galactomannan and (1–3)-β-d-glucan detection (CitationPazos et al 2005), as well as galactomannan and PCR (CitationMillon et al 2005; CitationFlorent et al 2006). Additionally, its usefulness for diagnosing and monitoring IC using (1–3)-β-d-glucan and CAGTA was evaluated in neutropenic adults at high risk. Both tests anticipated the clinical and radiological diagnosis, and the initiation of antifungal therapy in most patients. A combination of both tests improved specificity and positive predictive value to 100% (CitationPazos et al 2006). These studies suggest that a combination of two tests to detect antigen, antibodies, (1–3)-β-d-glucan and DNA will be needed to optimize the diagnosis of systemic fungal infections (CitationPonton and del Palacio 2007).
Culture-based microbiological tools
Diverse risk factors for IC, including prior Candida spp. colonization, could allow the detection of patients that may be potential candidates for preemptive antifungal therapy. Numerous patients are colonized in the ICU but only few subsequently develop systemic candidiasis. Screening for Candida colonization assessment is performed routinely in many ICUs. Nevertheless, the value of positive surveillance cultures and of several developed colonization indexes for the prediction of IC and the indication for preemptive antifungal therapy is currently under active investigation (CitationPiarroux et al 2004). Recently, a simple scoring system (“Candida score”) has been evaluated to assist clinicians in differentiating between Candida species colonization and proven Candida infection in nonneutropenic critically ill patients (CitationLeon et al 2006).
In conclusion, although substantial progress has been made in the diagnosis of IFIs, no single test has found widespread clinical use. There is a consensus in publications that results obtained from a panel of diagnostic tests in association with blood culture findings and clinical aspects of the patient will likely be the most useful strategy for the early diagnosis of patients with IFIs and the monitoring of therapeutic response.
The laboratory and the empirical antifungal therapy
In patients at high risk of fungal infection, the administration of empirical antifungal treatment must be considered since a relationship between the delay in the initiation of treatment and in clinical outcome and hospital mortality has been demonstrated (CitationMorrell et al 2005; CitationGarey et al 2006). Empirical antifungal therapy is defined as the treatment administered to patients who have several risk factors and clinical features for IFIs, when microbiological documentation, species identification or susceptibility data are still not available.
When this treatment is to be established, several factors must be taken into account, such as: 1) the hospital area epidemiology; 2) previous susceptibility data of species isolated in the hospital area; 3) the multicenter surveillance studies to predict the susceptibility patterns of isolates; 4) the potential risk of emergence of fluconazole resistance or appearance of candidiasis due to fluconazole-resistant species among patients receiving fluconazole prophylaxis (CitationAgresti et al 1994); 5) the presence of neutropenia; 6) the underlying patient conditions which can affect the metabolism of the drug; 7) the toxicity of the antifungal agent; and 8) previous experience in fungal treatment. Furthermore, it must be kept in mind that any individual isolate of any species may become resistant to any antifungal agent.
summarizes the in vitro susceptibility patterns of yeasts and moulds against the most frequently isolated species. These data are representative of those published in numerous in vitro studies.
Table 1 Usual susceptibility patterns for yeasts and moulds
In summary, as epidemiological data and resistance to antifungal agents depends on characteristics of patients and geographical localization, it is convenient, in all invasive mycoses, to perform both the identification of all isolates at species level and the antifungal susceptibility tests to identify the local epidemiology so as to apply the most appropriate empirical therapy in each institution.
Prophylaxis, empirical, pre-emptive or targeted therapy, which is the best in critically ill patients?
Approximately 10.4% of infections in an ICU are related to Candida spp. with the majority being nosocomial (CitationAlberti et al 2002). However, this rate could be underestimated due to the fact that at least 4% of critically ill patients who die in an ICU present an unexpected fungal infection during postmortem examination (CitationDimopoulos et al 2004). Furthermore, ICU admission itself has become an independent risk factor for the development of a Candida spp. infection (CitationPuzniak et al 2004; CitationTortorano et al 2004).
Candida infections are associated with a significant mortality rate, particularly among critically ill patients (CitationLeleu et al 2002). The crude mortality rate of these infections has been estimated at 40%–75%, and the mortality rate attributable to candidemia at 25%–38% (CitationNolla-Salas et al 1997; CitationPetri et al 1997; CitationTortorano et al 2004; CitationAlmirante et al 2005). A review of matched cohort and case-control studies has examined the mortality rate that could be linked to candidemia (CitationFalagas et al 2006). The data suggested that candidemia is indeed associated with a considerable mortality rate that can be attributed to the infection itself.
In recent years, the species of Candida that result in candidemia have shifted from C. albicans to non-C. albicans (NCA). Approximately half of the reported cases of candidemia are now caused by NCA species (CitationPfaller et al 2000; CitationTortorano et al 2004; CitationAlmirante et al 2005), and several publications have indicated that these cases have a worse prognosis than those caused by C. albicans (CitationBen Abraham et al 2004; CitationKlingspor et al 2004; CitationMorgan et al 2005; CitationDimopoulos et al 2008). This increase has been attributed to the use of fluconazole prophylaxis (CitationSendid et al 2006). Other adverse outcome predictors described in candidemia episodes are the length of ICU stay, renal failure, thrombocytopenia, hematologic malignancy, and the need for mechanical ventilation or inotropic support (CitationBen Abraham et al 2004; CitationAlmirante et al 2005). In a Spanish multicenter study involving ICU patients in 28 hospitals, an Acute Physiology and Chronic Health Evaluation (APACHE) II score of >20 at the time of candidemia was associated with a higher mortality rate (CitationNolla-Salas et al 1997), whereas early treatment with antifungal medication and the removal of central venous catheters were protective against death (CitationNolla-Salas et al 1997; CitationAlmirante et al 2005). Furthermore, inadequate empiric antibiotic treatment it is associated with IFIs and a worse prognosis (CitationParkins et al 2007). Two reports have demonstrated a strong association between a delay in the start of antifungal therapy and an increase in hospital mortality rates (CitationMorrell et al 2005; CitationGarey et al 2006); thus, it is necessary to recognize that time is of the utmost importance when considering the therapy of patients who are at risk for IFIs.
Prophylaxis of Candida infections in ICU
The implementation of targeted antifungal prophylaxis has been shown to be effective in certain ICU settings (CitationCalandra and Marchetti 2002). Results from controlled randomized trials (CitationEggimann et al 1999; CitationPelz et al 2001; CitationGarbino et al 2002) support the efficacy of azole prophylaxis in nonneutropenic high-risk ICU patients, diminishing the incidence of Candida infection but not mortality.
Three recently published meta-analysis have tried to evaluate the impact of fluconazole prophylaxis on the incidence of fungal infections and on mortality among critically ill surgical patients (CitationCruciani et al 2005; CitationShorr et al 2005; CitationPlayford et al 2006). The meta-analysis by CitationShorr and colleagues (2005) demonstrated that prophylactic fluconazole administration in surgical ICU patients appears to successfully decrease the rate of mycoses, but this strategy does not improve survival. The second meta-analysis by CitationCruciani and colleagues (2005) showed that patients who received azole prophylaxis (fluconazole and ketoconazole) experienced a 80% relative risk reduction in candidemia, 31.5% relative risk reduction in overall mortality, and 79.4% reduction in mortality attributable to Candida infections. Finally, CitationPlayford and colleagues (2006) reported reduction of the IFI incidence rate by about 50% and overall mortality by about 25%.
Currently, because of the potential for both resistance and emergence of non-albicans isolates, clinicians must properly consider these issues when evaluating fluconazole prophylaxis in ICU, although the meta-analyses published by CitationPlayford and colleagues (2006) describes above showed no evidence of epidemiological shifts after fluconazole prophylaxis.
In addition, it has been criticized that all controlled randomized trials included into the metaanalyses by Playford and Cruciani and colleagues were limited by their monocentric design and each of them focused on a group of clinically distinctive patients.
Under these circumstances, prophylactic use of fluconazole in high risk ICU patients cannot be generally recommended, but should be restricted to patients with multiple risk factors for developing IC, for instance as Playford recommend, if the cumulative incidence of IC in a certain subpopulation of the ICU approaches or exceeds 10%, in spite of active prevention, prophylaxis should be initiated. Using this approach, the number needed to treat to prevent one case of IC ranges between 17 (for a risk of 11%) and nine patients (for a risk of 20%). The subgroups of patients who might most benefit from prophylaxis in ICU may be patients with upper gastrointestinal perforation (CitationPelz et al 2001; CitationGarbino et al 2002), patients with heavy Candida colonization (CitationPiarroux et al 2004), and patients with severe acute pancreatitis (CitationDe Waele et al 2003).
Empirical antifungal treatment in ICU
Assessing risk
The early identification of risk factors for the development of candidemia such as peritonitis, abdominal surgery, previous administration of broad-spectrum antibiotics, parenteral nutrition, multiple lumen catheters, prior Candida spp. colonization, renal replacement therapy, and mechanical ventilation (CitationPetri et al 1997; CitationBlumberg et al 2001; CitationAlvarez-Lerma et al 2003), has become the cornerstone of empiric treatment of fungal infections in the ICU setting in order to reduce the high mortality rate associated with these infections (CitationIbanez-Nolla et al 2004; CitationGarnacho-Montero et al 2005).
The Ostrosky-Zeichner prediction rule
In a multicenter retrospective setting, CitationOstrosky-Zeichner and colleagues (2007) created a prediction rule for IC. The rule was obtained through analysis of a group of 2890 patients, in which incidence of IC was 3% (88 cases). Statistical modeling revealed a particularly high risk for patients under systemic antibiotic treatment (days 1–3) or with indwelling central venous catheter (days 1–3) and at least two of the following factors: total parenteral nutrition (days 1–3), any dialysis (days 1–3), any major surgery (days –7–0), pancreatitis (days –7–0), any use of steroids (days –7–3), or use of other immunosuppressive agents (days –7–0). The rule was associated with a sensitivity of 34%, a specificity of 90%, and a PPV and a NPV of 1% and 97%, respectively. This rule applies to approximately 10% of patients who stay in the unit for >4 days, and approximately 10% of patients to whom this rule is applied will develop proven or probable IC. In this study, patients with any combination of diabetes mellitus, new-onset hemodialysis, use of total parenteral nutrition, or receipt of broad-spectrum antibiotics had an IC rate of 16.6%. This compared with a rate of 5.1% in patients who lacked these characteristics (P = 0.001). Fifty-two percent of patients who stayed in the ICU for ≥4 days met this rule, and the rule captured 78% of patients who eventually developed IC.
The Candida score
A Spanish group reported on the development of a bedside scoring system that allows early antifungal treatment when candidemia is suspected in nonneutropenic ICU patients (CitationLeon et al 2006). This “Candida score” is based on the predictive value of previously reported risk factors. Using a logistic regression analysis and adjusting for possible confounding variables, the authors found several factors to be independently associated with a greater risk for proven candidal infection. The scores for the individual factors were: parenteral nutrition (+0.908), prior surgery (+0.997), multifocal Candida colonization (+1.112), and severe sepsis (+2.038). The authors concluded that a “Candida score” of >2.5 could accurately select patients who would benefit from early antifungal treatment (sensitivity 81%, specificity 74%).
Pre-emptive antifungal treatment in the ICU
Poor outcomes are, in part, associated with difficulties in establishing the microbiologic diagnosis at an early stage of infection. Blood culture results are positive in only 50% of invasive Candida and Fusarium infections, and are very rarely positive in cases of IA. Cultures of bronchoalveolar lavage fluid or brushing specimens are positive in <50% of subjects with invasive pulmonary aspergillosis. Finally, positive cultures of specimens from nonsterile body sites may be related to either colonization or infection, and distinguishing between these can be difficult. Nonculture-based diagnostic tests may provide a useful adjunct to these more traditional approaches.
Corrected colonization index
CitationPiarroux and colleagues (2004) assessed the efficacy of a pre-emptive antifungal therapy in preventing proven candidiasis in critically ill surgical patients, using a corrected colonization index (CCI) (ratio of highly positive samples to the total numbers of samples cultured) to measure the intensity of Candida mucosal colonization. Patients with a CCI value of ≥0.4 received early pre-emptive antifungal therapy with fluconazole, and the incidence of ICU-acquired proven candidiasis decreased significantly from 2.2 to 0%. However, it is possible that the overload of samples sent to the microbiology laboratory could limit the widespread use of this approach.
Targeted treatment of invasive candidiasis in the ICU
The best first-line treatment for candidemia in critically ill patients remains controversial. Clinical studies have shown that amphotericin B (AmB), fluconazole, echinocandins, and voriconazole have similar efficacy in the treatment of Candida bloodstream infections (CitationRex et al 1994; CitationPhillips et al 1997; CitationMora-Duarte et al 2002; CitationKullberg et al 2005). In accordance with the last IDSA guidelines (CitationPappas et al 2004), many experts favor initial treatment with AmB in severely ill or clinically unstable patients; although the recent published Swiss guidelines for fungal infections (CitationFluckiger et al 2006) do not support this statement. However, its renal toxicity could present a serious problem in these individuals, which may often preclude its use as first-line therapy (CitationBates et al 2001; CitationBlot et al 2002).
While the triazole, fluconazole, may be selected on the basis of its efficacy and safety (CitationRex et al 1994; CitationPhillips et al 1997), the increasing frequency of patients infected with Candida strains that are resistant to this drug highlight the need for initial treatment with a broader-spectrum agent, at least until the Candida spp. is identified, in order to avoid inadequate antifungal treatment and an associated increased mortality rate (CitationZaragoza and Peman 2006). Results from the first randomized, prospective, multicenter study in nonneutropenic patients with candidemia who were treated with either voriconazole alone or AmB deoxycholate followed by fluconazole have demonstrated equivalence of these two regimens with regard to efficacy and mortality rates (CitationKullberg et al 2005). Response rates were similar in the voriconazole and AmB/fluconazole arms; however, for C. tropicalis infection, the response rate was significantly higher in the group treated with voriconazole, despite in vitro susceptibility of these strains to AmB. These results can be easily applied to critically ill patients as approximately half of the patients included in the study were admitted to an ICU. The only limitation to the use of intravenous voriconazole in these patients could be the accumulation and toxicity of its excipient (cyclodextrin) in severe renal dysfunction, although there are no data regarding this concern in patients undergoing renal replacement therapy.
Published reports suggest that caspofungin is equivalent in efficacy to standard therapy with AmB in the treatment of Candida infections (CitationMora-Duarte et al 2002). CitationMora-Duarte and colleagues (2002) compared caspofungin to AmB in the treatment of IC in nonneutropenic (n = 200) and neutropenic patients (n = 24). Caspofungin was as efficacious as AmB, with favourable response rates of 73.4% and 61.7%, respectively. Micafungin has become the second available echinocandin approved for use in the USA and Japan for the treatment of esophageal candidiasis and prophylaxis in subjects with neutropenia (CitationChandrasekar and Sobel 2006). Recently, two different studies have been published for invasive candidiasis (CitationKuse et al 2007; CitationPappas et al 2007). In these studies, micafungin was as effective as liposomal AmB (CitationKuse et al 2007) or caspofungin (CitationPappas et al 2007) as first-line treatment of candidemia and IC and caused fewer adverse events than liposomal AmB. Finally, anidulafungin is the only antifungal compound that has been able to demonstrate superiority over fluconazole in IC (CitationReboli et al 2007), although we must be cautious with the results presented as the study involved was powered a priori for equivalency. However, anidulafungin’s efficacy and safety profile could indicate that it should be readily considered as a first-line option for the treatment of IC.
In the last years, some publications have shown a shift toward the use of antifungal drugs other than fluconazole due to the increasing number of non-C. albicans (NCA) isolates (CitationSendid et al 2006; CitationZaragoza and Peman 2006). Consequently, the application of an early de-escalation therapy in critically ill patients with fungal infection should be recommended (CitationZaragoza and Peman 2006). For this reason, voriconazole (due to its broad spectrum and good profile in the ICU setting), caspofungin, anidulafungin and micafungin (particularly in renal dysfunction) could be attractive options in critically ill patients. Finally, the choice of antifungal drug must be based on the individual characteristics of the patient, and particularly focus on the presence of renal or hepatic failure, hemodinamic instability and possible interactions with other drugs. The presence of hemodynamic instability is a mayor factor for choosing empirical therapy. This fact has been considered by the recent guidelines published enhancing a tailored therapy (de-escalation) especially with severe sepsis or septic shock (CitationPappas et al 2004; CitationFluckiger et al 2006). All these guidelines heavily recommend, including last ongoing IDSA guidelines, the use of echinocandins in noneutropenic patients with IC when hemodynamic inestability was present. The high rate of clinical success of these agents in candidemia, their low toxicity, their excellent safety profile and their broad spectrum against non-albicans spp. makes this recommendation feasible. A recent publication has corroborated the use of caspofungin in critically ill patients (CitationDiNubile et al 2007).
In conclusion, IFIs, especially in the critical care setting, have become an excellent target for prophylactic, empiric, and pre-emptive therapy interventions. summarizes the antifungal therapy strategies in ICU patients.
Table 2 Antifungal therapy strategies in ICU patients
Prophylaxis, empirical, pre-emptive or targeted therapy, which is the best in hematological patients?
Hematological patients are prone to IFI since most of them receive myelotoxic chemotherapy and usually have more than one of well-known risk factors for IFI (eg, long-lasting neutropenia, older age, active cancer, corticosteroid therapy, administration of broad spectrum antibiotics, allogeneic HSCT, central venous catheter, organ dysfunction). These patients are usually polymedicated and are thus exposed to harmful drug interactions. Furthermore, it is important to emphasize that the same individual patient will be at risk at several time points through the entire treatment plan for his/her underlying disease. Thus, the planned antineoplastic treatment should be kept in mind when designing antifungal strategies for hematological patients.
Prophylaxis of IFI in hematological patients
Historically, outcomes for IFI have been disappointing and associated with a high mortality rate. Because of this, prophylaxis has been the first option to consider in hematological patients. Since the early 90’s, fluconazole prophylaxis has reduced infections caused by Candida spp. but dramatic changes in the epidemiology of IFI have occurred, with Aspergillus spp. and NCA species becoming increasingly common. These changes have affected the selection of antifungals for first-line or prophylactic use, as not all agents have the spectrum of activity required. At this point, it should be noted that evidence based recommendations are to be interpreted with caution and so we should always consider the moment when they were formulated. For example, benefit of antifungal prophylaxis with fluconazole (400 mg/day) in allogeneic HSCT recipients continues to be an A-I recommendation but today C. albicans is not longer the predominant pathogen in this setting. However, newer azoles have the potential to provide protection against mould infections that are difficult to treat once they occur. Two large randomized, fluconazole- or itraconazole-based controlled clinical trials in acute leukemia patients (CitationCornely et al 2007b) and in HSCT recipients with graft-versus-host disease (GVHD) (CitationUllmann et al 2007) have demonstrated the efficacy of oral posaconazole (200 mg thrice daily) for reducing invasive mould infections. Ideally prophylaxis should usually be initiated in parallel with the administration of cytotoxic therapy in order to ensure a protective effect at the time of maximal neutropenia and intestinal epithelial damage, but concerns over drug interactions make advisable to start triazole-based prophylaxis after the administration of cytotoxic therapy, especially in HSCT recipients. The ability to switch from the oral to an intravenous formulation for the same antifungal product in the setting of severe oral mucositis is an advantage. Both oral solution and intravenous formulation of itraconazole are suitable for prophylaxis in high-risk patients, but compliance with the oral solution is poor (CitationMarr et al 2004b). A preliminary report of a large trial to assess prophylactic voriconazole in allogeneic HSCT recipients (oral or IV 200 mg BID) was recently presented (CitationWingard et al 2007). Prophylaxis with micafungin (Citationvan Burik et al 2004) and caspofungin (CitationMattiuzzi et al 2006) have been studied at daily IV doses of 50 mg but echinocandins are preferred in the clinical practice for therapy instead of prophylaxis. The end of prophylaxis should be dictated by the termination of the specific risk. Mould-active prophylaxis may require administration into the late post-engraftment period in allogeneic HSCT for those patients with higher risk due to acute or chronic GVHD requiring augmented immunosuppressive therapy (CitationMarr et al 2004b; CitationUllmann et al 2007). Finally, the use of aerosolized lipid-based formulations of AmB may prove to be useful as prophylaxis for mould infection in the respiratory tract, the major portal of entry of fungal conidia (CitationRijnders et al 2008). The combination of nebulized AmB with fluconazole is an interesting approach to avoid drug interactions (eg, in patients receiving vinca alkaloids).
Empirical therapy of IFI in hematological patients
For lower risk patients or when IFI is suspected in patients receiving prophylaxis, empirical antifungal therapy is often employed following a predefined duration of fever. During the early 1980s one quarter to one third of severely neutropenic cancer patients with persistent or relapsing fever despite broad-spectrum antibacterial therapy developed IFI. Empirical AmB deoxycholate reduced the incidence of IFI and overall mortality by 50%–80% and 23%–45%, respectively. Fever is a poorly predictive surrogate upon which to base an intervention such as empirical antifungal therapy (CitationDe Pauw 2005). Although a substantial number of leukemia patients and HSCT recipients are given empirical antifungal therapy, proven IFI occurs in only 2% to 15% (CitationKanda et al 2000; CitationBow et al 2002; CitationUllmann et al 2002; CitationCornely et al 2007b) suggesting that the current guidelines (CitationHughes et al 2002) based upon persistent neutropenic fever are significantly flawed and may result in unjustifiable excess treatment-related toxicities and resource expenses (CitationDe Pauw 2005).
Currently, caspofungin and liposomal AmB are the first-line option for treating patients with febrile neutropenia (CitationWalsh et al 1999, Citation2004). The role of voriconazole as an empirical treatment for patients with febrile neutropenia remains unresolved, and in fact, voriconazole is currently not licensed for this indication. The results of a large, international, multicenter, randomized study that compared voriconazole with liposomal AmB for the empirical treatment of febrile neutropenic patients have been controversial (CitationWalsh et al 2002). According to the predefined end point of this study (noninferiority), voriconazole did not achieve this goal relative to liposomal AmB; the overall success rates were 26% and 30.6%, respectively. Breakthrough fungal infections occurred in eight patients (1.9%) in the voriconazole group compared with 21 patients (5%) in the liposomal AmB group (P = 0.02). However, more patients died in the voriconazole group and a claimed significant reduction in the number of breakthrough fungal infections disappeared when patients arbitrarily excluded from analysis by the trial authors were included (CitationJorgensen et al 2006).
Continuous infusion administration AmB has been proposed over the past few years, claiming it could reduce the risk of nephrotoxicity associated to this agent. The preliminary results of an open study, including 80 neutropenic patients during 4 hour infusion versus continuous infusion, suggest more advantages for continuous infusion. A significantly lower incidence in reactions related with the infusion was found in the case of continous administration, which also favored the creatinine clearance values (CitationEriksson et al 2001). Another cohort study on 81 febrile neutropenic hematological patients with a higher IFI risk, evaluated the administration of AmB in intermittent infusion during 4h, compared to a continuous infusion (CitationPeleg and Woods 2004). Kidney failure was seen in 45% and 10% of the patients, respectively (OR 0.14; IC 95% 0.04–0.5; P<0.001). The result was similar in patients with allogeneic transplant and in those which were given other nephrotoxic drugs. A multivariate logistic regression showed that the continuous infusion was the only variable significantly associated to kidney failure, with a protective effect.
Nevertheless, the use of continuous infusion is still controversial. Whilst some authors defend this strategy before opting for lipid or liposomic formulations (CitationJohnson 2004; CitationSchneemann and Bachli 2004), others do not consider it totally acceptable because of limited experience. CitationAltmannsberger and colleagues (2007), when repeating the experience provided by CitationPeleg and Woods (2004), found no significant advantages between intermittent and continuous infusion in patients with a high IFI risk.
Pre-emptive therapy of IFI in hematological patients
A more refined approach is pre-emptive therapy where treatment is only initiated upon positive identification of a surrogate marker of infection in combination with clinical and radiological signs. This pre-emptive therapy will subject fewer patients to toxic and expensive treatments. The interest in this strategy is based upon the observation that early detection is associated with better outcomes. A study in acute leukemia patients receiving fluconazole prophylaxis examined an algorithm-based pre-emptive approach based upon serial diagnostic testing and clinical monitoring (CitationMaertens et al 2005). Only patients with ≥2 positive serum GM assays or CT and or bronchoscopic evidence for mould infection received antifungal therapy. A total of 41 of 117 febrile neutropenic episodes (35%) had persistent neutropenic fever; however, only 9 patients (22% of the 41 persistent neutropenic fevers; 8% of the original febrile neutropenic episodes) satisfied the pre-defined criteria for antifungal therapy. Despite these promising observations, the appearance of a clinical or radiological marker such as a suggestive nodular pulmonary infiltrate on computerized thoracic tomography in a high-risk patient will compel the anxious physician to initiate antimould therapy independent of molecular markers. Pre-emptive strategies are intellectually attractive because they combine in an elegant manner newer diagnostic tools in order to give antifungal therapy only to patients who really deserve it. However, in the real world, they are difficult to carry out. Furthermore, nonculture methods such is serum GM assay lose diagnostic power if an anti-mould prophylaxis is given, which in turn will be given to higher risk patients. Thus pre-emptive therapy based on sensitive diagnostic non-culture methods needs further validation in larger randomized trials before becoming a standard.
Targeted therapy of IFI in hematological patients
Targeted therapy is used in patients with confirmed IFI. Again hematological patients deserve especial consideration as they are commonly at high risk of hemorrhagic complications that render them unfit for aggressive diagnostic procedures including biopsies. Therefore many suspected cases will be diagnosed as probable IFI at the most.
A study comparing voriconazole to conventional AmB in 277 patients (CitationHerbrecht et al 2002) demonstrated higher response rates among voriconazole recipients (52.8% versus 31.6%), a 67% improvement. Patients with early lesions characterized by pulmonary nodules with halos had higher treatment response rates (52.4% versus 29.1%) (CitationGreene 2005). Moreover, a survival advantage for voriconazole recipients was observed (70.8% compared to 57.9%, P = 0.024). Despite this, response among allogeneic HSCT recipients remained suboptimal (32.4% for voriconazole versus 13.3% for conventional AmB). A study of dose-intense (10 mg/kg/d for 14 days followed by 3 mg/kg/d vs 3 mg/kg/d) of liposomal AmB as primary therapy for IA demonstrated similar response rates (46% vs 50%, respectively), but more nephrotoxicity (31% versus 14%), hypokalemia (30% versus 16%), and higher mortality in the dose-intense group (41% versus 28%) (CitationCornely et al 2007a). Based on this experience, the value of dose-intensity for IA appears limited.
Poor response rates for primary and salvage monotherapy therapy for IFI and the availability of increasingly safer agents with differing mechanisms of action have prompted hematologists to use early combination therapy. Arguments for considering combination therapy include enhanced fungal killing (synergy), an enhanced spectrum of activity, prevention of development of resistance, and reduction of drug-related toxicities (CitationKontoyiannis and Lewis 2004). Favorable responses were observed among HSCT patients failing polyene-based therapy for IA with a combination of voriconazole and caspofungin compared to voriconazole monotherapy (CitationMarr et al 2004a). Recently, a French multicenter randomized study comparing combination therapy with liposomal AmB (3 mg/kg/d) plus caspofungin (70 mg day 1 and 50 mg/d thereafter) versus high-dose liposomal AmB monotherapy (10 mg/kg/d) for primary treatment of IA was published (CitationCaillot et al 2007). A favorable overall response was observed in 67% combination therapy recipients compared to 27% high-dose therapy recipients (P = 0.028). The results of this small pilot, representing the first prospective study of combination therapy in IA, are encouraging but need confirmation. Combination antifungal therapies are expensive and potentially toxic and there are limited well-designed randomized-controlled trials to guide the practicing clinician faced with managing these problems.
In conclusion, risk-adapted prophylaxis is the best option in hematological patients. resumes the antifungal therapy strategies in these patients.
Table 3 Antifungal therapy strategies in hematological patients
Prophylaxis, empirical, pre-emptive or targeted therapy, which is the best in solid organ transplant recipients (SOT)?
Renal, liver, heart, and lung transplantation are now considered to be the standard therapeutic interventions in patients with end-stage organ failure. The use of newer more potent immunosuppressive regimens as well as widespread use of antifungal drugs has changed the landscape of fungal infections. The incidence of invasive mycoses following SOT ranges from 5% to 42% depending on the organ transplanted (CitationMarik 2006; CitationSingh 2004; CitationSolé and Salavert 2007). Fungal infections in SOT recipients continue to be a significant cause of morbidity and mortality. The clinical and epidemiological characteristics of IFIs in recipients of nonpulmonary solid organ transplantation (NP-SOT) are very different from which occurs in the patients with lung transplantation (LT) or HSCT. The incidence of invasive mycoses varies with type of SOT, though Candida spp. and Aspergillus spp. account for most IFI in SOT recipients (CitationSilveira and Husain 2007). Liver transplant recipients have highest reported incidence of Candida infections while LT recipients have highest rate of Aspergillus infections (CitationSingh 2005; CitationSingh and Paterson 2005; CitationSolé et al 2005; CitationSolé and Salavert 2008). Recent epidemiological studies suggest the emergence of resistant strains of Candida as well as mycelial fungi other than Aspergillus in these patients. Moreover, significant percentages of fungal infections are occurring late in the course of transplantation. SOT recipients also are at risk for Cryptococcus infections (CitationSingh et al 2007) and reactivation of endemic mycoses such as histoplasmosis and coccidiomycosis.. Emergence of newer and more potent antifungal agents with lower toxicity potentially changes the concept of antifungal prophylaxis (Citationvan Burik 2005; CitationMetcalf and Dockrell 2007).
Prophylaxis and pre-emptive therapy of IFI in solid organ transplant patients
Several prophylactic strategies with antifungal drugs have been reported to result in a decreased incidence and mortality of fungal disease in LT recipients (CitationCovarrubias and Milstone 2005; CitationHusain et al 2006b; CitationMagill and Dropulic 2006); however, there has not been a uniform approach, data are limited, and besides there is a considerable variation in anti-fungal prophylaxis practices among LT centres throughout the world. The majority of LT programs are using universal antifungal prophylaxis in the postoperative period; about 30% use a pre-emptive approach for patients with pre- and/or post-transplant fungal airway colonization. As the antifungal agent used as the duration of prophylaxis varies substantially from center to center (CitationHusain et al 2006b). It is clear that there is considerable uncertainty to which approach (prophylaxis or pre-emptive therapy) is most appropriate, which agent is the best, and what duration of prophylaxis or pre-emptive therapy is needed. Antifungal prophylaxis in LT recipients should be taken into account the incidence of colonization, anastomoses healing, chronic rejection, and the time of LT, thus providing a rationale for the duration of therapy.
To prevent invasive pulmonary aspergillosis, multiple strategies and antifungal drugs have been utilized such as oral itraconazole, voriconazole or aerosolized AmB used alone or in combination. Aerosolized medication regimens are an attractive option, as drug interactions and systemic toxicities are likely to be limited (CitationDrew 2006). Lipid preparations of AmB appear to be ideal for inhalational administration; however, there are not rigorous pharmacokinetic studies in LT recipients, to determine the appropriate dose and schedule of their administration. Monforte and colleagues have demonstrated that aerosolized AmB and lipid preparations of AmB are safe and achieve high concentrations in BAL fluid for the first 24 hours and 14 days, respectively, following nebulization (CitationMonforte et al 2003, Citation2005). These lipid formulations let a delayed administration (every 7–14 days), which is rebounded and better accomplished by patient. Several centers have reported on the safety of aerosolized AmB with a variety of dosing regimens (CitationReichenspurner et al 1997; CitationCalvo et al 1999; CitationMonforte et al 2003), and others with aerosolized AmB lipid formulations (CitationPalmer et al 2001; CitationDrew et al 2004; CitationLowry et al 2007). Our group has used aerosolized AmB as part of the post-LT protocol since 1994 (CitationCalvo et al 1999). Since three years ago we also are using AmB lipid complex, with the same respiratory tolerability and safety that aerosolized AmB, but AmB lipid complex results more comfortable for long periods of time (50 mg inhaled/weekly), and patients have better adherence to treatment. Recently, in vitro suitability of caspofungin for aerosol administration has been characterized (CitationWong-Beringer et al 2005). Caspofungin solution appeared to have physicochemical and aerodynamic characteristics suitable for aerosolization. However, further in vivo testing is warranted. Although the incidence of IFI seems to be reduced with aerosolized AmB prophylaxis, the efficacy of this approach has not been determined in a large prospective clinical trial. Furthermore, without detectable levels of AmB in the circulation, extrapulmonary fungal infections may not be prevented by this strategy. Besides, it is important to take into consideration the type of delivery systems used for inhaled drugs (CitationCorcoran et al 2006; CitationHagerman et al 2006). In addition, contamination of the nebulization systems used in the prophylaxis with AmB nebulized in LT has been described (CitationMonforte et al 2003). The contamination of the nebulizing systems may be the origin of respiratory infections and it is frequent when no strict cleaning and disinfection protocol is followed.
In conclusion, aerosolized antifungal therapy is a promising route of drug delivery for pulmonary aspergillosis due to attainment of high localized concentrations (CitationMohammad and Klein 2006; CitationSolé and Nieto 2007).
Respect to oral prophylaxis, recently a study that examined the efficacy and toxicity of a strategy of universal de novo antifungal prophylaxis with voriconazole compared to targeted antifungal prophylaxis has been published (CitationHusain et al 2006a). The main finding of this study was that the overall rate of IA at 1 year decreased to 1.5% with universal voriconazole prophylaxis as compared to 23.5% with a targeted prophylaxis strategy. Interestingly, the rate of Candida colonization, particularly non-albicans spp. in the voriconazole group was significantly higher. In the voriconazole prophylaxis cohort, 27% of the LT recipients had normal liver enzymes throughout the course of the study. The main handicap of this azole therapy is the strong interaction with immunosuppressors that obliges to monitoring calcineurin inhibitors to avoid toxicity or rejection. Other interesting finding was that universal voriconazole prophylaxis did not increase the rate of non-Aspergillus fungal infections (specially, zygomycosis).
Available echinocandins (caspofungin, micafungin, anidulafungin) may have an important role in prophylaxis because of their antifungal profile, pharmacokinetics and security; however, they are expensive and need intravenous administration.
Another question is how long should be prophylaxis maintained? The majority of centers agree to apply universal prophylaxis during first period post transplant (3 months) after this time, each center use a tailored prophylaxis. Besides, it is recommended to use nebulized antifungal prophylaxis and/or preemptive therapy with antifungal agents (voriconazole) in patients with chronic rejection and respiratory samples positive for Aspergillus, even without clinical or radiological signs, mainly in single LT patients due to the high risk of IA (CitationSolé et al 2005). This preemptive treatment should last for at least 6 months, the time period over which colonization has been shown to precede disseminated infection (CitationSingh and Husain 2003) and in some cases for life. shows antifungal therapy strategies in LT patients.
Table 4 Antifungal therapy strategies in lung transplantation (LT) recipients
Due to the difficulty in obtaining a proven diagnosis of IFI, empiric and pre-emptive therapy plays an important role in NP-SOT patients () (CitationLeather and Wingard 2006; CitationBow 2008). As it is mentioned in , some of NP-SOT recipients have additional risk factors that increases the probability of suffer an IFI caused by Aspergillus spp. (CitationEchaniz-Quintana et al 2004; CitationSingh 2005; CitationSingh et al 2006b, Citation2006c). For this reason, the incorporation of a strategy of antifungal prophylaxis adapted-to-risk is accepted and high risk patients need to be identified in order to prevent the development of the disease (CitationPlayford et al 2004; CitationCastroagudin et al 2005; CitationHellinger et al 2005).
Table 5 Antifungal therapy strategies in recipients of nonpulmonary solid organ transplantation (NP-SOT)
Table 6 Etiological and clinical characteristics of IFI according to the type of SOT
Since the IFI risk is lower in the cardiac and kidney recipient patients, we will make reference mainly to patients with orthotopic liver transplantation (OLT) as paradigm of the indication of antifungal prophylaxis in NP-SOT patients. However, prophylaxis of candidiasis in SOT recipients is a challenging topic. Strategies to prevent IC should be based on institutional trends and, when appropriate, should target high-risk patients only (CitationSingh 2000). Only a few well-designed studies have been conducted and all of the controlled trials were performed in liver transplant recipients. Oral prophylaxis with nonabsorbable antifungal agents (nystatin, clotrimazole, AmB) has shown inconsistent results (CitationWiesner et al 1988; CitationArnow et al 1996; CitationHjortrup et al 1997; CitationHellinger et al 2002). Two randomized controlled trials have shown the efficacy of fluconazole in the prophylaxis of IC. In one study, comparing fluconazole 100 mg/day for the first 4 weeks following liver transplantation to oral nystatin, fluconazole was associated with a reduction in Candida colonization and superficial infections, as well as a trend toward reduction of invasive infections (CitationLumbreras et al 1996). In a randomized, placebo-controlled study, fluconazole 400 mg/day for 10 weeks after liver transplantation prevented most types of Candida infection, except those caused by C. glabrata and C. krusei. In another double blind randomized control trial itraconazole decreased the rate of fungal infection from 24% to 4% in liver transplant recipients (CitationSharpe et al 2003). A recently published meta-analysis showed that antifungal prophylaxis in liver transplant recipients significantly reduced the total episodes of superficial and IFI caused by yeasts (at least for C. albicans), as well as mortality attributable to fungal infections; however, it did not affect overall mortality or the need for empirical antifungal treatment (CitationCruciani et al 2006). Compared to controls, patients receiving prophylaxis experienced a higher proportion of episodes of Candida non-albicans infections. Prophylactic strategies against invasive Candida infections should be reassessed periodically because risk factors continue to evolve in liver transplant recipients (CitationHusain et al 2003).
Antifungal prophylaxis to prevent IA remains an unsettled issue in NP-SOT. To date, no prospective randomized studies have demonstrated that antifungal prophylaxis prevents IA in OLT. A recent meta-analysis of antifungal prophylaxis in OLT demonstrated no beneficial effect on IA. Observational studies, however, suggest that targeted prophylaxis with lipid formulations of AmB may be effective in preventing IA in high-risk OLT recipients (CitationSingh et al 2001; CitationFortun et al 2003). Lipid formulations of AmB at a dose of 5 mg/kg/day have been shown to be efficacious in reducing IFI in high-risk OLT recipients on renal replacement therapy, although there was no reduction in mortality. A study of universal prophylaxis with cumulative doses of 1–1.5 g of liposomal or lipid complex AmB showed a reduction in the incidence of IA, which was most significant among patients receiving renal replacement therapy (0% vs 32% on controls). However, low doses of lipid formulations of AmB failed to prevent IA (CitationTollemar et al 1995).
The efficacy of the antifungal prophylaxis oral solution of itraconazole as has been assessed in two reports on OLT recipients. A randomized, controlled trial of itraconazole in an oral solution (200 mg every 12 h) versus intravenous or oral fluconazole (400 mg every 24 h) documented no significant difference in the incidence of IA (CitationWinston and Busuttil 2002).
On the other hand, the Spanish group GESITRA has communicated recently the preliminary results of a prospective, noncomparative, open label trial on antifungal prophylaxis with caspofungin in high-risk OLT recipients (CitationFortun et al 2007). Overall, caspofungin prophylaxis was successful in 87.8% of the patients. These results suggest promise for the prophylactic use of echinocandins in high risk OLT.
However, it is controversial the election of antifungal agent for prophylaxis in these patients. The high cost and the need of parenteral infusion limits the use of lipid formulations of AmB and caspofungin. Despite in vitro data suggesting that caspofungin in combination with calcineurin or TOR-inhibitors may have enhanced activity against Aspergillus, breakthrough infections in patients on caspofungin have been noted (CitationKontoyiannis et al 2003). The recommendations for prophylaxis against Aspergillus in OLT are a lipid formulation of AmB, voriconazole or caspofungin, with a duration of 3–4 weeks or until resolution of risk factors. In heart transplant recipients routine antifungal prophylaxis is not warranted, but in patients deemed to be at high risk (), itraconazole at 400 mg daily administered orally from day 5 after transplantation for 3 to 6 months is associated with a significantly lower incidence of IA (CitationMunoz et al 2004).
Randomized studies in high-risk patients are needed to determine the efficacy of targeted antifungal prophylaxis for the prevention of IA. In the meantime, each centre needs to evaluate its own recipient risk factors and rates of IA to determine whether these strategies are appropriate for its patients (CitationBiancofiore et al 2002).
In conclusion, targeted prophylaxis against Candida and Aspergillus spp. is recommended in all SOT. Fluconazole should be used for prophylaxis against Candida spp. unless the institution has a high rate of non-albicans infections; conversely, voriconazole is recomended for prophylaxis against Aspergillus spp. allthough its significant interactions with immunosuppressive agents could be a potential limiting factor.
Targeted therapy of IFI in solid organ transplant patients
With regard to the treatment, voriconazole, an extended spectrum highly lipophilic triazole with 98% oral bioavailability, is actually the first choice for initial therapy of IA in LT patients and other immunosuppressed hosts. Furthermore, the first experiences with LT patients also evidenced that continuous infusion of AmB is well tolerated, safe and efficient (CitationSpeich et al 2002). Other potentially effective therapies include lipid formulations of AmB and echinocandins. Combination therapy using a triazole and an echinocandin has been evaluated in SOT, with a significant reduction in mortality in those patients with renal failure and infected by A. fumigatus (CitationSingh et al 2006a).
In conclusion, IFIs, especially immunocompromised and critical care patients, have become an excellent target for prophylactic, empiric, and pre-emptive therapy interventions due to high morbidity and mortality rates, an increasing incidence, and associated health care costs. Early diagnosis and treatment are associated with a better prognosis. In all cases the choice of antifungal drug must be based on the individual characteristics of the patient. A tailored therapy (de-escalation) must also be considered in the ICU setting. Furthermore, risk-adapted prophylaxis is the best option in hematological and SOT patients.
Disclosure
The authors report no conflicts of interest in this work.
References
- AgrestiMGDe BernardisFMondelloF1994Clinical and mycological evaluation of fluconazole in the secondary prophylaxis of esophageal candidiasis in AIDS patients. An open, multicenter studyEur J Epidemiol1017227957784
- AkamatsuNSugawaraYKanekoJ2007Preemptive treatment of fungal infection based on plasma (1 – >3) beta-d-glucan levels after liver transplantationInfection353465117885729
- AlbertiCBrun-BuissonCBurchardiH2002Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort studyIntensive Care Med281082111907653
- AlmiranteBRodriguezDParkBJ2005The Barcelona Candidemia Project Study Group. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003J Clin Microbiol4318293515815004
- AltmannsbergerPHollerEAndreesenR2007Amphotericin B deoxycholate: no significant advantage of a 24 h over a 6 h infusion scheduleJ Antimicrob Chemother60180217537868
- Alvarez-LermaFPalomarMLeonC2003Colonización y/o infección fúngica en unidades de cuidados intensivos. Estudio multicéntrico de 1.562 pacientesMed Clin (Barc)121161612867000
- ArnowPMCarandangGCZabnerR1996Randomized controlled trial of selective bowel decontamination for prevention of infections following liver transplantationClin Infect Dis2299710038783700
- AsciogluSRexJHde PauwB2002Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensusClin Infect Dis3471411731939
- BatesDWSuLYuDT2001Mortality and costs of acute renal failure associated with amphotericin B therapyClin Infect Dis326869311229835
- Ben AbrahamRKellerNTeodorovitchNPredictors of adverse outcome from candidal infection in a tertiary care hospitalJ Infect493172315474630
- BiancofioreGBindiMLBaldassarriR2002Antifungal prophylaxis in liver transplant recipients: a randomized placebo-controlled studyTranspl Int15341712122510
- BlotSIVandewoudeKHHosteEA2002Effects of nosocomial candidemia on outcomes of critically ill patientsAm J Med113480512427497
- BlumbergHMJarvisWRSoucieJM2001Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. The National Epidemiology of Mycosis SurveyClin Infect Dis331778611418877
- BowEJ2008Considerations in the approach to invasive fungal infection in patients with haematological malignanciesBr J Haematol1401335218173752
- BowEJLaverdiereMLussierN2002Antifungal prophylaxis for severely neutropenic chemotherapy recipients: a meta analysis of randomized-controlled clinical trialsCancer9432304612115356
- CaillotDThiebautAHerbrechtR2007Liposomal amphotericin B in combination with caspofungin for invasive aspergillosis in patients with hematologic malignancies: a randomized pilot study (Combistrat trial)Cancer1102740617941026
- CalandraTMarchettiO2002Antifungal prophylaxis for intensive care unit patients: let’s fine tune itIntensive Care Med28169870012580153
- CalvoVBorroJMMoralesP1999Antifungal prophylaxis during the early postoperative period of lung transplantation. Valencia Lung Transplant GroupChest1151301410334143
- CastroagudinJFPontonCBustamanteM2005Prospective interventional study to evaluate the efficacy and safety of liposomal amphotericin B as prophylaxis of fungal infections in high-risk liver transplant recipientsTransplant Proc373965716386598
- ChandrasekarPHSobelJD2006Micafungin: a new echinocandinClin Infect Dis421171816575738
- CorcoranTEVenkataramananRMihelcKM2006Aerosol deposition of lipid complex amphotericin-B (Abelcet) in lung transplant recipientsAm J Transplant627657317049064
- CordonnierCMaurySRibaudP2006A grading system based on severity of infection to predict mortality in allogeneic stem cell transplant recipientsTransplantation82869216861946
- CornelyOAMaertensJBresnikM2007aLiposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial)Clin Infect Dis4412899717443465
- CornelyOAMaertensJWinstonDJ2007bPosaconazole vs fluconazole or itraconazole prophylaxis in patients with neutropeniaN Engl J Med3563485917251531
- CovarrubiasMBMilstoneAB2005An overview of fungal prophylaxis in lung transplantationCurr Opin Organ Transplant1022732
- CrucianiMde LallaFMengoliC2005Prophylaxis of Candida infections in adult trauma and surgical intensive care patients: a systematic review and meta-analysisIntensive Care Med3114798716172847
- CrucianiMMengoliCMalenaM2006Antifungal prophylaxis in liver transplant patients: a systematic review and meta-analysisLiver Transpl12850816628697
- De PauwBE2005Between over- and undertreatment of invasive fungal diseaseClin Infect Dis411251316206098
- De WaeleJJVogelaersDBlotS2003Fungal infections in patients with severe acute pancreatitis and the use of prophylactic therapyClin Infect Dis372081312856213
- DimopoulosGNtzioraFRachiotisG2008Candida albicans versus non-albicans intensive care unit-acquired bloodstream infections: differences in risk factors and outcomeAnesth Analg106523918227310
- DimopoulosGPiagnerelliMBerreJ2004Post mortem examination in the intensive care unit: still useful?Intensive Care Med302080515480565
- DiNubileMJLupinacciRJStrohmaierKM2007Invasive candidiasis treated in the intensive care unit: observations from a randomized clinical trialJ Crit Care222374417869975
- DrewR2006Potential role of aerosolized amphotericin B formulations in the prevention and adjunctive treatment of invasive fungal infectionsInt J Antimicrob Agents27Suppl 1364416713192
- DrewRHDoddsAEBenjaminDKJr2004Comparative safety of amphotericin B lipid complex and amphotericin B deoxycholate as aerosolized antifungal prophylaxis in lung-transplant recipientsTransplantation77232714742987
- Echaniz-QuintanaAPita-FernandezSOtero-FerreiroA2004Risk factors associated with invasive fungal infection in orthotopic liver transplantationMed Clin (Barc)122444815104954
- EggimannPFrancioliPBilleJ1999Fluconazole prophylaxis prevents intra-abdominal candidiasis in high-risk surgical patientsCrit Care Med2710667210397206
- ErikssonUSeifertBSchaffnerA2001Comparison of effects of amphotericin B deoxycholate infused over 4 or 24 hours: randomised controlled trialBMJ3225798211238151
- FalagasMEApostolouKEPappasVD2006Attributable mortality of candidemia: a systematic review of matched cohort and case-control studiesEur J Clin Microbiol Infect Dis254192516773391
- FlorentMKatsahianSVekhoffA2006Prospective evaluation of a polymerase chain reaction-ELISA targeted to Aspergillus fumigatus and Aspergillus flavus for the early diagnosis of invasive aspergillosis in patients with hematological malignanciesJ Infect Dis193741716453271
- FluckigerUMarchettiOBilleJ2006Treatment options of invasive fungal infections in adultsSwiss Med Wkly1364476316937323
- FortunJMartin-DavilaPMorenoS2003Prevention of invasive fungal infections in liver transplant recipients: the role of prophylaxis with lipid formulations of amphotericin B in high-risk patientsJ Antimicrob Chemother52813914563893
- FortunJMontejoMMartin-DavilaP2007Prospective, multicentre study of caspofungin for prophylaxis in high-risk liver transplantation [abstract]17th European Congress of Clinical Microbiology and Infectious DiseasesICC, Munich, GermanyMarch 31–April 31733463
- GarbinoJLewDPRomandJA2002Prevention of severe Candida infections in nonneutropenic, high-risk, critically ill patients: a randomized, double-blind, placebo-controlled trial in patients treated by selective digestive decontaminationIntensive Care Med2817081712447512
- GareyKWRegeMPaiMP2006Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional studyClin Infect Dis43253116758414
- Garnacho-MonteroJLeónCAlmiranteB2005Recomendaciones terapéuticas para infecciones fúngicas en el paciente crítico no neutropénico. Conferencia de consenso. ConclusionesMed Intensiva3Suppl 14352
- GreeneR2005The radiological spectrum of pulmonary aspergillosisMed Mycol43Suppl 1S147S15416110807
- HagermanJKHancockKEKlepserME2006Aerosolised antibiotics: a critical appraisal of their useExpert Opin Drug Deliv3718616370941
- HellingerWCBonattiHYaoJD2005Risk stratification and targeted antifungal prophylaxis for prevention of aspergillosis and other invasive mold infections after liver transplantationLiver Transpl116566215915482
- HellingerWCYaoJDAlvarezS2002A randomized, prospective, double-blinded evaluation of selective bowel decontamination in liver transplantationTransplantation731904912131685
- HerbrechtR2002Improving the outcome of invasive aspergillosis: new diagnostic tools and new therapeutic strategiesAnn Hematol81Suppl 2S52S5312611078
- HerbrechtRDenningDWPattersonTF2002Voriconazole versus amphotericin B for primary therapy of invasive aspergillosisN Engl J Med3474081512167683
- HjortrupARasmussenAHansenBA1997Early bacterial and fungal infections in liver transplantation after oral selective bowel decontaminationTransplant Proc293106109365684
- HopeWWWalshTJDenningDW2005Laboratory diagnosis of invasive aspergillosisLancet Infect Dis56092216183515
- HughesWTArmstrongDBodeyGP20022002 guidelines for the use of antimicrobial agents in neutropenic patients with cancerClin Infect Dis347305111850858
- HusainSPatersonDLStuderS2006aVoriconazole prophylaxis in lung transplant recipientsAm J Transplant630081617062003
- HusainSTollemarJDominguezEA2003Changes in the spectrum and risk factors for invasive candidiasis in liver transplant recipients: prospective, multicenter, case-controlled studyTransplantation752023912829905
- HusainSZaldonisDKusneS2006bVariation in antifungal prophylaxis strategies in lung transplantationTranspl Infect Dis8213817116134
- Ibanez-NollaJNolla-SalasMLeonMA2004Early diagnosis of candidiasis in non-neutropenic critically ill patientsJ Infect481819214720495
- JarqueISalavertMRomaE2004Hospital Universitario La Fe Guide to the prophylaxis and treatment of fungal infections in immunodepressed patients or in patients requiring special careRev Esp Quimioter173578915696227
- JohnsonJR2004Reduction of nephrotoxicity associated with amphotericin B deoxycholateClin Infect Dis38303714699469
- JorgensenKJGotzschePCJohansenHK2006Voriconazole versus amphotericin B in cancer patients with neutropeniaCochrane Database Syst Rev1CD00470716437492
- KandaYYamamotoRChizukaA2000Prophylactic action of oral fluconazole against fungal infection in neutropenic patients. A meta-analysis of 16 randomized, controlled trialsCancer8916112511013378
- KlingsporLTornqvistEJohanssonA2004A prospective epidemiological survey of candidaemia in SwedenScand J Infect Dis3652515000560
- KontoyiannisDPLewisRE2004Toward more effective antifungal therapy: the prospects of combination therapyBr J Haematol1261657515238137
- KontoyiannisDPLewisREOsherovN2003Combination of caspofungin with inhibitors of the calcineurin pathway attenuates growth in vitro in Aspergillus speciesJ Antimicrob Chemother51313612562696
- KullbergBJSobelJDRuhnkeM2005Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trialLancet36614354216243088
- KuseERChetchotisakdPda CunhaCA2007Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trialLancet36915192717482982
- LeatherHLWingardJR2006New strategies of antifungal therapy in hematopoietic stem cell transplant recipients and patients with hematological malignanciesBlood Rev202678716781028
- LeleuGAegerterPGuidetB2002Systemic candidiasis in intensive care units: a multicenter, matched-cohort studyJ Crit Care171687512297992
- LeonCRuiz-SantanaSSaavedraP2006A bedside scoring system (“Candida score”) for early antifungal treatment in nonneutropenic critically ill patients with Candida colonizationCrit Care Med34730716505659
- LowryCMMartyFMVargasSO2007Safety of aerosolized liposomal versus deoxycholate amphotericin B formulations for prevention of invasive fungal infections following lung transplantation: a retrospective studyTranspl Infect Dis9121517461997
- LumbrerasCCuervas-MonsVJaraP1996Randomized trial of fluconazole versus nystatin for the prophylaxis of Candida infection following liver transplantationJ Infect Dis17458388769617
- MaertensJDeerenDDierickxD2006Preemptive antifungal therapy: still a way to goCurr Opin Infect Dis19551617075330
- MaertensJTheunissenKVerhoefG2005Galactomannan and computed tomography-based preemptive antifungal therapy in neutropenic patients at high risk for invasive fungal infection: a prospective feasibility studyClin Infect Dis4112425016206097
- MaertensJVerhaegenJLagrouK2001Screening for circulating galactomannan as a noninvasive diagnostic tool for invasive aspergillosis in prolonged neutropenic patients and stem cell transplantation recipients: a prospective validationBlood9716041011238098
- MagillSSDropulicLK2006Antifungal prophylaxis in transplant recipients: where do we go from here?Transpl Infect Dis8187917116131
- MarikPE2006Fungal infections in solid organ transplantationExpert Opin Pharmacother729730516448324
- MarrKABoeckhMCarterRA2004aCombination antifungal therapy for invasive aspergillosisClin Infect Dis3979780215472810
- MarrKACrippaFLeisenringW2004bItraconazole versus fluconazole for prevention of fungal infections in patients receiving allogeneic stem cell transplantsBlood10315273314525770
- MattiuzziGNAlvaradoGGilesFJ2006Open-label, randomized comparison of itraconazole versus caspofungin for prophylaxis in patients with hematologic malignanciesAntimicrob Agents Chemother50143716377679
- MeerssemanWLagrouKMaertensJ2008Galactomannan in bronchoalveolar lavage fluid: a tool for diagnosing aspergillosis in intensive care unit patientsAm J Respir Crit Care Med177273417885264
- Mennink-KerstenMAKlontRRWarrisA2004Bifidobacterium lipoteichoic acid and false ELISA reactivity in aspergillus antigen detectionLancet363325714751710
- MetcalfSCDockrellDH2007Improved outcomes associated with advances in therapy for invasive fungal infections in immunocompromised hostsJ Infect552879917697716
- MillonLPiarrouxRDeconinckE2005Use of real-time PCR to process the first galactomannan-positive serum sample in diagnosing invasive aspergillosisJ Clin Microbiol43509710116207969
- MohammadRAKleinKC2006Inhaled amphotericin B for prophylaxis against invasive Aspergillus infectionsAnn Pharmacother4021485417148653
- MonforteVRomanAGavaldaJ2005Prophylaxis for Aspergillus infection in lung transplantation: Pharmacokinetics, safety and efficacy [abstract]J Heart Lung Transplant
- MonforteVRomanAGavaldaJ2003Nebulized amphotericin B concentration and distribution in the respiratory tract of lung-transplanted patientsTransplantation751571412792517
- Mora-DuarteJBettsRRotsteinC2002Comparison of caspofungin and amphotericin B for invasive candidiasisN Engl J Med3472020912490683
- MoraguesMDOrtizNIruretagoyenaJR2004Evaluation of a new commercial test (Candida albicans IFA IgG) for the serodiagnosis of invasive candidiasisEnferm Infecc Microbiol Clin2283814756989
- MorganJMeltzerMIPlikaytisBD2005Excess mortality, hospital stay, and cost due to candidemia: a case-control study using data from population-based candidemia surveillanceInfect Control Hosp Epidemiol26540716018429
- MorrellMFraserVJKollefMH2005Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortalityAntimicrob Agents Chemother493640516127033
- MunozPRodriguezCBouzaE2004Risk factors of invasive aspergillosis after heart transplantation: protective role of oral itraconazole prophylaxisAm J Transplant46364315023157
- Nolla-SalasJSitges-SerraALeon-GilC1997Candidemia in non-neutropenic critically ill patients: analysis of prognostic factors and assessment of systemic antifungal therapy. Study Group of Fungal Infection in the ICUIntensive Care Med2323309037636
- OdabasiZMattiuzziGEsteyE2004Beta-d-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndromeClin Infect Dis3919920515307029
- Ostrosky-ZeichnerLAlexanderBDKettDH2005Multicenter clinical evaluation of the (1 – >3) beta-d-glucan assay as an aid to diagnosis of fungal infections in humansClin Infect Dis41654916080087
- Ostrosky-ZeichnerLSableCSobelJ2007Multicenter retrospective development and validation of a clinical prediction rule for nosocomial invasive candidiasis in the intensive care settingEur J Clin Microbiol Infect Dis26271617333081
- PalmerSMDrewRHWhitehouseJD2001Safety of aerosolized amphotericin B lipid complex in lung transplant recipientsTransplantation72545811502995
- PappasPGRexJHSobelJD2004Guidelines for treatment of candidiasisClin Infect Dis381618914699449
- PappasPGRotsteinCMBettsRF2007Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasisClin Infect Dis458839317806055
- ParkinsMDSabudaDMElsayedS2007Adequacy of empirical antifungal therapy and effect on outcome among patients with invasive Candida species infectionsJ Antimicrob Chemother60613817576697
- PazosCMoraguesMDQuindosG2006Diagnostic potential of (1,3)-beta-d-glucan and anti-Candida albicans germ tube antibodies for the diagnosis and therapeutic monitoring of invasive candidiasis in neutropenic adult patientsRev Iberoam Micol232091517388644
- PazosCPontonJdel PalacioA2005Contribution of (1 – >3)-beta-d-glucan chromogenic assay to diagnosis and therapeutic monitoring of invasive aspergillosis in neutropenic adult patients: a comparison with serial screening for circulating galactomannanJ Clin Microbiol4329930515634986
- PelegAYWoodsML2004Continuous and 4 h infusion of amphotericin B: a comparative study involving high-risk haematology patientsJ Antimicrob Chemother54803815308606
- PelzRKHendrixCWSwobodaSM2001Double-blind placebo-controlled trial of fluconazole to prevent candidal infections in critically ill surgical patientsAnn Surg233542811303137
- PerkinsJD2007Use of Aspergillus galactomannan enzyme-linked immunosorbent assay (ELISA) in liver transplant patientsLiver Transpl13304517354292
- PetriMGKonigJMoeckeHP1997Epidemiology of invasive mycosis in ICU patients: a prospective multicenter study in 435 non-neutropenic patients. Paul-Ehrlich Society for Chemotherapy, Divisions of Mycology and Pneumonia ResearchIntensive Care Med23317259083235
- PfallerMAJonesRNDoernGV2000Bloodstream infections due to Candida species: SENTRY antimicrobial surveillance program in North America and Latin America, 1997–1998Antimicrob Agents Chemother447475110681349
- PfeifferCDFineJPSafdarN2006Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysisClin Infect Dis4214172716619154
- PhillipsPShafranSGarberG1997Multicenter randomized trial of fluconazole versus amphotericin B for treatment of candidemia in non-neutropenic patients. Canadian Candidemia Study GroupEur J Clin Microbiol Infect Dis16337459228472
- PiarrouxRGrenouilletFBalvayP2004Assessment of preemptive treatment to prevent severe candidiasis in critically ill surgical patientsCrit Care Med322443915599149
- PickeringJWSantHWBowlesCA2005Evaluation of a (1 – >3)-beta-d-glucan assay for diagnosis of invasive fungal infectionsJ Clin Microbiol4359576216333082
- PlayfordEGWebsterACSorellTC2004Antifungal agents for preventing fungal infections in solid organ transplant recipientsCochrane Database Syst Rev3CD00429115266524
- PlayfordEGWebsterACSorrellTC2006Antifungal agents for preventing fungal infections in non-neutropenic critically ill and surgical patients: systematic review and meta-analysis of randomized clinical trialsJ Antimicrob Chemother576283816459344
- PontonJdel PalacioA2007Advances and limitations in the early diagnosis of invasive yeast infectionsRev Iberoam Micol24181617874854
- PontonJQuindosGArillaMC1994Simplified adsorption method for detection of antibodies to Candida albicans germ tubesJ Clin Microbiol3221798126184
- PuzniakLTeutschSPowderlyW2004Has the epidemiology of nosocomial candidemia changed?Infect Control Hosp Epidemiol256283315357152
- QuindosGMoraguesMDPontonJ2004Is there a role for antibody testing in the diagnosis of invasive candidiasis?Rev Iberoam Micol2110415458356
- ReboliACRotsteinCPappasPG2007Anidulafungin versus fluconazole for invasive candidiasisN Engl J Med35624728217568028
- ReichenspurnerHGambergPNitschkeM1997Significant reduction in the number of fungal infections after lung-, heart-lung, and heart transplantation using aerosolized amphotericin B prophylaxisTransplant Proc2962789123449
- RexJHBennettJESugarAM1994A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. Candidemia Study Group and the National InstituteN Engl J Med3311325307935701
- RijndersBJCornelissenJJSlobbeL2008Aerosolized liposomal amphotericin B for the prevention of invasive pulmonary aspergillosis during prolonged neutropenia: a randomized, placebo-controlled trialClin Infect Dis461401818419443
- SchneemannMBachliEB2004Continuous infusion of amphotericin B deoxycholate: a cost-effective gold standard for therapy of invasive fungal infections?Clin Infect Dis38303414699468
- SendidBCotteauAFrancoisN2006Candidaemia and antifungal therapy in a French University Hospital: rough trends over a decade and possible linksBMC Infect Dis68016670011
- SharpeMDGhentCGrantD2003Efficacy and safety of itraconazole prophylaxis for fungal infections after orthotopic liver transplantation: a prospective, randomized, double-blind studyTransplantation769778314508365
- ShorrAFChungKJacksonWL2005Fluconazole prophylaxis in critically ill surgical patients: a meta-analysisCrit Care Med3319283516148461
- SilveiraFPHusainS2007Fungal infections in solid organ transplantationMed Mycol453052017510855
- SinghN2000Antifungal prophylaxis for solid organ transplant recipients: seeking clarity amidst controversyClin Infect Dis315455310987719
- SinghN2004Antifungal prophylaxis in solid-organ transplant recipients: considerations for clinical trial designClin Infect Dis39Suppl 4S200S20615546118
- SinghN2005Invasive aspergillosis in organ transplant recipients: new issues in epidemiologic characteristics, diagnosis, and managementMed Mycol43Suppl 1S267S27016110819
- SinghNAlexanderBDLortholaryO2007Cryptococcus neoformans in organ transplant recipients: impact of calcineurin-inhibitor agents on mortalityJ Infect Dis1957566417262720
- SinghNHusainS2003Aspergillus infections after lung transplantation: clinical differences in type of transplant and implications for managementJ Heart Lung Transplant222586612633692
- SinghNLimayeAPForrestG2006aCombination of voriconazole and caspofungin as primary therapy for invasive aspergillosis in solid organ transplant recipients: a prospective, multicenter, observational studyTransplantation81320616477215
- SinghNLimayeAPForrestG2006bLate-onset invasive aspergillosis in organ transplant recipients in the current eraMed Mycol44445916882611
- SinghNPatersonDL2005Aspergillus infections in transplant recipientsClin Microbiol Rev18446915653818
- SinghNPatersonDLGayowskiT2001Preemptive prophylaxis with a lipid preparation of amphotericin B for invasive fungal infections in liver transplant recipients requiring renal replacement therapyTransplantation71910311349726
- SinghNPruettTLHoustonS2006cInvasive aspergillosis in the recipients of liver retransplantationLiver Transpl121205916598780
- SoléAMorantPSalavertM2005Aspergillus infections in lung transplant recipients: risk factors and outcomeClin Microbiol Infect113596515819861
- SoléANietoM2007Nebulized antifungal prophylaxis:Role of amphotericin B lipid complexMed Intensiva56470
- SoléASalavertM2007Voriconazole for the therapy of mycoses in recipients of solid organ transplantsRev Iberoam Micol242172217874859
- SoléASalavertM2008Fungal infections after lung transplantationTransplant Rev (Orlando)228910418631862
- SpeichRDutlyANaefR2002Tolerability, safety and efficacy of conventional amphotericin B administered by 24-hour infusion to lung transplant recipientsSwiss Med Wkly132455812457304
- SulahianATouratierSRibaudP2003False positive test for aspergillus antigenemia related to concomitant administration of piperacillin and tazobactamN Engl J Med3492366714668472
- TollemarJHockerstedtKEriczonBG1995Liposomal amphotericin B prevents invasive fungal infections in liver transplant recipients. A randomized, placebo-controlled studyTransplantation5945507839427
- TortoranoAMPemanJBernhardtH2004Epidemiology of candidaemia in Europe: results of 28-month European Confederation of Medical Mycology (ECMM) hospital-based surveillance studyEur J Clin Microbiol Infect Dis233172215029512
- UllmannAJHeusselCPCornelyOA2002Voriconazole versus liposomal amphotericin B for empirical antifungal therapyN Engl J Med3461745712041526
- UllmannAJLiptonJHVesoleDH2007Posaconazole or fluconazole for prophylaxis in severe graft-versus-host diseaseN Engl J Med3563354717251530
- van BurikJA2005Role of new antifungal agents in prophylaxis of mycoses in high risk patientsCurr Opin Infect Dis184798316258319
- van BurikJARatanatharathornVStepanDE2004Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantationClin Infect Dis3914071615546073
- WalshTJFinbergRWArndtC1999Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study GroupN Engl J Med3407647110072411
- WalshTJPappasPWinstonDJ2002Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent feverN Engl J Med3462253411807146
- WalshTJTepplerHDonowitzGR2004Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropeniaN Engl J Med351139140215459300
- WiesnerRHHermansPERakelaJ1988Selective bowel decontamination to decrease gram-negative aerobic bacterial and Candida colonization and prevent infection after orthotopic liver transplantationTransplantation4557043279582
- WingardJRCarterSLWalshTJ2007Results of a randomized, double-blind trial of fluconazole vs. voriconazole for the prevention of invasive fungal infections in 600 allogeneic blood and marrow transplant patientsBlood110163
- WinstonDJBusuttilRW2002Randomized controlled trial of oral itraconazole solution versus intravenous/oral fluconazole for prevention of fungal infections in liver transplant recipientsTransplantation746889512352887
- WinstonDJPakrasiABusuttilRW1999Prophylactic fluconazole in liver transplant recipients. A randomized, double-blind, placebo-controlled trialAnn Intern Med1317293710577295
- Wong-BeringerALambrosMPBeringerPM2005Suitability of caspofungin for aerosol delivery: physicochemical profiling and nebulizer choiceChest12837111616304338
- ZaragozaRPemanJ2006Invasive fungal infections in critically ill patients: different therapeutic options and a uniform strategyRev Iberoam Micol23596316854178
- ZaragozaRPemanJRamirezP2006Clinical and management utility of Candida germ tube antibody detection in critically ill patients. Preliminary data [abstract]46th Interscience Conference on Antimicrobial Agents and ChemotherapySan Francisco, CASept 27–30M-1618
