Abstract
1. Chronic oxidative stress and inflammation are major mediators of chronic kidney disease (CKD) and result in impaired activation of the cytoprotective transcription factor Nrf2. Given the role of oxidative stress and inflammation in CKD pathogenesis, strategies aimed at restoring Nrf2 activity may attenuate CKD progression.
2. The present study investigated whether the synthetic triterpenoid RTA dh404 (2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid-9,11-dihydro-trifluoroethyl amide or CDDO-dhTFEA) would afford renal protection in a 5/6 nephrectomized rat model of CKD. RTA dh404 (2 mg/kg/day) was orally administered once daily for 12 weeks after 5/6 nephrectomy surgery.
3. The remnant kidneys from the vehicle-treated CKD rats showed activation of nuclear factor kappaB (NF-κB), upregulation of NAD(P)H oxidase, glomerulosclerosis, interstitial fibrosis and inflammation, as well as marked reductions in Nrf2 and its target gene products (i.e. catalase, heme oxygenase-1, thioredoxin 1, thioredoxin reductase 1 and peroxiredoxin 1). The functional and structural deficits in the kidney were associated with increased (∼30%) mean arterial pressure (MAP). Treatment with RTA dh404 restored MAP, increased Nrf2 and expression of its target genes, attenuated activation of NF-κB and transforming growth factor-β pathways, and reduced glomerulosclerosis, interstitial fibrosis and inflammation in the CKD rats.
4. Thus, chronic treatment with RTA dh404 was effective in restoring Nrf2 activity and slowing CKD progression in rats following 5/6 nephrectomy.
Introduction
Oxidative stress and inflammation play a critical role in the pathogenesis and progression of chronic kidney disease (CKD) (Fujihara et al., Citation2007; Manning et al., Citation2005; Quiroz et al., Citation2008; Remuzzi et al., Citation2006; Vaziri et al., Citation1998). Under physiological conditions, oxidative and electrophilic stress elicit upregulation of endogenous antioxidant and cytoprotective enzymes and proteins, which all work in concert to prevent and limit inflammation, tissue injury and dysfunction, a process controlled primarily by activation of the transcription factor nuclear factor-erythroid-2-related factor 2 (Nrf2) (Li et al., Citation2008; Wakabayashi et al., Citation2010).
Unfortunately, Nrf2 activity and expression of its target gene products are markedly reduced in the kidney in different models of CKD, including Imai rats with spontaneous focal segmental glomerulosclerosis (Kim et al., Citation2011), rats with CKD induced by 5/6 nephrectomy (Kim & Vaziri, Citation2010) and adenine-induced chronic interstitial nephropathy (Aminzadeh et al., Citation2013). Given the central role of Nrf2 in the regulation of cellular antioxidant and anti-inflammatory machinery (Yoh et al., Citation2001, Citation2008), strategies aimed at restoring Nrf2 activity are effective in preventing or hindering CKD progression (Ruiz et al., Citation2013). In fact, a number of studies have shown salutary effects of natural compounds with weak Nrf2 activating properties such as curcumin, the green tea polyphenol (−)-epigallocatechin-3-gallate, resveratrol, antroquinonol and ankaflavin, as well as the synthetic antioxidant tertbutylhydroquinone, in experimental animals with CKD of diverse etiologies (Ghosh et al., Citation2009; Lee et al., Citation2012; Li et al., Citation2011; Palsamy & Subramanian, Citation2011; Peng et al., Citation2011; Soetikno et al., Citation2013; Tapia et al., Citation2012; Tsai et al., Citation2011). As the 5/6 nephrectomy model is the one that produces pressure overload, it is worthy to note that blood pressure was lowered in the above studies which included it as a measured parameter (Ghosh et al., Citation2009; Soetikno et al., Citation2013).
Bardoxolone methyl and its analogs are oleanolic acid-derived synthetic triterpenoid compounds which potently activate the Nrf2-Keap1 pathway (Dinkova-Kostova et al., Citation2005; Liby et al., Citation2007). Via interaction with the Nrf2 repressor molecule, Keap1, bardoxolone methyl and its analogs promote translocation of Nrf2 to the nucleus, where it binds to antioxidant response elements in the promoter region of its target genes, leading to induction of many antioxidant and cytoprotective enzymes and related proteins (Ichikawa et al., Citation2009; Sporn et al., Citation2011; Yates et al., Citation2007). Bardoxolone methyl and its analogs are also potent inhibitors of the nuclear factor kappaB (NF-κB) inflammatory pathway through both direct (i.e. inhibition of IKKβ kinase activity) and indirect mechanisms (i.e. detoxification of reactive oxygen species) (Liby & Sporn, Citation2012). Previous studies have shown that bardoxolone methyl and its analog, RTA 405 (or CDDO-ethyl amide), ameliorate murine ischemic acute kidney injury and attenuate renal interstitial inflammation and fibrosis in mice with protein overload proteinuria (Wu et al., Citation2011). It is now recognized that rodent-specific adverse metabolite formation precludes chronic dosing of bardoxolone methyl in rodents, and therefore, analogs where such adverse metabolite formation does not occur must be used for longer term studies (Reisman et al., Citation2012, Citation2013). Additionally, in a phase 2, double-blind, randomized, placebo-controlled trial enrolling patients with type 2 diabetes and mild to moderate CKD, bardoxolone methyl-treatment for 52 weeks resulted in a significant increase in the estimated glomerular filtration rate (Pergola et al., Citation2011). However, due to unforeseen complications, the nature of which is currently under investigation, the BEACON trial (de Zeeuw et al., Citation2013), a large randomized, placebo-controlled, phase 3 study which had enrolled patients with type 2 diabetes and stage 4 CKD was terminated.
The specific reason for the early termination of the BEACON trial has not yet been publicly communicated. Notably, many previously conducted rodent studies with bardoxolone methyl analogs and a one-year non-human primate study demonstrated tolerability and efficacy at markedly higher doses than used in the BEACON trial (20 mg/day) (Chin et al., Citation2013; Reisman et al., Citation2012, Citation2013; Xing et al., Citation2012). Moreover, the maximum-tolerated dose described in a phase 1 oncology trial with bardoxolone methyl was 900 mg/day (Hong et al., Citation2012). Non-clinically, there are conflicting reports of tolerability to bardoxolone methyl analogs in Zucker diabetic fatty (ZDF) rats, where one study by Zoja et al. (Citation2013) demonstrated poor tolerability characterized by kidney and liver injury. In a separate report by Chin et al. (Citation2013), chronic administration of either RTA dh404 or RTA 405 was well-tolerated by ZDF rats, streptozotocin-induced type I diabetic rats, and high fat diet-induced obese mice, while also producing salutary effects. With the data presented by Chin et al. strongly questioning the interpretation and relative importance of the results published by Zoja et al., further studies investigating the tolerability of bardoxolone methyl analogs in non-clinical models are warranted. Therefore, the present study was undertaken to determine the effects of long-term (12 weeks) administration of RTA dh404 on kidney structure and function, as well as oxidative, inflammatory, fibrosis and Nrf2 pathways in Sprague-Dawley rats with CKD induced by 5/6 nephrectomy.
Methods
Materials
All reagents, unless otherwise specified, were purchased from Sigma Aldrich (St. Louis, MO). RTA dh404 was synthesized and provided by Reata Pharmaceuticals, Inc. (Irving, TX). The chemical name for RTA dh404 is CDDO-9,11-dihydro-trifluoroethyl amide (CDDO-dhTFEA).
Animals
Male Sprague-Dawley rats, weighing 225–250 g, were purchased from Harlan Laboratories (Indianapolis, IN). They were housed in a climate-controlled and light-regulated facility with 12:12-h day-night cycles. The animals were fed regular rat chow (Purina Mills, Brentwood, MO) and water ad libitum and randomly assigned to the CKD or normal control groups. The animals assigned to the CKD group were subjected to 5/6 nephrectomy by surgical resection using dorsal incisions, as described previously (Vaziri et al., Citation2007). The animals assigned to the control group were subjected to sham operation (n = 6). All surgical procedures were carried out, while the animals were under general anesthesia (ketamine/xylazine, 100/5 mg/kg, i.p.). Rats with CKD were orally administered RTA dh404 (2 mg/kg) or vehicle (sesame oil) once daily for 12 weeks (n = 9/group) starting immediately prior to surgery. The selection of the 2 mg/kg/day dose was based on a previous study where similar oral doses of the RTA dh404 analog CDDO-Im produced significant induction of Nrf2 targets in kidneys of mice and afforded significant protection from cisplatin-induced nephrotoxicity (Aleksunes et al., Citation2010). At the conclusion of the observation period, the animals were placed in metabolic cages for a 24-h urine collection. They were then anesthetized (ketamine/xylazine, 100/5 mg/kg, i.p.) and euthanized by exsanguination using cardiac puncture. The kidneys were immediately removed and processed for histological evaluation, according to standard techniques for using hematoxylin and eosin (H&E) and periodic acid Schiff (PAS)-staining, as well as western blot analyses. Serum creatinine, urine protein and hematocrit were determined using standard laboratory procedures. Blood pressure was determined three days prior to sacrifice by tail plethysmography as described previously (Vaziri et al., Citation2002). Plasma malondialdehyde (MDA) was measured by the method of Naito & Yamanaka (Citation1978). All experiments were approved by the University of California-Irvine Institutional Committee for the Use and Care of Experimental Animals.
Western blot analyses
Cytoplasmic and nuclear extracts were prepared as previously described (Kim et al., Citation2011). Target proteins in the cytoplasmic and nuclear fractions of the kidney tissue were measured by western blot analysis using the following antibodies: rabbit antibodies against rat NF-κB p65, Nrf2, Keap1, modulatory and catalytic subunits of glutamate-cysteine ligase (Gclm and Gclc), cyclooxygenase-2 (COX-2) and heme oxygenase-1 (HO-1) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against phosphorylated IκB-α (p-IκB-α; Cell Signaling Technology, Denver, CO), catalase (EMD Chemicals, Gibbstown, NJ), inducible nitric oxide synthase (iNOS), 3-Nitrotyrosine (NT) (Abcam, Cambridge, MA), α-smooth muscle actin (α-SMA), transforming growth factor-β (TGF-β), SMAD7, phosphorylated extracellular signal-regulated kinase 1/2 (ERK1/2), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Sigma-Aldrich, St. Louis, MO), P22Phox, gp91phox and Rac1 (BD Bioscience, San Jose, CA) were purchased from the cited sources. Antibodies to histone H1 (Santa Cruz Biotechnology) and GAPDH were used for measurements of the housekeeping proteins for nuclear and cytosolic target proteins, respectively.
Briefly, aliquots containing 50 μg proteins were fractionated on 8 and 4–20% Tris-glycine gel (Novex, San Diego, CA) at 120 V for 2 h and transferred to a Hybond-ECL membrane (Amersham Life Science, Arlington Heights, IL). The membrane was incubated for 1 h in blocking buffer (1 × Tris-buffered saline (TBS), 0.05% Tween-20 and 5% non-fat milk) and then overnight in the same buffer containing the given antibodies. The membrane was washed three times for 5 min in 1 × TBS, 0.05% Tween-20 before a 2-h incubation in a buffer (1 × TBS, 0.05% Tween-20 and 3% non-fat milk) containing horseradish peroxidase-linked anti-rabbit IgG and anti-mouse IgG (Amersham Life Science) at 1:1000 dilution. The membrane was washed four times and developed by autoluminography using ECL chemiluminescent reagents (Amersham Life Science).
Messenger RNA analyses
Messenger RNA was quantified, as previously described (Reisman et al., Citation2009), using the Quantigene™ Plex 2.0 assay from Affymetrix (Santa Clara, CA). A modified panel (Catalog #31177) with targets designed against the rat genome was used. Descriptions of the panels with accession numbers can be found at http://www.panomics.com. Thioredoxin reductase 1 (Txnrd1), thioredoxin 1 (Txn1) and peroxiredoxin 1 (Prdx1) were quantified. The mRNA expression data were standardized to the internal control ribosomal protein l19 (Rpl19) and presented as fold the mean vehicle control.
Statistical analysis
Data were analyzed using SPSS software version 17.0 (SPSS, Chicago, IL) to perform a one-way analysis of variance, followed by a Bonfferoni post-hoc test with significance set at p < 0.05. Data are expressed as mean ± standard error of the mean (SEM).
Results
General data
Data are summarized in . Compared with the sham control group, the vehicle-treated CKD group exhibited weight loss, proteinuria, polyuria, hypertension (evaluated as MAP) and anemia. Administration of RTA dh404 at 2 mg/kg/day resulted in significant reduction of urinary protein excretion, and amelioration of hypertension and anemia in the treated CKD animals. This was associated with significant reduction in plasma lipid peroxidation product, MDA, denoting amelioration of oxidative stress. No significant differences were observed in body weight, serum creatinine or urine volume between the vehicle-treated CKD rats and the RTA dh404-treated CKD rats. Furthermore, RTA dh404 appeared to be well-tolerated with no apparent adverse effects (e.g. clinical or macroscopic) observed (data not shown).
Table 1. General parameters.
Oxidative and inflammatory pathways
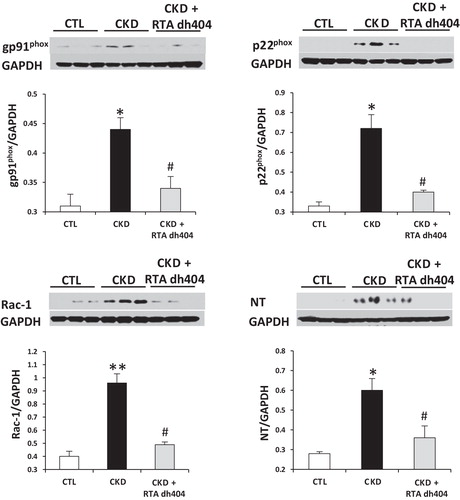
Data are shown in and . Compared with the control group, the vehicle-treated CKD group showed a significant increase in phosphorylated IκB and elevated nuclear content of p65 subunit, indicating activation of NF-κB in kidney. This was accompanied by significant upregulation of the NADPH oxidase subunits (i.e. gp91phox, p22phox and Rac1), COX-2, and iNOS, and accumulation of NT, a biomarker of oxidative/nitrosative stress. Administration of RTA dh404 was associated with attenuated NF-κB activity, reduced expression of NADPH oxidase subunits, COX-2, and iNOS, and lowered kidney tissue NT abundance in rats with CKD.
Figure 1. Representative western blots and group data depicting protein abundance of phospho-IκB and nuclear contents of p65 active subunit of NF-κB, iNOS and COX-2 in the renal tissues of sham-operated control (CTL; n = 6) and a 5/6 nephrectomized rat CKD treated with vehicle (CKD; n = 9) or RTA dh404 (CKD + RTA dh404; n = 9); *p < 0.05 versus CTL, #p < 0.05 versus CKD.
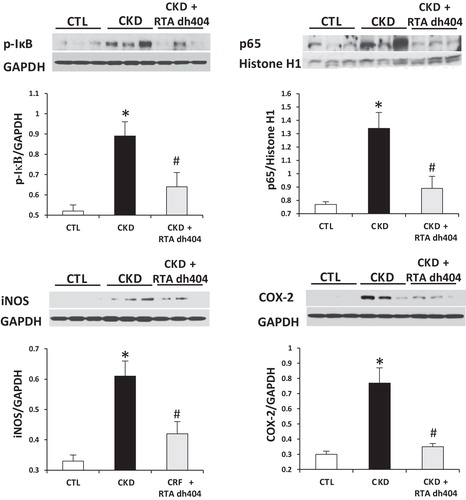
Figure 2. Representative western blots and group data depicting protein abundance of NAD(P)H oxidase subunits (p22phox, gp91phox and rac1) and NT in the renal tissues of sham-operated control (CTL; n = 6) and a 5/6 nephrectomized rat CKD treated with vehicle (CKD; n = 9) or RTA dh404 (CKD + RTA dh404; n = 9); *p < 0.05 versus CTL, **p < 0.01 versus CTL, #p < 0.05 versus CKD.
Nrf2/Keap1 pathway
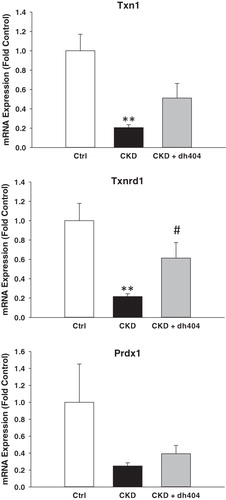
Data are shown in and . In confirmation of our earlier studies (Kim & Vaziri, Citation2010; Kim et al., Citation2011), the nuclear Nrf2 content was markedly reduced and Keap1 abundance was significantly increased in the kidneys of the vehicle-treated CKD rats, as compared with those found in the control animals. This was associated with significant down-regulation of Nrf2 target gene products, including protein expression of catalase, HO-1, Gclc, and Gclm and mRNA expression of Txn1, Txnrd1 and Prdx1 (p < 0.1) in kidneys of vehicle-treated CKD rats, indicating impaired Nrf2 activation in this model. Administration of RTA dh404 to rats with CKD was associated with increased nuclear Nrf2 abundance, decreased Keap1 abundance and increased protein expression of catalase, HO-1, Gclc and Gclm. The mRNA expression of Txnrd1 was significantly increased, while Txn1 and Prdx1 tended to be increased in kidneys of CKD rats treated with RTA dh404 compared with CKD rats treated with vehicle.
Figure 3. Representative western blots and group data depicting protein abundance of Nrf2, Keap1 and Nrf2 downstream gene products: catalase, HO-1 and catalytic (GCLC) and modulatory (GCLM) subunits of glutamate-cysteine ligase in the renal tissues of sham-operated control (CTL; n = 6) and a 5/6 nephrectomized rat CKD treated with vehicle (CKD; n = 9) or RTA dh404 (CKD + RTA dh404; n = 9); *p < 0.05 versus CTL, **p < 0.01 versus CTL, #p < 0.05 versus CKD.
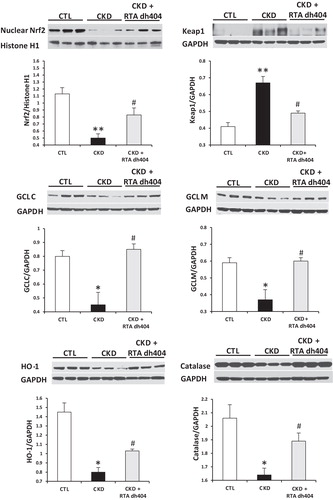
Figure 4. Messenger RNA expression of peroxiredoxin 1 (Prdx1), thioredoxin 1 (Txn1) and thioredoxin reductase 1 (Txnrd1) in the renal tissues of sham-operated control (CTL; n = 6) and a 5/6 nephrectomized rat CKD treated with vehicle (CKD; n = 9) or RTA dh404 (CKD + RTA dh404; n = 9); **p < 0.01 versus CTL, #p < 0.05 versus CKD.
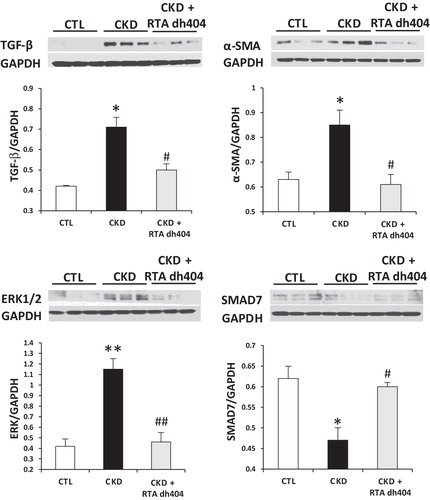
TGF-β, α-SMA, SMAD7 and ERK1/2 protein expression
Data are illustrated in . Compared with the control rats, the vehicle-treated CKD group showed marked increase in TGF-β, ERK1/2 and α-SMA abundance, and a significant decrease in the SMAD7 abundance in the remnant kidney. These abnormalities were mitigated by RTA dh404 administration to rats with CKD.
Figure 5. Representative western blots and group data depicting protein abundance of TGF-β, α-SMA, extracellular signal-regulated kinase 1/2 (ERK 1/2) and SMAD7 in the renal tissues of sham-operated control (CTL; n = 6) and a 5/6 nephrectomized rat CKD treated with vehicle (CKD; n = 9) or RTA dh404 (CKD + RTA dh404; n = 9); *p < 0.05 versus CTL, **p < 0.01 versus CTL, #p < 0.05 versus CKD, ##p < 0.01 versus CKD.
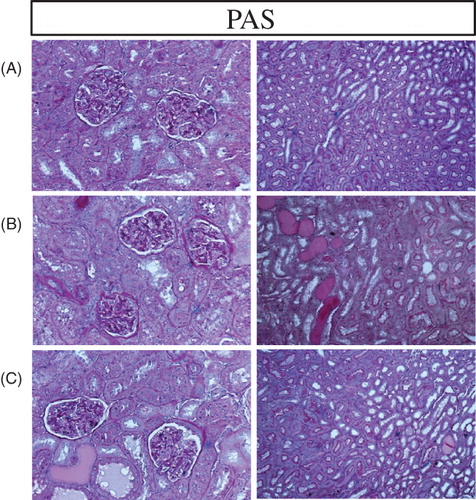
Histological data
Representative photomicrographs of kidney histology are presented in and , where similar results extended to all animals in each group. As expected the vehicle-treated CKD rats exhibited marked interstitial fibrosis and leukocyte accumulation, tubular atrophy and dilatation, and glomerular sclerosis. Long-term therapy with RTA dh404 attenuated interstitial inflammation and fibrosis and glomerulosclerosis in rats with CKD.
Figure 6. Representative photomicrographs of the H&E stained renal tissue (20X) in a sham-operated control (A) and a 5/6 nephrectomized rat (CKD)] treated with vehicle (B) or RTA dh404 (C). The remnant kidney in the CKD animals (B) exhibited significant glomerulosclerosis, tubulo-interstitial injury and heavy inflammatory cell infiltration. RTA dh404 treatment-reduced inflammatory cell infiltration and glomerular and tubulo-interstitial injury (C).
![Figure 6. Representative photomicrographs of the H&E stained renal tissue (20X) in a sham-operated control (A) and a 5/6 nephrectomized rat (CKD)] treated with vehicle (B) or RTA dh404 (C). The remnant kidney in the CKD animals (B) exhibited significant glomerulosclerosis, tubulo-interstitial injury and heavy inflammatory cell infiltration. RTA dh404 treatment-reduced inflammatory cell infiltration and glomerular and tubulo-interstitial injury (C).](/cms/asset/ce72df2c-e8a6-483d-b4cb-dc8ebdcda8c8/ixen_a_852705_f0006_b.jpg)
Figure 7. Representative photomicrographs of the PAS-stained renal tissue in a sham-operated control (A) and a 5/6 nephrectomized rat (CKD) treated with vehicle (B) or RTA dh404 (C). The remnant kidney in the CKD animals exhibited significant fibrosis (B) which was reduced with RTA dh404 treatment (C). 20 × magnification.
Discussion
As expected, the vehicle-treated CKD rats employed in the present study exhibited heavy interstitial infiltration of inflammatory cells, interstitial fibrosis, tubular atrophy and dilatation, and glomerulosclerosis. This was accompanied by activation of NF-κB, the master regulator of the inflammatory cascade, upregulation of NAD(P)H oxidase, COX-2, and iNOS, and accumulation of NT, pointing to oxidative stress and inflammation in the remnant kidney tissue. Interstitial fibrosis and glomerulosclerosis in the vehicle-treated CKD rats were associated with upregulation of pro-fibrotic mediators, TGF-β and α-SMA, and down-regulation of anti-fibrosis factor, SMAD7. These cellular and structural deficits resulted in increased serum creatinine (SCr), proteinuria and MAP.
In confirmation of previous findings (Kim & Vaziri, Citation2010), oxidative stress and inflammation in the remnant kidney tissue was associated with impaired activation of Nrf2 and depressed expression of its key target gene products in the remnant kidneys of the vehicle-treated rats with CKD. In fact, nuclear Nrf2 content was markedly reduced, tissue abundance of Nrf2 repressor molecule, Keap1, was significantly elevated, and expression of the Nrf2 target gene products, catalase, HO-1, Gclc Gclm, Txn1, Txnrd1 and Prdx1 was reduced in the vehicle-treated rats with CKD. These findings support the hypothesis that defective Nrf2 function contributes to the pathogenesis of oxidative stress and inflammation and progression of kidney disease. This supposition is also supported by the occurrence of renal disease in Nrf2-deficient mice and the reported salutary action of natural compounds with Nrf2 activating properties on progression of kidney disease of various etiologies in experimental animals (Ghosh et al., Citation2009; Lee et al., Citation2012; Li et al., Citation2011; Palsamy & Subramanian, Citation2011; Peng et al., Citation2011; Ruiz et al., Citation2013; Soetikno et al., Citation2013; Tapia et al., Citation2012; Tsai et al., Citation2011).
The synthetic triterpenoid, bardoxolone methyl, is a potent activator of the Nrf2-Keap1 pathway (Liby et al., Citation2007; Sporn et al., Citation2011; Yates et al., Citation2007). Via interaction with Keap1, this compound and its analogs interfere with binding of Nrf2 by Keap1, thereby enhancing nuclear translocation of Nrf2 and up-regulation of its target genes encoding antioxidant and cytoprotective molecules (Dinkova-Kostova et al., Citation2005; Liby et al., Citation2007; Sporn et al., Citation2011). Short-term administration of bardoxolone methyl attenuates ischemia-reperfusion-induced acute kidney injury in rats and reduces renal interstitial inflammation and fibrosis in mice with protein overload proteinuria (Wu et al., Citation2011). Given the role of Nrf2 dysfunction in the pathogenesis of oxidative stress and inflammation, which are the driving force behind progression of CKD, it seemed plausible that long-term administration of the potent Nrf2 activator and bardoxolone methyl analog RTA dh404 might prevent or retard progression of kidney disease by restoring Nrf2 activity.
Treatment of CKD rats with RTA dh404 restored Nrf2 protein expression, which was associated with Keap1 and target gene expression more similar to controls that underwent sham surgery only. RTA dh404 also attenuated activation of pro-inflammatory NF-κB and pro-fibrotic TGF-β pathways, which is consistent with the known inhibitory effects of bardoxolone methyl and its analogs on NF-κB (Liby & Sporn, Citation2012). Collectively, restoration of Nrf2 function and inhibition of NF-κB activation was associated with reduced glomerulosclerosis, interstitial fibrosis and inflammation in the CKD rats.
Overall, this study revealed that long-term administration of the RTA dh404, at a dose of 2 mg/kg/day, yielded salutary effects in the 5/6 nephrectomy rat model. In summary, long-term administration of the bardoxolone methyl analog, RTA dh404, resulted in attenuation of oxidative stress, inflammation and fibrosis in the remnant kidneys of rats with CKD induced by 5/6 nephrectomy, which was accompanied by an increase or a rescue of Nrf2 and its target genes, and decreased activation and expression of NF-κB and its target genes.
Declaration of interest
S.A. Reisman and C.J. Meyer are employed by and have a financial interest in Reata Pharmaceuticals, Inc. M.A. Aminzadeh, N.D. Vaziri, J. Yuan and M. Khazaeli have received an unrestricted research grant from Reata Pharmaceuticals, Inc.
References
- Aleksunes LM, Goedken MJ, Rockwell CE, et al. (2010). Transcriptional regulation of renal cytoprotective genes by Nrf2 and its potential use as a therapeutic target to mitigate cisplatin-induced nephrotoxicity. J Pharmacol Exp Ther 335:2–12
- Aminzadeh MA, Nicholas SB, Norris KC, et al. (2013). Role of impaired Nrf2 activation in the pathogenesis of oxidative stress and inflammation in chronic tubulo-interstitial nephropathy. Nephrol Dial Transplant 28:2038–45
- Chin M, Lee CY, Chuang JC, et al. (2013). Bardoxolone methyl analogs RTA 405 and dh404 are well tolerated and exhibit efficacy in rodent models of Type 2 diabetes and obesity. Am J Physiol Renal Physiol 304:F1438–46
- de Zeeuw D, Akizawa T, Agarwal R, et al. (2013). Rationale and trial design of bardoxolone methyl evaluation in patients with chronic kidney disease and type 2 diabetes: the occurrence of renal events (BEACON). Am J Nephrol 37:212–22
- Dinkova-Kostova AT, Liby KT, Stephenson KK, et al. (2005). Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci USA 102:4584–9
- Fujihara CK, Antunes GR, Mattar AL, et al. (2007). Chronic inhibition of nuclear factor-kappaB attenuates renal injury in the 5/6 renal ablation model. Am J Physiol Renal Physiol 292:F92–9
- Ghosh SS, Massey HD, Krieg R, et al. (2009). Curcumin ameliorates renal failure in 5/6 nephrectomized rats: role of inflammation. Am J Physiol Renal Physiol 296:F1146–57
- Hong DS, Kurzrock R, Supko JG, et al. (2012). A phase I first-in-human trial of bardoxolone methyl in patients with advanced solid tumors and lymphomas. Clin Cancer Res 18:3396–406
- Ichikawa T, Li J, Meyer CJ, et al. (2009). Dihydro-CDDO-trifluoroethyl amide (dh404), a novel Nrf2 activator, suppresses oxidative stress in cardiomyocytes. PLoS ONE 4:e8391
- Kim HJ, Vaziri ND. (2010). Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol 298:F662–71
- Kim HJ, Sato T, Rodriguez-Iturbe B, et al. (2011). Role of intrarenal angiotensin system activation, oxidative stress, inflammation, and impaired nuclear factor-erythroid-2-related factor 2 activity in the progression of focal glomerulosclerosis. J Pharmacol Exp Ther 337:583–90
- Lee BH, Hsu WH, Chang YY, et al. (2012). Ankaflavin: a natural novel PPARgamma agonist upregulates Nrf2 to attenuate methylglyoxal-induced diabetes in vivo. Free Radic Biol Med 53:2008–16
- Liby KT, Sporn MB. (2012). Synthetic oleanane triterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol Rev 64:972–1003
- Liby KT, Yore MM, Sporn MB. (2007). Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer 7:357–69
- Li H, Zhang L, Wang F, et al. (2011). Attenuation of glomerular injury in diabetic mice with tert-butylhydroquinone through nuclear factor erythroid 2-related factor 2-dependent antioxidant gene activation. Am J Nephrol 33:289–97
- Li W, Khor TO, Xu C, et al. (2008). Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol 76:1485–9
- Manning RD Jr, Tian N, Meng S. (2005). Oxidative stress and antioxidant treatment in hypertension and the associated renal damage. Am J Nephrol 25:311–17
- Naito C, Yamanaka T. (1978). Lipid peroxides in atherosclerotic diseases (author’s transl). Nihon Ronen Igakkai Zasshi 15:187–91
- Palsamy P, Subramanian S. (2011). Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochim Biophys Acta 1812:719–31
- Peng A, Ye T, Rakheja D, et al. (2011). The green tea polyphenol (−)-epigallocatechin-3-gallate ameliorates experimental immune-mediated glomerulonephritis. Kidney Int 80:601–11
- Pergola PE, Raskin P, Toto RD, et al. (2011). Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med 365:327–36
- Quiroz Y, Ferrebuz A, Romero F, et al. (2008). Melatonin ameliorates oxidative stress, inflammation, proteinuria, and progression of renal damage in rats with renal mass reduction. Am J Physiol Renal Physiol 294:F336–44
- Reisman SA, Chertow GM, Hebbar S, et al. (2012). Bardoxolone methyl decreases megalin and activates nrf2 in the kidney. J Am Soc Nephrol 23:1663–73
- Reisman SA, Ward KW, Klaassen CD, et al. (2013). CDDO-9,11-dihydro-trifluoroethyl amide (CDDO-dhTFEA) induces hepatic cytoprotective genes and increases bile flow in rats. Xenobiotica 43:571–8
- Reisman SA, Yeager RL, Yamamoto M, et al. (2009). Increased Nrf2 activation in livers from Keap1-knockdown mice increases expression of cytoprotective genes that detoxify electrophiles more than those that detoxify reactive oxygen species. Toxicol Sci 108:35–47
- Remuzzi G, Benigni A, Remuzzi A. (2006). Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest 116:288–96
- Ruiz S, Pergola PE, Zager RA, et al. (2013). Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int 83:1029–41
- Soetikno V, Sari FR, Lakshmanan AP, et al. (2013). Curcumin alleviates oxidative stress, inflammation, and renal fibrosis in remnant kidney through the Nrf2-keap1 pathway. Mol Nutr Food Res 57:1649–59
- Sporn MB, Liby KT, Yore MM, et al. (2011). New synthetic triterpenoids: potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J Nat Prod 74:537–45
- Tapia E, Soto V, Ortiz-Vega KM, et al. (2012). Curcumin induces Nrf2 nuclear translocation and prevents glomerular hypertension, hyperfiltration, oxidant stress, and the decrease in antioxidant enzymes in 5/6 nephrectomized rats. Oxid Med Cell Longev 2012:269039
- Tsai PY, Ka SM, Chao TK, et al. (2011). Antroquinonol reduces oxidative stress by enhancing the Nrf2 signaling pathway and inhibits inflammation and sclerosis in focal segmental glomerulosclerosis mice. Free Radic Biol Med 50:1503–16
- Vaziri ND, Bai Y, Ni Z, et al. (2007). Intra-renal angiotensin II/AT1 receptor, oxidative stress, inflammation, and progressive injury in renal mass reduction. J Pharmacol Exp Ther 323:85–93
- Vaziri ND, Ni Z, Oveisi F, et al. (2002). Enhanced nitric oxide inactivation and protein nitration by reactive oxygen species in renal insufficiency. Hypertension 39:135–41
- Vaziri ND, Oveisi F, Ding Y. (1998). Role of increased oxygen free radical activity in the pathogenesis of uremic hypertension. Kidney Int 53:1748–54
- Wakabayashi N, Slocum SL, Skoko JJ, et al. (2010). When NRF2 talks, who’s listening? Antioxid Redox Signal 13:1649–63
- Wu QQ, Wang Y, Senitko M, et al. (2011). Bardoxolone methyl (BARD) ameliorates ischemic AKI and increases expression of protective genes Nrf2, PPARgamma, and HO-1. Am J Physiol Renal Physiol 300:F1180–92
- Xing Y, Niu T, Wang W, et al. (2012). Triterpenoid dihydro-CDDO-trifluoroethyl amide protects against maladaptive cardiac remodeling and dysfunction in mice: a critical role of Nrf2. PLoS ONE 7:e44899
- Yates MS, Tauchi M, Katsuoka F, et al. (2007). Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol Cancer Ther 6:154–62
- Yoh K, Hirayama A, Ishizaki K, et al. (2008). Hyperglycemia induces oxidative and nitrosative stress and increases renal functional impairment in Nrf2-deficient mice. Genes Cells 13:1159–70
- Yoh K, Itoh K, Enomoto A, et al. (2001). Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int 60:1343–53
- Zoja C, Corna D, Nava V, et al. (2013). Analogs of bardoxolone methyl worsen diabetic nephropathy in rats with additional adverse effects. Am J Physiol Renal Physiol 304:F808–19

