Abstract
Purpose: The purpose of this study was to monitor tumour blood flow with power Doppler ultrasound following antiangiogenic therapy with bevacizumab in order to optimally time the application of radiofrequency (RF) ablation to increase ablation diameter.
Materials and methods: Athymic nude mice bearing human hepatocellular carcinoma xenografts were treated with bevacizumab and imaged daily with power Doppler ultrasound to quantify tumour blood flow. Mice were treated with RF ablation alone or in combination with bevacizumab at the optimal time, as determined by ultrasound. Ablation diameter was measured with histology and tumour microvascular density was calculated with immunohistochemistry. A computational thermal model of RF ablation was used to estimate ablation volume.
Results: A maximum reduction of 27.8 ± 8.6% in tumour blood flow occurred on day 2 following antiangiogenic therapy, while control tumours increased 29.3 ± 17.1% (p < 0.05). Tumour microvascular density was similarly reduced by 45.1 ± 5.9% on day 2 following antiangiogenic therapy. Histology demonstrated a 13.6 ± 5.6% increase in ablation diameter (40 ± 21% increase in volume) consistent with a computational model.
Conclusion: Quantitative power Doppler ultrasound is a useful biomarker to monitor tumour blood flow following antiangiogenic treatment and to guide the application of RF ablation as a drug plus device combination therapy.
Introduction
Radiofrequency (RF) ablation is a US Food and Drug Administration (FDA)-cleared device for the cauterisation of soft tissue and is used to treat primary and secondary solid tumours in various locations including liver, kidney, bone, and lung Citation[1–5]. Percutaneous RF ablation of hepatic tumours has demonstrated impressive 5-year local control rate for lesions <3 cm of approximately 85% Citation[1], Citation[5–7]. However, RF ablation's ability to treat large tumours (>3 cm diameter) has been limited Citation[1], Citation[5–7], in part due to blood perfusion mediated cooling. This includes cooling by large vessels (>3 mm) which causes local ablation zone deviations (‘heat sink effect’) Citation[8], Citation[9] that are often the cause of perivascular tumour recurrence Citation[7], Citation[10], as well as microvascular perfusion cooling that affects overall ablation zone size and shape Citation[11]. This phenomenon represents a significant drawback when treating highly vascular tumours such as hepatocellular carcinoma (HCC) of the liver. To overcome the effects of vascular mediated cooling, more powerful RF generators may be used Citation[12], Citation[13], while pharmacological manipulation, such as antiangiogenic and antivascular agents, can limit tumour perfusion Citation[14].
Angiogenesis describes the formation of new blood vessels from existing blood vessels Citation[15–18]. The goal of antiangiogenic agents is to limit the growth of new blood vessels while antivascular agents, also known as vascular disrupting agents, simply destroy the existing tumour vasculature and often result in drastic reductions in tumour perfusion Citation[19–22]. The antiangiogenic agent bevacizumab (Avastin®), a humanised monoclonal antibody against vascular endothelial growth factor (VEGF), is FDA approved for the treatment of metastatic colorectal and kidney cancers, as well as non-small cell lung cancer, and glioblastoma only in combination with other therapies Citation[23–26]. Bevacizumab is also gaining popularity in novel combination therapies Citation[18], Citation[24], Citation[27]. An emerging theory in antiangiogenic therapy suggests that bevacizumab and similar agents may transiently ‘normalise’ the abnormal structure and function of tumour vasculature, improving the delivery of oxygen and drugs for a brief window of time Citation[28], Citation[29] and potentially increasing tumour blood flow. Improved blood flow associated within the normalisation window may theoretically decrease the ablation diameter by promoting dissipation of local heat.
Goldberg and colleagues have used both the antiangiogenic and antiproliferative agent sorafenib Citation[30], Citation[31] and the antivascular agent arsenic trioxide in animals Citation[14], Citation[32] to limit tumour perfusion and increase ablation diameter. Although it was demonstrated that applying antiangiogenic/antivascular therapy to reduce tumour perfusion improved tumour ablation diameters, the optimum timing between applying pharmacologic therapy and ablation is unknown. The various effects of antiangiogenic/antivascular therapy, i.e. vascular normalisation and enhanced perfusion versus classical antiangiogenesis and reduced perfusion, require a surrogate method to monitor changes in blood flow during a treatment to determine the optimal time, or minimum level of blood flow, to initiate treatment with RF ablation. Furthermore, the ability to monitor tumour perfusion in a cost-effective and clinically meaningful manner has not been clearly established. Numerous imaging modalities sensitive to tumour perfusion have the potential to provide essential insight on timing and implementation of this combination therapy. Dynamic positron emission tomography (PET) with [15O]H2O is perhaps, ‘the gold standard’ of perfusion imaging analysis techniques Citation[33] but is challenging to implement in all hospitals. Multi-detector computed tomography (MDCT) is suitable to quantify tumour perfusion Citation[31], Citation[34], but may require excessive radiation exposure when performed over the course of antiangiogenic/antivascular treatment (hours to weeks). Magnetic resonance imaging (MRI) Citation[34–38] and Doppler ultrasound Citation[34], Citation[39–42] or dynamic contrast enhanced ultrasound Citation[43] are alternative modalities to quantify tumour perfusion without excessive radiation exposure associated with CT.
The purpose of this study was to monitor tumour blood flow changes with power Doppler ultrasound following antiangiogenic treatment with bevacizumab in a human HCC xenograft model in mice. Changes in microvascular density (MVD) and tumour vascular architecture with bevacizumab therapy were also quantified. Finally, ablations were performed at the estimated minimum in tumour perfusion following antiangiogenic therapy, and ablation diameters were compared to untreated controls.
Materials and methods
Animal studies were conducted under a protocol approved by the National Institutes of Health Clinical Center Animal Care and Use Committee. These experiments were separated into two sequential parts: 1) Initial assessment of tumour blood flow with power Doppler ultrasound imaging for 1 week following antiangiogenic therapy with bevacizumab to determine the minimum blood flow, and 2) RF ablation at the minima of blood flow as ascertained from part 1. Histological analyses were performed to determine tumour microvasculature changes following antiangiogenic therapy and to measure ablation diameters following RF ablation. A heat transfer model was used to quantify changes in ablation volume as a function of tumour perfusion. Additional details may be found in the supplementary information.
Tumour cell line and animal model
Human HCC (HepG2) cells were purchased from American Type Culture Collection (ATCC, Manassas, VA). Cells were grown in Eagle's Minimum Essential Medium (ATCC) supplemented with 10% fetal bovine serum and penicillin/streptomycin. The cell line was maintained in a 5% CO2 incubator at 37°C. Athymic nude mice (BALB/c nu/nu) were inoculated with 100 µL of tumour cell suspension (5 × 106 cells). When the tumour xenografts reached a minimum of 8 mm in the shortest dimension, mice were treated with a single dose of 100 µg bevacizumab or saline via tail vein injection. The weight and condition of the mice were monitored daily, and tumour size was measured with a digital caliper (length and width).
Power Doppler
Tumours were imaged using a VisualSonics Vevo 770 ultrasound (Toronto, Ontario) at 0 h, 6 h, 1 day, 2 days, 3 days, 4 days, 5 days, 6 days, and 7 days post-treatment. Prior to and during imaging, mice were anaesthetised with ∼2% isoflurane as needed with a mixture of air and oxygen. The ultrasound transducer used for power Doppler imaging (RMV708) transmitted a central frequency of 55 MHz with a 4.5 mm focal length. The entire tumour xenograft was imaged with the aid of a z-motor (step size = 0.1 mm) using the power Doppler mode with consistent gain (30.00 dB), velocity (slow), wall filter (0.8 mm/s), and scan speed (0.5 mm/s). Raw image data was processed using custom MATLAB scripts (Mathworks, Natick, MA). These scripts were employed to 1) separate B-mode ultrasound and power Doppler data, 2) manually identify and draw the region of interest (ROI) to select tumour area, and 3) quantify vascular parameters within specified ROIs. Vascular parameters were obtained from the power Doppler images by thresholding with a consistent value to isolate functionally perfused area. Regions deeper than 7.2 mm (240 lines) were not included in the analysis due to a lack of signal at these depths. Vascular parameters used to estimate blood flow were mean colour level (MCL) and vessel fractional area (FA). The MCL is the average intensity of the Doppler signal above the consistent threshold chosen to isolate vascular structures within a tumour ROI. The FA is the fraction of a tumour ROI that contained perfused area as identified by positive Doppler signal. The product of MCL and FA, or the colour-weighted fractional area (CWFA), was averaged over the tumour volume and used as an approximation of tumour blood flow for each animal. Each animal was treated as an independent sample and data are presented as mean ± SEM (n = 5–6).
Radiofrequency ablation
Percutaneous RF ablation was administered with the goal of producing partial ablation resulting in a thermal lesion equal to approximately half of the tumour volume. Briefly, a 22-gauge RF ablation needle with a 5-mm active tip (Radionics, Burlington, MA) was used to create an ablation in the centre of the tumour (90°C for 50 s).
Ablation histological analysis
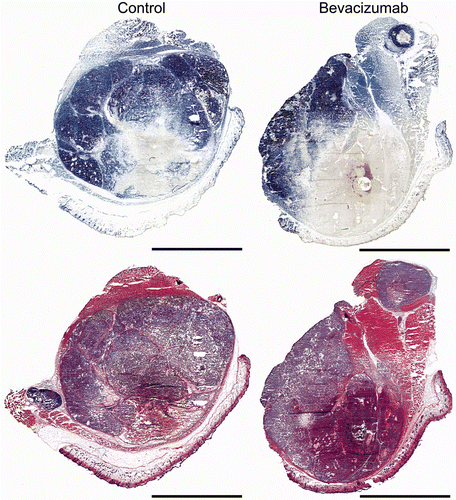
Briefly, a nitroblue tetrazolium (NBT) staining protocol was used to identify viable (blue) and non-viable (white/clear) tissue to quantify ablation diameter. The slides were imaged using brightfield illumination (Zeiss, Axio Imager.M1, Thornwood, NY) with a motorised scanning stage and mosaic stitching software (Axiovision, Zeiss). The ablation diameter was quantified by selecting for analysis the three largest ablation zones in continuous regions for each tumour. Correction for tissue shrinkage due to fixation was not applied. Each tumour was considered an independent sample and ablation diameters are reported as individual data points (n = 5–7).
Microvascular analysis
Animals were euthanised at day 0, 2 days, and 7 days and tumours were harvested and flash-frozen using liquid nitrogen. The tumour endothelial cells were identified with an anti-CD31 immunohistochemistry staining. Vascular properties were determined including MVD, blood vessel size, tumour area, and parameters that describe vascular architecture such as median distance from a tumour pixel to the nearest vascular surface. The median distance was calculated by a 2D Euclidean distance transform of a binary blood vessel mask. A smaller median distance indicates a more favourable transport environment Citation[44], Citation[45]. The microvascular analysis was performed with a custom script in MATLAB.
Heat transfer model
A thermal model of a needle electrode immersed in tumour with axi-symmetric geometry was created. The model included temperature-dependent electrical and thermal tissue properties including perfusion similar to previous studies Citation[9], Citation[11]. A parametric study was performed where absolute perfusion was varied in the range typical for both normal liver and liver tumours (0–1.4 mL/min/mL) Citation[11], Citation[46], Citation[47] where constant power was applied to obtain 90°C for a 22-gauge needle electrode with a 5-mm active tip (50 s ablation, similar to in vivo experiments) or a 17-gauge cooled needle electrode with 3-cm active tip (12 min ablation, similar to clinical practice). Ablation zone diameter was defined as tissue regions with temperature above 50°C. Prior ablation studies have demonstrated that 50°C provides an adequate estimate of the threshold for necrosis after ablation Citation[48], Citation[49], and prior modelling studies have employed this threshold temperature to estimate ablation zone dimensions Citation[50], Citation[51].
Statistics
All statistical analyses were performed in GraphPad Prism 5 (GraphPad Software, La Jolla, CA). The power Doppler CWFA are compared with a two-way repeated measures ANOVA (Factors: time and treatment) and comparison between the two groups at each time point were made with a t test. MVD and median distance were analysed with a one-way ANOVA and Student-Newman-Keuls (SNK) post-hoc test between multiple groups. Ablation diameters were compared with a t test. Data are reported as mean ± SEM. P values less than 0.05 were considered statistically significant and all statistical tests were two-sided.
Results
Power Doppler ultrasound imaging
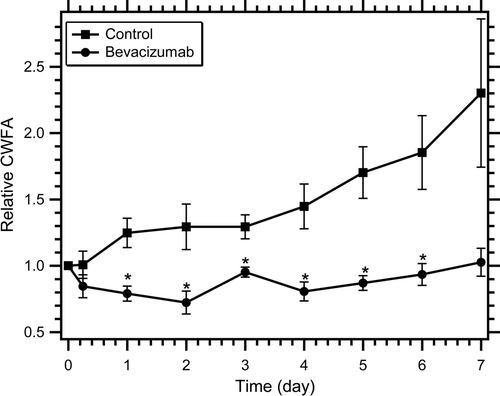
The tumour blood flow was investigated with power Doppler ultrasound over 1 week as shown in . The Doppler signal above a consistent but arbitrary threshold is designated as tumour blood flow indicated by red colour overlaid on a B-mode image shown in greyscale. Although the MCL was relatively constant for both groups over time, the vessel FA increased for the control animals over 1 week and remained constant or decreased for the bevacizumab-treated animals. The relative tumour blood flow is probably best described by the CWFA that accounts for both velocity and blood volume Citation[52] as shown in . The bevacizumab treatment had a significant effect on CWFA (P value < 0.05, ANOVA). Control animals demonstrated a steady increase in CWFA over 1 week while bevacizumab animals demonstrated an immediate decrease after only 6 h, although not significant (P value > 0.05, t test). CWFA in bevacizumab-treated animals decreased 27.8 ± 8.6% on day 2 to a minimum level, then subsequently returned to pretreatment levels for the remainder of the observation period. In contrast to bevacizumab-treated animals, CWFA of control tumours increased 29.3 ± 17.1% on day 2. The CWFA of bevacizumab-treated animals was significantly lower (P value < 0.05, t test) than control animals from day 1 to day 6.
Figure 1. Power Doppler ultrasound images of tumours treated with saline (control) and bevacizumab at 0 h, 2 days, and 7 days after administration. Representative images are shown at a similar location for the same tumour with B-mode images (greyscale) overlaid with power Doppler (red). Doppler signal above an arbitrary threshold is only shown for clarity. Power Doppler images represent a relative tumour blood flow.
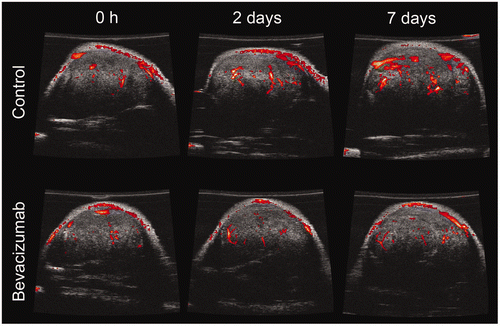
Figure 2. Colour-weighted fractional area (CWFA) indicating relative tumour blood flow for control and bevacizumab-treated animals over 1 week. Animals were treated with saline (control) or bevacizumab at day 0 and all data were normalised by the day 0 value. CWFA approximates tumour blood flow. Data are shown as mean ± SEM (n = 5–6) and * indicates P value < 0.05.
Microvascular analysis
Tumour perfusion in macroscopic blood vessels, detected by power Doppler ultrasound, was compared to microvascular changes, investigated by immunohistochemistry, as shown in . CD31 staining of endothelial cells in whole tumour sections is shown in the top row of , demonstrating the distribution of blood vessels throughout the entire tumour. The tumour appears to grow in a lobular fashion with a greater density of blood vessels surrounding the tumour lobules and periphery of the tumour. The MVD may be better appreciated at higher magnification as shown in the second row of . As expected following bevacizumab therapy, a qualitative decrease in MVD is observed for bevacizumab-treated animals at days 2 and 7. The MVD was quantified in whole tumour sections and shown in A. Bevacizumab treatment significantly reduced the MVD at days 2 and 7 versus control tumours (P value < 0.05, ANOVA; P value < 0.05, SNK) corresponding to a reduction of 33.1 ± 7.2 and 32.4 ± 5.4%, respectively.
Figure 3. Microvascular analysis of control and bevacizumab-treated tumours. Top row: Whole tumour sections with CD31 immunohistochemistry identifying endothelial cells (red) and DAPI indicating tumour nuclei (blue). Middle row: Higher magnification images of tumour sections shown in the top row. Bottom row: Distance maps indicating the distance between a tumour cell and the nearest vascular surface. Large distances (up to 200 µm) are shown in red while short distances are shown in blue. Bar is 2 mm for top and bottom row and 200 µm for middle row.
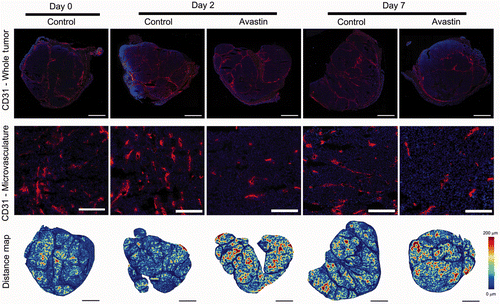
Figure 4. Microvascular density (A) and median distance from a tumour cell to the nearest vascular surface (B) calculated from whole tumour sections for control and bevacizumab-treated tumours. Data are shown as mean ± SEM (n = 4–5) and * indicates P value < 0.05 versus control on the same day.
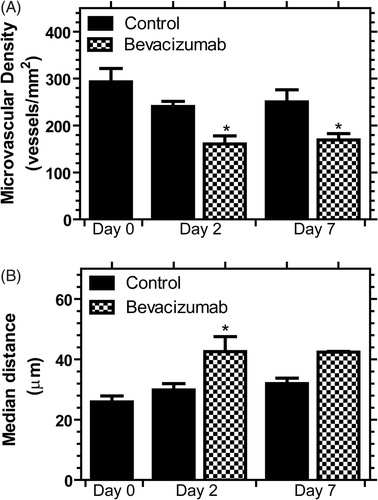
The vascular architecture may be described by distance maps, the distance a tumour cell is from the nearest vascular surface, shown in the bottom row of . Large distances shown by the red colour most likely indicate regions of poor vascular density and organisation, while blue areas are regions of better organisation and higher vascular density. The bevacizumab-treated tumours demonstrated a greater fraction of large distances suggesting a sparser and poorly organised vascular network for delivery of oxygen and drugs as well as for the removal of heat. The median distance between a tumour cell and the nearest vascular surface is shown in B and is consistent with the MVD findings of a sparser vascular network for bevacizumab-treated animals. This distance is significantly increased by 42.7 ± 16.7% (P value < 0.05, SNK) on day 2 following bevacizumab treatment suggesting that antiangiogenic therapy induced a less efficient vascular network for the removal of heat.
Tumour ablation
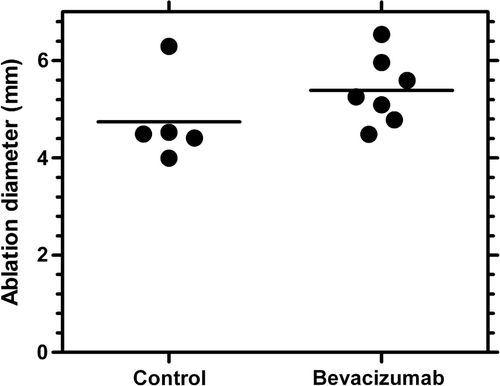
RF ablation was performed on day 2 following treatment with saline or bevacizumab, which corresponded to the minima in blood flow for the bevacizumab treatment determined by power Doppler ultrasound. The size of the ablation was determined with a cellular viability stain and shown in . In comparison to the haematoxylin and eosin (H&E) images, a well demarcated zone of necrosis, indicated by the clear area or lack of blue stain, can be easily identified with the viability stain from which the ablation diameters were determined. Although not statistically significant (P value =0.1909, t test), the ablation diameter increased 13.6 ± 5.6% (4.7 to 5.4 mm) when RF ablation was combined with bevacizumab as shown in . Assuming a spherical ablation diameter, this corresponded to a 40 ± 21% volume increase for the combined therapy.
Heat transfer model
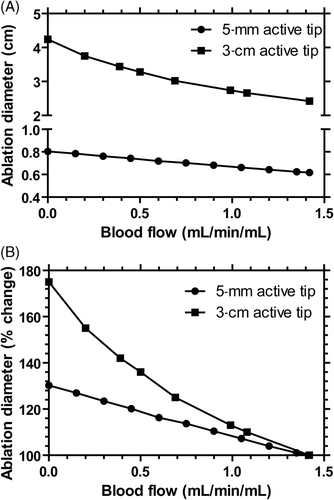
A computational thermal model was used to predict ablation diameter, and most importantly, relate perfusion changes to expected ablation diameter. For a 5-mm active tip, ablation zone diameter increased 9% from 6.1 mm to 6.6 mm when perfusion was reduced by 30% similar to the results obtained with bevacizumab. Within the examined range of perfusion values there was an approximate linear relationship between perfusion and ablation zone diameter (A). In contrast, a longer ablation of 12 min with a 3-cm active tip (common clinical practice) resulted in a non-linear increase in ablation diameter up to 75% when perfusion was eliminated, much greater than a 30% increase with a shorter ablation time (B).
Figure 7. Computational modelling of ablation diameter (A), and percentage change in ablation diameter (B) for a 5-mm and 3-cm active tip electrode. The ablation was performed for 50 s for the 5-mm active tip (similar to in vivo protocol) and for 12 min for the 3-cm active tip (similar to routine clinical practice).
Discussion
The perfect sequencing of drug plus device combination therapies in the clinic remains uncertain. It has been shown by Goldberg and colleagues as well as this study that antiangiogenic/antivascular agents produce larger ablations Citation[14], Citation[30], Citation[32]. Many techniques, including MRI and MDCT Citation[31], Citation[34–38] may be used to monitor changes in tumour blood perfusion following antiangiogenic/antivascular treatment. We chose to use power Doppler ultrasound Citation[34], Citation[39], Citation[40], Citation[42] due to its low cost, high sensitivity to flow, and ability to serially monitor tumours without radiation exposure. The predominant factor that changed in response to treatment was FA. A comparison between and demonstrates that power Doppler does not capture all tumour blood vessels but is limited to only large blood vessels due to sensitivity, voxel size, and partial volume effects. It is probable that the blood flow detected in the large blood vessels is connected with small tumour microvasculature as depicted in ; in other words, blood flows within a tumour from large blood vessels to smaller ones. In control tumours, FA increased over the course of a week as the tumour grew and blood flow detected in large blood vessels compensated by increasing size to feed a greater tumour volume and associated microvasculature. This scenario is supported by similar MVD values in the control tumours over a week. In contrast, bevacizumab treatment of tumours resulted in significantly reduced MVD leaving fewer small blood vessels for the large ones to flow into, reducing overall blood flow to the tumour as detected by power Doppler ultrasound. This suggests that power Doppler ultrasound indirectly reports on changes in the tumour microvasculature. Such a tool could also be valuable for early determination of candidate drug efficacy.
The use of antiangiogenic treatments continues to grow in popularity Citation[18], but these agents may have various effects on a tumour. It has been proposed that antiangiogenic agents may transiently ‘normalise’ the vasculature to improve the delivery of oxygen and drugs, making the tumour more suitable for chemotherapy and radiotherapy Citation[29], Citation[53]. If normalised tumour vasculature has greater perfusion, then ablation diameters may be reduced with improperly timed RF ablation. This normalisation strategy has been shown in numerous models but is dependent on agent identity, agent dose, timing, and tumour histology Citation[27], Citation[54–58]. In our study, antiangiogenic treatment reduced perfusion. Furthermore, mild hyperthermia during RF ablation in the sublethal region of the tumour may also limit microvascular density Citation[59], and in combination with bevacizumab, this hyperthermia treatment may limit tumour growth.
Vascular mediated cooling can be broadly categorised into two separate effects: 1) large vessels cooling (‘heat sink effect’) that results in deviation of the ablation zone shape near the vessel Citation[8], Citation[9] leading to potential recurrence of tumours proximal to vessels Citation[7], and 2) microvascular perfusion-mediated cooling, which affects overall size and shape of the ablation zone Citation[11]. Computational modelling suggests that a further increase in ablation diameter may be gained by longer heating or reducing the perfusion to even lower levels than here (see ), suggesting the use of antivascular or embolic agents rather than antiangiogenic agents may further increase ablation size. Another approach is the combination of RF ablation with chemotherapeutics such as liposomal doxorubicin to increase ablation size by reducing required temperature for necrosis Citation[60–65].
This study has several limitations. The preclinical models cannot capture the complexity and geometry of clinical RF ablation. The electrodes and tumour size were much smaller than in clinical practice. Furthermore, small blood vessels are predominant in this animal model, while in clinical practice large blood vessels (>3 mm) have a high potential to modify the shape of the ablation. The increase in ablation diameter with bevacizumab was not statistically significant, possibly due to a high inherent variability found in tumour blood flow. This explanation is supported by the high coefficient of variance (standard deviation/mean) in CWFA of 0.46 for all animals. The use of ultrasound microbubble contrast has the advantage of increased signal and may report on smaller diameter blood vessels, but in our hands, had greater variability with time following injection than using power Doppler without ultrasound contrast.
Quantitative power Doppler ultrasound is a useful biomarker to monitor tumour blood flow following antiangiogenic treatment and guide the optimised application of RF ablation as a drug plus device combination therapy. If a patient-specific estimate of tumour and surrounding tissue blood flow can be obtained, computational modelling may be valuable to provide an estimate of ablation diameter due to the therapy. Such personalisation of drug and device combinations might prove a rational approach, although further clinical work with antiangiogenic or antivascular agents is ongoing and could clarify this strategy.
Acknowledgements
We thank Pavel Yarmolenko and Sergio Dromi for their useful discussion. We would also like to thank James Coad for providing us with a viability staining protocol.
Declaration of interest: This research was supported in part by the Center for Interventional Oncology in the Intramural Research Program of the National Institutes of Health (NIH) and the Howard Hughes Medical Institute NIH Research Scholars Program (AAT). The authors report no other conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Friedman M, Mikityansky I, Kam A, Libutti SK, Walther MM, Neeman Z, et al. Radiofrequency ablation of cancer. Cardiovasc Intervent Radiol 2004; 27: 427–434
- Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma: Part 1, Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. Am J Roentgenol 2005; 185: 64–71
- Grenier N, Douws C, Perot V, Ferriere JM, Ravaud A. Combined radiofrequency ablation and antiangiogenic drug for the treatment of recurrent renal tumor. Urology 2009; 73: e11–12
- Hines-Peralta A, Goldberg SN. Review of radiofrequency ablation for renal cell carcinoma. Clin Cancer Res 2004; 10: S6328–6334
- Wood BJ, Ramkaransingh JR, Fojo T, Walther MM, Libutti SK. Percutaneous tumor ablation with radiofrequency. Cancer 2002; 94: 443–451
- Mulier S, Ni Y, Jamart J, Michel L, Marchal G, Ruers T. Radiofrequency ablation versus resection for resectable colorectal liver metastases: Time for a randomized trial?. Ann Surg Oncol 2008; 15: 144–157
- Mulier S, Ni YC, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation – Multivariate meta-analysis and review of contributing factors. Ann Surg 2005; 242: 158–171
- Lu DS, Raman SS, Vodopich DJ, Wang M, Sayre J, Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: Assessment of the ‘heat sink’ effect. Am J Roentgenol 2002; 178: 47–51
- Haemmerich D. Effects of micro- and macro-vascular perfusion during radiofrequency tumor ablation. Proc SPIE 2009; 7181: R1–11
- Lu DS, Yu NC, Raman SS, Limanond P, Lassman C, Murray K, et al. Radiofrequency ablation of hepatocellular carcinoma: Treatment success as defined by histologic examination of the explanted liver. Radiology 2005; 234: 954–960
- Schutt DJ, Haemmerich D. Effects of variation in perfusion rates and of perfusion models in computational models of radio frequency tumor ablation. Med Phys 2008; 35: 3462–3470
- Mertyna P, Hines-Peralta A, Liu ZJ, Halpern E, Goldberg W, Goldberg SN. Radiofrequency ablation: Variability in heat sensitivity in tumors and tissues. J Vasc Interv Radiol 2007; 18: 647–654
- Mertyna P, Dewhirst MW, Halpern E, Goldberg W, Goldberg SN. Radiofrequency ablation: The effect of distance and baseline temperature on thermal dose required for coagulation. Int J Hyperthermia 2008; 24: 550–559
- Horkan C, Ahmed M, Liu Z, Gazelle GS, Solazzo SA, Kruskal JB, et al. Radiofrequency ablation: Effect of pharmacologic modulation of hepatic and renal blood flow on coagulation diameter in a VX2 tumor model. J Vasc Interv Radiol 2004; 15: 269–274
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000; 407: 249–257
- Li WW. Tumor angiogenesis: Molecular pathology, therapeutic targeting, and imaging. Acad Radiol 2000; 7: 800–811
- Patan S, Munn LL, Jain RK. Intussusceptive microvascular growth in a human colon adenocarcinoma xenograft: A novel mechanism of tumor angiogenesis. Microvasc Res 1996; 51: 260–272
- Kerbel RS. Tumor angiogenesis. N Engl J Med 2008; 358: 2039–2049
- Siemann DW, Chaplin DJ, Horsman MR. Vascular-targeting therapies for treatment of malignant disease. Cancer 2004; 100: 2491–2499
- Stevenson JP, Rosen M, Sun W, Gallagher M, Haller DG, Vaughn D, et al. Phase I trial of the antivascular agent combretastatin A4 phosphate on a 5-day schedule to patients with cancer: Magnetic resonance imaging evidence for altered tumor blood flow. J Clin Oncol 2003; 21: 4428–4438
- Zhao D, Chang CH, Kim JG, Liu H, Mason RP. In vivo near-infrared spectroscopy and magnetic resonance imaging monitoring of tumor response to combretastatin A-4-phosphate correlated with therapeutic outcome. Int J Radiat Oncol Biol Phys 2011; 80: 574–581
- Thorpe PE. Vascular targeting agents as cancer therapeutics. Clin Cancer Res 2004; 10: 415–427
- Jenab-Wolcott J, Giantonio BJ. Bevacizumab: Current indications and future development for management of solid tumors. Expert Opin Biol Ther 2009; 9: 507–517
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350: 2335–2342
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006; 355: 2542–2550
- Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 2007; 357: 2666–2676
- Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 2004; 10: 145–147
- Huang G, Chen L. Tumor vasculature and microenvironment normalization: A possible mechanism of antiangiogenesis therapy. Cancer Biother Radiopharm 2008; 23: 661–667
- Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005; 307: 58–62
- Hakime A, Hines-Peralta A, Peddi H, Atkins MB, Sukhatme VP, Signoretti S, et al. Combination of radiofrequency ablation with antiangiogenic therapy for tumor ablation efficacy: Study in mice. Radiology 2007; 244: 464–470
- Sabir A, Schor-Bardach R, Wilcox CJ, Rahmanuddin S, Atkins MB, Kruskal JB, et al. Perfusion MDCT enables early detection of therapeutic response to antiangiogenic therapy. Am J Roentgenol 2008; 191: 133–139
- Hines-Peralta A, Sukhatme V, Regan M, Signoretti S, Liu ZJ, Goldberg SN. Improved tumor destruction with arsenic trioxide and radiofrequency ablation in three animal models. Radiology 2006; 240: 82–89
- Van der Veldt AA, Lubberink M, Bahce I, Walraven M, de Boer MP, Greuter HN, et al. Rapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: Implications for scheduling of anti-angiogenic drugs. Cancer Cell 2012; 21: 82–91
- Drevs J, Schneider V. The use of vascular biomarkers and imaging studies in the early clinical development of anti-tumour agents targeting angiogenesis. J Intern Med 2006; 260: 517–529
- Miller JC, Pien HH, Sahani D, Sorensen AG, Thrall JH. Imaging angiogenesis: Applications and potential for drug development. J Natl Cancer Inst 2005; 97: 172–187
- Padhani AR. MRI for assessing antivascular cancer treatments. Br J Radiol 2003; 76: S60–80
- Schor-Bardach R, Alsop DC, Pedrosa I, Solazzo SA, Wang X, Marquis RP, et al. Does arterial spin-labeling MR imaging-measured tumor perfusion correlate with renal cell cancer response to antiangiogenic therapy in a mouse model?. Radiology 2009; 251: 731–742
- Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: Standardized quantities and symbols. J Magn Reson Imaging 1999; 10: 223–232
- Forsberg F, Ro RJ, Potoczek M, Liu JB, Merritt CR, James KM, et al. Assessment of angiogenesis: Implications for ultrasound imaging. Ultrasonics 2004; 42: 325–330
- Gee MS, Saunders HM, Lee JC, Sanzo JF, Jenkins WT, Evans SM, et al. Doppler ultrasound imaging detects changes in tumor perfusion during antivascular therapy associated with vascular anatomic alterations. Cancer Res 2001; 61: 2974–2982
- Jugold M, Palmowski M, Huppert J, Woenne EC, Mueller MM, Semmler W, et al. Volumetric high-frequency Doppler ultrasound enables the assessment of early antiangiogenic therapy effects on tumor xenografts in nude mice. Eur Radiol 2008; 18: 753–758
- Goertz DE, Yu JL, Kerbel RS, Burns PN, Foster FS. High-frequency Doppler ultrasound monitors the effects of antivascular therapy on tumor blood flow. Cancer Res 2002; 62: 6371–6375
- Lavisse S, Lejeune P, Rouffiac V, Elie N, Bribes E, Demers B, et al. Early quantitative evaluation of a tumor vasculature disruptive agent AVE8062 using dynamic contrast-enhanced ultrasonography. Invest Radiol 2008; 43: 100–111
- Hardee ME, Eapen RJ, Rabbani ZN, Dreher MR, Marks J, Blackwell KL, et al. Her2/neu signaling blockade improves tumor oxygenation in a multifactorial fashion in Her2/neu+tumors. Cancer Chemother Pharmacol 2009; 63: 219–228
- Wang S, Shin IS, Hancock H, Jang BS, Kim HS, Lee SM, et al. Pulsed high intensity focused ultrasound increases penetration and therapeutic efficacy of monoclonal antibodies in murine xenograft tumors. J Control Realease 2012; 162: 218–224
- Tsushima Y, Funabasama S, Aoki J, Sanada S, Endo K. Quantitative perfusion map of malignant liver tumors, created from dynamic computed tomography data. Acad Radiol 2004; 11: 215–223
- Van Beers BE, Leconte I, Materne R, Smith AM, Jamart J, Horsmans Y. Hepatic perfusion parameters in chronic liver disease: Dynamic CT measurements correlated with disease severity. Am J Roentgenol 2001; 176: 667–673
- Breen MS, Breen M, Butts K, Chen L, Saidel GM, Wilson DL. MRI-guided thermal ablation therapy: Model and parameter estimates to predict cell death from MR thermometry images. Ann Biomed Eng 2007; 35: 1391–1403
- Solazzo S, Mertyna P, Peddi H, Ahmed M, Horkan C, Goldberg SN. RF ablation with adjuvant therapy: Comparison of external beam radiation and liposomal doxorubicin on ablation efficacy in an animal tumor model. Int J Hyperthermia 2008; 24: 560–567
- Haemmerich D, Chachati L, Wright AS, Mahvi DM, Lee FT, Jr, Webster JG. Hepatic radiofrequency ablation with internally cooled probes: Effect of coolant temperature on lesion size. IEEE Trans Biomed Eng 2003; 50: 493–500
- Prakash P, Diederich CJ. Considerations for theoretical modelling of thermal ablation with catheter-based ultrasonic sources: Implications for treatment planning, monitoring and control. Int J Hyperthermia 2012; 28: 69–86
- Osaki T, Takagi S, Hoshino Y, Okumura M, Fujinaga T. Antitumor effects and blood flow dynamics after photodynamic therapy using benzoporphyrin derivative monoacid ring A in KLN205 and LM8 mouse tumor models. Cancer Lett 2007; 248: 47–57
- Le Serve AW, Hellmann K. Metastases and the normalization of tumour blood vessels by ICRF 159: A new type of drug action. Br Med J 1972; 1: 597–601
- Ansiaux R, Baudelet C, Jordan BF, Crokart N, Martinive P, DeWever J, et al. Mechanism of reoxygenation after antiangiogenic therapy using SU5416 and its importance for guiding combined antitumor therapy. Cancer Res 2006; 66: 9698–9704
- Dickson PV, Hamner JB, Sims TL, Fraga CH, Ng CY, Rajasekeran S, et al. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res 2007; 13: 3942–3950
- Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res 2004; 64: 3731–3736
- Vlahovic G, Rabbani ZN, Herndon JE, 2nd, Dewhirst MW, Vujaskovic Z. Treatment with imatinib in NSCLC is associated with decrease of phosphorylated PDGFR-beta and VEGF expression, decrease in interstitial fluid pressure and improvement of oxygenation. Br J Cancer 2006; 95: 1013–1019
- Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: Role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 2004; 6: 553–563
- Roca C, Primo L, Valdembri D, Cividalli A, Declerck P, Carmeliet P, et al. Hyperthermia inhibits angiogenesis by a plasminogen activator inhibitor 1-dependent mechanism. Cancer Res 2003; 63: 1500–1507
- Goldberg SN, Kamel IR, Kruskal JB, Reynolds K, Monsky WL, Stuart KE, et al. Radiofrequency ablation of hepatic tumors: Increased tumor destruction with adjuvant liposomal doxorubicin therapy. Am J Roentgenol 2002; 179: 93–101
- Ahmed M, Liu Z, Lukyanov AN, Signoretti S, Horkan C, Monsky WL, et al. Combination radiofrequency ablation with intratumoral liposomal doxorubicin: Effect on drug accumulation and coagulation in multiple tissues and tumor types in animals. Radiology 2005; 235: 469–477
- Ahmed M, Monsky WE, Girnun G, Lukyanov A, D’Ippolito G, Kruskal JB, et al. Radiofrequency thermal ablation sharply increases intratumoral liposomal doxorubicin accumulation and tumor coagulation. Cancer Res 2003; 63: 6327–6333
- Monsky WL, Kruskal JB, Lukyanov AN, Girnun GD, Ahmed M, Gazelle GS, et al. Radio-frequency ablation increases intratumoral liposomal doxorubicin accumulation in a rat breast tumor model. Radiology 2002; 224: 823–829
- Solazzo SA, Ahmed M, Schor-Bardach R, Yang W, Girnun GD, Rahmanuddin S, et al. Liposomal doxorubicin increases radiofrequency ablation-induced tumor destruction by increasing cellular oxidative and nitrative stress and accelerating apoptotic pathways. Radiology 2010; 255: 62–74
- Wood BJ, Poon RT, Locklin JK, Dreher MR, Ng KK, Eugeni M, et al. Phase I study of heat-deployed liposomal doxorubicin during radiofrequency ablation for hepatic malignancies. J Vasc Interv Radiol 2012; 23: 248–255