Abstract
Purpose: The recent discoveries in the field of human small heat shock proteins (sHSPs) clearly point to the important roles played by these adenosine triphosphate (ATP)-independent chaperones in the regulation of a large spectrum of vital cellular processes and in pathological diseases. These proteins are therefore considered as very attractive therapeutic targets. Aims: To understand the functions of the stress-inducible members of the sHSP family, HspB1, HspB5 and HspB8, and be able to therapeutically modulate their activities, researchers are faced with the complex oligomerisation and phosphorylation properties of these proteins and with their ability to interact with each other and with specific protein targets. Here, we have integrated, in a functionally orientated way, the up-to-date literature data concerning HspB1, HspB5 and HspB8 protein interactions which reflect their numerous crucial cellular functions. We also present data supporting the idea that specific phospho-oligomeric domains of HspB1 are involved in the interaction with particular client proteins. Conclusions: More information concerning the interactions between client protein targets and sHSPs or the multiple combinatorial chimeric oligomeric complexes formed by different sHSPs are urgently required to elaborate a comprehensive sHSPs protein interactome and propose efficient and pathology-specific therapeutic approaches.
Introduction
The human family of small heat shock proteins sHSPs (also known as HSPB) contains ten members (HspB1 to HspB10) [Citation1]. They share the C-terminal alpha-crystallin domain which characterises mammalian alphaAB-crystallin polypeptides [Citation2–4]. Their N-terminal domain is decorated with a hydrophobic WD/PF motif and phosphoserine sites [Citation5] while their C-terminal domain contains the conservative tripeptide (I/V/L)-X-(I/V/L) motif and a flexible tail [Citation6–8]. This motif can interact with a hydrophobic groove on the surface of the core alpha-crystallin domain of a neighbouring dimer, and therefore can modulate the structural plasticity of sHSP oligomers [Citation8]. Only three, HspB1 (Hsp27), HspB5 (αB-crystallin) and HspB8 are stress inducible and therefore belong to the family of heat shock proteins. These three proteins, plus HspB4 (αA-crystallin), bear a conserved ATP-independent chaperone activity [Citation9–12]. Recent observations also suggest a weak chaperone activity associated to two other members of the family: HspB6 and HspB7 [Citation13–15]. Elevated expression of these sHSPs induces a cellular protection against different stresses (as heat shock) that are known to alter protein folding [Citation4]. In these conditions, sHSPs trap misfolded proteins through a so-called holdase activity and therefore avoid aggregation of the misfolded members. A cooperation with the Hsp70-Hsp90 ATP refoldase machine is then required for refolding or proteolytic elimination of the altered proteins [Citation9,Citation11,Citation16–25]. The trapping of damaged proteins in large structures depends on the sHSPs’ ability to form reversible, phosphorylation-regulated, polydispersed large oligomers (up to 800 kDa, depending on the sHSP). At least in the case of HspB1, the dynamic structural plasticity of this protein could be considered as a sensor of the cellular environment [Citation26–28].
An important discovery was the finding that HspB1, HspB5 and HspB8 are, similarly to the other members of the sHSPs family, constitutively expressed in many tissues [Citation29–31]. The recent findings revealed that these constitutively expressed sHSPs have an incredible number of crucial roles in normal and pathological cells. Indeed, they play important roles in signal transduction, transcription, and translation mechanisms. Moreover, they are key factors that maintain the integrity of the cytoskeleton architecture, they have anti-oxidant, anti-apoptotic, tumorigenic and metastasis properties, and they can contribute to cardiac cell hypertrophy and survival [Citation10,Citation31–38]. In addition, they can attenuate the aggregation or fibrillation of pathological proteins (i.e. mutant synuclein, parkin, Aβ-amyloid, polyQ-huntingtin) and participate in the regulation of proteolysis [Citation10,Citation21,Citation31]. Hence, their expression is often up-regulated during cell differentiation [Citation39] or in pathological conditions, such as those that characterise neurodegeneration [Citation10,Citation31,Citation34] myopathies [10,31,43], cardiomyopathies [10,31,43], cataracts [Citation10,Citation31,Citation34], inflammatory diseases [Citation10,Citation31,Citation34] and cancers [Citation31,Citation32,Citation34,Citation38]. Hence, depending on the pathology, the up-regulated expression of sHSPs can be either beneficial or deleterious to the patients [Citation10,Citation32,Citation34,Citation37]. Moreover, when mutated, several sHSPs have been described as responsible for the development of neurodegenerative [Citation10,Citation20,Citation40,Citation41], myopathic and caratact diseases [Citation10,Citation42,Citation43]. It has recently been proposed that sHSPs can achieve such a huge endeavour through their ability to recognise, interact and modulate the activity and/or half-life of many different proteins. In that respect, the dynamic plasticity of sHSPs’ structure is probably the key factor that allows the recognition of the more appropriated client proteins in a given specific situation [Citation27,Citation36–38,Citation44].
It is now well established that a clear understanding of the function of a protein requires information about its interactions with other proteins. This consideration is even more acute if the studied protein is a chaperone which displays apparent pleotropic activities resulting from its ability to modulate many crucial regulators. In that respect, individual experimental approaches are too limited to reveal an interactome comprehensively, and far more data are needed that can be obtained from the collective effort of the scientific community. As has been demonstrated in the case of Hsp90 [Citation45], integrated data from the existing and future literature will be required to build an interaction network of the human sHSPs molecular chaperone machines. The task will be quite intense, since, when they are expressed in the same cells, sHSPs can often interact with each other and form polydispersed hetero-oligomeric chimeric structures [Citation46–53] that may have different interactome properties than the parental sHSPs. A first approach towards this endeavour is presented here by listing the many proteins that we and many others have discovered to interact with either HspB1, HspB5 or HspB8. Interacting proteins are classified depending on their particular function in the cell. We also indicate, when they are known, the phospho-oligomeric organisation and/or the sequence domain of sHSPs involved in the interaction.
HspB1 (Hsp27)
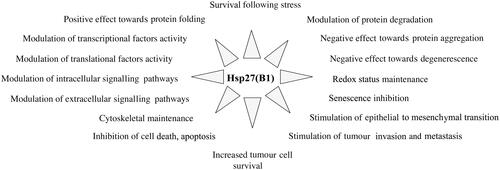
HspB1 (previously denominated Hsp27 or Hsp28) has been intensively studied, since it is one of the first human sHSPs that has been characterised and purified [Citation26,Citation54]. As described above, in stress conditions HspB1 is an important player that traps mis-folded polypeptides, avoids their aggregation, and can indirectly promote their refolding or proteolytic degradation. This protein is also constitutively expressed in most tissues. It is particularly abundant in heart, colon, lung, prostate, brain and muscular tissues [Citation31,Citation37,Citation55] as well as in pathological cells such as cancer cells [Citation38]. Studies analysing the effects associated with its over- or under-expression have concluded that HspB1 has multiple and apparently unrelated cellular functions (). For example, HspB1 has been reported to act as a modulator of transcription, translation, transduction pathways, apoptosis, oxido-resistance, redox status, tumour cell survival and invasion, senescence, cellular degenerescence and cytoskeleton integrity. These activities are supposed to result from HspB1’s ability to interact with a large number of protein partners. Moreover, when mutated, it plays a significant role in the development of certain neurodegenerative disorders [Citation56]. In spite of its broad effects on the biology of the cell, HspB1 is considered as an important therapeutic target, particularly in some cancer pathologies [Citation10,Citation38].
Figure 1. Cellular functions of HspB1. In addition to its well-known ability to protect cells against heat shock and other types of injuries, constitutively expressed HspB1 plays a major role in many different cellular processes, such as those listed in the figure.
Structural and phosphorylation changes of HspB1 modulate its ability to recognise protein targets
HspB1 is phosphorylated at the level of three serine sites (15, 78 and 82), in the N-terminal part of the polypeptide, by mitogen-activated protein kinase-associated protein kinases (MAPKAP kinases 2,3) which are themselves activated by phosphorylation by MAP p38 protein kinase [Citation57]. Amongst the different sHSPs, HspB1 is probably the protein that displays the most intense dynamic changes in its phosphorylation and oligomerisation in response to physiological alterations of the cellular environment [Citation27]. This leads to the conclusion that HspB1 structural organisation is an intracellular sensor that has multiple and complex strategies to respond to specific events. For example, in a defined physiological situation, conformational and phosphorylation changes accompanied by association/dissociation of oligomers may reprogramme HspB1 and favour its ability to interact with other and more appropriate client protein partners in order to modulate their folding/activity and/or half-life. This phenomenon could indirectly link HspB1 to multiple cellular functions. It is therefore of prime importance to have a clear understanding of what the interacting partners of HspB1 are in a particular cellular situation and to decipher HspB1 structural organisations aimed at interacting with specific protein targets. This type of information will be crucial to design therapeutic strategies aimed at modulating HspB1 specific functions. As an approach towards this task, summarises the different protein targets that have been described in the literature to interact with HspB1 and the modulating effect towards these targets. When it is known, the oligomeric/phosphorylated form of interacting HspB1 is indicated, but this parameter has been determined in only a very few cases. Only a few of the interacting targets (AR, Her2, Stat-2, Stat-3, HDAC-6, pro-caspase-3, Snail, HDM2) appear stabilised by HspB1. The stabilisation criterion was that these polypeptides are proteolytically degraded by the ubiquitin-proteasome machinery in the absence of HspB1. In reference to some Hsp90 interacting partners [Citation58], these interacting proteins can be considered as ‘clients’ of HspB1 [Citation44]. Other interacting partners show an enhanced degradation or a positive or negative modulation of their activity. Some can also be direct modulators of HspB1 chaperone activity. Of interest, HspB1 interacts with mutant proteins and positively interferes with their ability to aggregate or form fibrils. Some of the sHSPs, in particular HspB5 and HspB6, can form complex hetero-oligomers with HspB1 when they are expressed in the same cells. The phenomenon usually induces a reciprocal chaperoning effect towards the two partners. Formation of hetero-oligomeric complexes does not appear, at least in vitro, to alter HspB1 chaperone activity, but can mutually affect the structure of both partners and modulate their ability to interact with specific protein targets [Citation59] or could generate the recognition of new protein targets. HspB1 expression is also associated with other changes in the cell physiology, as for example the activity of anti-oxidant enzymes and NF-κB or the efficiency of splicing recovery after heat shock. However, these effects are described in in a separate section since the protein targets that are directly modulated by HspB1 are not yet characterised.
Table I. HspB1 interactome.
Specific phospho-oligomeric structures of HspB1 recognise different protein clients
Despite the fact that HspB1 interacting sequences with non-sHSP-specific target proteins have not yet been documented, our recent observations support the hypothesis that, in the same cell, specific phospho-oligomeric structures can interact with different protein clients. In growing HeLa cells, HspB1 is the major constitutively expressed sHSP. Analysis of its native size using a gel filtration column revealed that HspB1 is mainly recovered in three distinct structural organisations: oligomers whose size is smaller than 200 kDa that are phosphorylated at the level of serine 15 and 82, oligomers that display a native size comprising between 200 and 400 kDa that are exclusively phosphorylated at the level of serine 78, and oligomers that have a larger size and which contain the remaining of serine 82 phosphorylation (). The positions of three client proteins were detected and immunoprecipitation studies confirmed that pro-caspase-3 interacts the HspB1 small oligomers and HDAC6 with the large ones suggesting that different phosphorylation/oligomerisation organisations of HspB1 are required for the respective binding of these two clients. In contrast, STAT2 interacted with more complex and less defined HspB1 structural organisations with native size comprising between 200 and about 700 kDa [Citation27,Citation44]. Hence, in addition to its role in controlling HspB1 oligomerisation, phosphorylation may also be a signalling mechanism which favours the recognition of specific target polypeptides.
Figure 2. Native size and phosphorylation of HspB1 and structure-specific interaction with client protein targets. HeLa cells were lysed and the 10 000 × g cytosolic fraction containing all the cellular content of HspB1 was analysed by gel filtration column as previously described [Citation27]. Immunoblot analysis of two-by-two pooled fractions was performed using antibodies that are specific to either total HspB1 or phosphorylated (phospho-Ser15, phospho-Ser78 or phospho-Ser82) HspB1. The presence of three client proteins that interact with HspB1 was detected using specific antibodies recognising Pro-caspase-3, HDAC6 and STAT2. Three native size fractions could be defined depending on HspB1 phosphorylation: 50–200 kDa, phosphorylation at the level of serines 15 and 82, 200–400 kDa, phosphorylation at the level of serine 78 and 400–700 kDa oligomers containing phosphorylated serine 82. Note that pro-caspase-3 co-eluted mainly with the serine 15 phosphorylated small oligomers. HDAC6 was at the level of the large serine 82 phosphorylated oligomers while STAT2 had a less defined elution profile between the medium and large sized oligomers. Interactions of these proteins with different phospho-oligomeric structures of HspB1 was confirmed by co-immunoprecipitation [Citation44].
![Figure 2. Native size and phosphorylation of HspB1 and structure-specific interaction with client protein targets. HeLa cells were lysed and the 10 000 × g cytosolic fraction containing all the cellular content of HspB1 was analysed by gel filtration column as previously described [Citation27]. Immunoblot analysis of two-by-two pooled fractions was performed using antibodies that are specific to either total HspB1 or phosphorylated (phospho-Ser15, phospho-Ser78 or phospho-Ser82) HspB1. The presence of three client proteins that interact with HspB1 was detected using specific antibodies recognising Pro-caspase-3, HDAC6 and STAT2. Three native size fractions could be defined depending on HspB1 phosphorylation: 50–200 kDa, phosphorylation at the level of serines 15 and 82, 200–400 kDa, phosphorylation at the level of serine 78 and 400–700 kDa oligomers containing phosphorylated serine 82. Note that pro-caspase-3 co-eluted mainly with the serine 15 phosphorylated small oligomers. HDAC6 was at the level of the large serine 82 phosphorylated oligomers while STAT2 had a less defined elution profile between the medium and large sized oligomers. Interactions of these proteins with different phospho-oligomeric structures of HspB1 was confirmed by co-immunoprecipitation [Citation44].](/cms/asset/375c23d3-279a-4314-b429-82b606ff3108/ihyt_a_792956_f0002_b.jpg)
HspB5 (alphaB-crystallin)
HspB5 is an ATP-independent chaperone which interacts with HspB4 (alphaA-crystallin) to form (in a 1:3 HspB5:HspB4 ratio) the oligomeric alpha-crystallin molecule which is one of the most important polypeptides involved in the refractive and light focusing properties of the lens [Citation43]. In contrast to HspB4, HspB5 is a stress inducible sHSP that is also constitutively expressed in several non-lens tissues such as those from the heart, the colon, muscles, lungs, and kidneys [Citation37]. As HspB1, HspB5 has numerous cellular functions (cytoskeleton, cell growth and adhesion, signalling mechanisms, protein transport, apoptosis, proteolysis and transcription) which all result from HspB5 interaction with a large spectrum of protein partners. See , which lists the protein targets that have already been reported in the literature to interact with HspB5. Only a few of the interacting targets appear stabilised by HspB5 to avoid their degradation. HspB5 mainly acts by modulating the activity of the protein targets or by attenuating their aggregation or fibrillation. HspB5 is particularly efficient at the level of the cytoskeleton, particularly intermediate filament proteins. Of interest, by mass spectral analysis, approximately 70 polypeptides (acute phase proteins, coagulation factors and proteins of the complement) were precipitated by HspB5 from plasma from patients with multiple sclerosis, rheumatoid arthritis and amyloidosis, and mice with experimental allergic encephalomyelitis [Citation60]. This interesting study clearly illustrates how large the spectrum of HspB5 interacting proteins can be. No such analysis has yet been performed concerning extra-cellular HspB1. HspB5 expression is up-regulated in several pathologies, in particular those of cancer origin [Citation38,Citation61,Citation62]. Several HspB5 mutations have been characterised that result in cataracts, cardiomyopathies and myofibrillar myopathies [Citation43]. Hence, HspB5 is considered as a therapeutic target, particularly in myopathies and cancer pathologies [Citation10,Citation38,Citation42].
Table II. HspB5 interactome.
HspB5 phosphorylation and interacting domains
HspB5 is phosphorylated at three sites (serines 19, 45 and 59). The MAPKAPK2/3 kinases are responsible for the phosphorylation of serine 59 while p42/p44 MAPKinase phosphorylates serine 45. HspB5 structural organisation differs from that of HspB1 since its oligomers are less dynamic and mainly recovered with native sizes ranging from about 400 to 700 kDa [Citation63]. It is not yet known whether changes in HspB5 native size could modulate its ability to recognise specific targets. However, information already exists about HspB5 interacting domains that are effective, at least in vitro, to recognise specific target proteins (see ). The sequences of these domains are not listed in but can be obtained in the cited references. For example, the DRFSVNLDVKHFS and HGKHEERQDE peptide domains in HspB5 alpha crystallin C-terminal domain appear involved in the inhibition of alpha-synuclein amyloid-beta fibrillation [Citation64].
HspB8 (Hsp22)
HspB8, a recently described phospho-oligomeric member of the family of human sHSPs [Citation65], bears a chaperone activity and is up-regulated in stress conditions. HspB8 is widely expressed in different human tissues, predominantly skeletal muscles, heart and nerves. As HspB1 and HspB5, HspB8 is also characterised by its pleotropic cellular roles. It is involved, directly or indirectly, in the regulation of apoptosis, ribonucleoprotein processing, cell differentiation and proliferation, carcinogenesis, cardiac cell hypertrophy and inflammatory process in rheumatoid arthritis [Citation31,Citation41,Citation66,Citation67]. Moreover, point mutations that alter HspB8 chaperone activity were found to correlate with the development of distal motor neurodegenerative diseases [Citation68]. In that respect, one of the most prominent roles of HspB8 is linked to its ability to counteract, more efficiently than HspB1 or HspB5, the aggregation of misfolded/denatured proteins and to participate in the regulation of their proteolysis [Citation20]. This high efficiency depends on HspB8’s ability to interact with Bag3, a co-chaperone stimulator of macroautophagy. In the HspB8-Bag3 cooperative complex, HspB8 is responsible for the recognition of the damaged proteins, while Bag3 is involved in macroautophagy activation [Citation11]. In addition, the HspB8-Bag3 complex activates, through phosphorylation and a non-chaperone-like mechanism, the eIF2alpha signalling pathway that leads to protein synthesis inhibition and autophagy stimulation [Citation24,Citation69]. Other studies have revealed that the autophagic removal of misfolded proteins may occur through a larger multiheteromeric complex made of HspB8, Bag3, Hsc70 and the E3 ligase CHIP [Citation70] plus also HspB6 [Citation71]. In response to the deleterous accumulation of misfolded proteins in response to drastic heat shock treatments, the Bag3-HspB8 complex is up-regulated through a stress-activated NF-κB dependent event [Citation72].
HspB8 interact with many different protein targets
HspB8 is present cellularly in the form of small homo-oligomers. However, it is recovered in polydispersed oligomeric complexes consequently due to its interactions with other members of the family (HspB1, HspB5, HspB6, HspB3 and HspB2) [Citation48,Citation49,Citation73,Citation74]. As HspB1 and HspB5, HspB8 interacts with many target proteins that are different from those interacting with these two sHSPs [Citation75]. These interactions are regulated by HspB8 phosphorylation (Serine 24 and Threonine 87 by extra signal cellular regulated kinase 1, ERK1) which modulates the structure and chaperone activity of this protein [Citation75]. The polypeptides that interact with HspB8 and which are linked to the multiples roles played by this protein are presented in . They are less abundant compared to HspB1 or HspB5. This is probably a consequence of the recent discovery of this fascinating sHSP.
Table III. HspB8 interactome.
Areas for future work
Here, we have analysed the interactomes of the three major stress inducible sHSPs. This choice was made because there is still little information available concerning the interactomes of the seven other members of the family of sHSPs. Most of these sHSPs are not stress inducible and bear only a weak, or no chaperone activity. However, some of them are interesting, such as HspB6 and HspB7 [Citation14,Citation31] and HspB4 (alphaA-crystallin) which can act as a chaperone towards HspB5 [Citation43,Citation52]. Hence, future work will certainly bring new information concerning the interactomes of these proteins. Another field of research that is still obscure concerns the effects induced by the interaction between sHSPs [Citation49,Citation53,Citation76]. Indeed, if several sHSPs are expressed in the same cell, they can form multiple combinatorial chimeric oligomeric complexes that could bear new protein target recognition abilities and modulate those of the parental molecules. Another consequence could be the dominant effect of a mutated sHSP towards other interacting members of the family [Citation77]. Unfortunately, only very few data are available and new studies are urgently required to analyse these complex interactions and their effects on the recognition of protein targets.
Conclusion
For years, sHSPs have been thought to act mainly as specialised molecular chaperones to attenuate cellular damage by inducing the storage of the altered proteins until they could be refolded by the major ATP-dependent chaperone machines (i.e. Hsp70, Hsp90), or degraded. Their constitutive expression in a large number of normal and pathological tissues and the discovery of mutations that are responsible for pathologies as diverse as neurodegeneration, myopathies, cardiomyopathies and cataracts have suggested that their role in the cell is more complex than it was originally proposed. This assumption was confirmed by experiments aimed at analysing the cellular effects induced by either up- or down-regulating their constitutive expression. Indeed, numerous reports in the literature describe that these proteins are involved in an incredible number of crucial, but often unrelated, cellular functions. As recently shown, these activities result from the holdase type of chaperone function of sHSPs which allows them to recognise, interact and modulate the activity and/or half-life of many specific proteins. Nowdays, the number of the proteins that interact with these HSPs is growing exponentially. So, the aim of this publication was to list the proteins that have already been described to interact with the three major stress inducible sHSP chaperones HspB1, HspB5 and HspB8 which are known to play important role in pathologies [Citation10,Citation20,Citation32,Citation34,Citation37,Citation38]. From this study we can conclude that today we are still far from being able to build a comprehensive overall dynamic interactome of sHSPs. The major disadvantage of this situation concerns the search for therapeutic drugs that could alter the interaction of a specific pathological protein target with a defined sHSP, or on the other hand, promote its interaction with a beneficial one. Indeed, despite some positive attempts to specifically modulate the HspB1 interactome [Citation78–80], we may remain stuck for a while with the use of broad approaches which, through general alteration of sHSP’s dynamic interactomes, could induce off-target mediated side-effects.
Declaration of interest
B.G.’s post doctoral fellowship is supported by the Association Contre le Cancer. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
Apologies to those whose work has not been cited due to lack of space. We thank Valerie Arrigo for comments on the manuscript and Patrick Mehlen for his support.
References
- Kappe G, Franck E, Verschuure P, Boelens WC, Leunissen JA, de Jong WW. The human genome encodes 10 alpha-crystallin-related small heat shock proteins: HspB1-10. Cell Stress Chaperones 2003;8:53–61
- Ingolia TD, Craig EA. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci USA 1982;79:2360–4
- de Jong W, Leunissen J, Voorter C. Evolution of the alpha-crystallin/small heat-shock protein family. Mol Biol Evol 1993;10:103–26
- Arrigo A-P, Landry J. Expression and function of the low-molecular-weight heat shock proteins. In: Morimoto RI, Tissieres A, Georgopoulos C, eds. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. pp 335–73
- Theriault JR, Lambert H, Chavez-Zobel AT, Charest G, Lavigne P, Landry J. Essential role of the NH2-terminal WD/EPF motif in the phosphorylation-activated protective function of mammalian Hsp27. J Biol Chem 2004;279:23463–71
- Takemoto L, Emmons T, Horwitz J. The C-terminal region of a-crystallin: Involvement in protection against heat-induced denaturation. Biochem J 1993;294:435–8
- Pasta SY, Raman B, Ramakrishna T, Rao Ch M. The IXI/V motif in the C-terminal extension of alpha-crystallins: Alternative interactions and oligomeric assemblies. Mol Vis 2004;10:655–62
- Sudnitsyna MV, Mymrikov EV, Seit-Nebi AS, Gusev NB. The role of intrinsically disordered regions in the structure and functioning of small heat shock proteins. Curr Protein Pept Sci 2012;13:76–85
- Horwitz J, Huang Q-L, Ding L-L. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA 1992;89:10449–53
- Arrigo A-P, Simon S, Gibert B, Kretz-Remy C, Nivon M, Czekalla A, et al. Hsp27 (HspB1) and alphaB-crystallin (HspB5) as therapeutic targets. FEBS Lett 2007;581:3665–74
- Carra S, Seguin SJ, Landry J. HspB8 and Bag3: A new chaperone complex targeting misfolded proteins to macroautophagy. Autophagy 2008;4:237–9
- Yang Z, Lu Y, Liu J, Wang Y, Zhao X. The chaperone-like activity of rat HspB8/Hsp22 and dynamic molecular transition related to oligomeric architectures in vitro. Protein Pept Lett 2012;19:353–9
- Seit-Nebi AS, Gusev NB. Versatility of the small heat shock protein HSPB6 (Hsp20). Cell Stress Chaperones 2010;15(3):233–6
- Vos MJ, Zijlstra MP, Kanon B, van Waarde-Verhagen MA, Brunt ER, Oosterveld-Hut HM, et al. HSPB7 is the most potent polyQ aggregation suppressor within the HSPB family of molecular chaperones. Hum Mol Genet 2010;19:4677–93
- Ke L, Meijering RA, Hoogstra-Berends F, Mackovicova K, Vos MJ, Van Gelder IC, et al. HSPB1, HSPB6, HSPB7 and HSPB8 protect against RhoA GTPase-induced remodeling in tachypaced atrial myocytes. PLoS One 2011;6:e20395
- Jakob U, Gaestel M, Engels K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem 1993;268:1517–20
- Ganea E. Chaperone-like activity of alpha-crystallin and other small heat shock proteins. Curr Protein Pept Sci 2001;2:205–25
- Boelens WC, Croes Y, de Jong WW. Interaction between alphaB-crystallin and the human 20S proteasomal subunit C8/alpha7. Biochim Biophys Acta 2001;1544:311–19
- den Engelsman J, Keijsers V, de Jong WW, Boelens WC. The small heat-shock protein alpha B-crystallin promotes FBX4-dependent ubiquitination. J Biol Chem 2003;278:4699–704
- Carra S, Sivilotti M, Chavez Zobel AT, Lambert H, Landry J. HspB8, a small heat shock protein mutated in human neuromuscular disorders, has in vivo chaperone activity in cultured cells. Hum Mol Genet 2005;14:1659–69
- Parcellier A, Brunet M, Schmitt E, Col E, Didelot C, Hammann A, et al. HSP27 favors ubiquitination and proteasomal degradation of p27Kip1 and helps S-phase re-entry in stressed cells. FASEB J 2006;20:1179–81
- Bellyei S, Szigeti A, Pozsgai E, Boronkai A, Gomori E, Hocsak E, et al. Preventing apoptotic cell death by a novel small heat shock protein. Eur J Cell Biol 2007;86:161–71
- Barbash O, Lin DI, Diehl JA. SCF Fbx4/alphaB-crystallin cyclin D1 ubiquitin ligase: A license to destroy. Cell Div 2007;2:2
- Carra S. The stress-inducible HspB8-Bag3 complex induces the eIF2alpha kinase pathway: Implications for protein quality control and viral factory degradation. Autophagy 2009;5:428–9
- Markossian KA, Yudin IK, Kurganov BI. Mechanism of suppression of protein aggregation by alpha-crystallin. Int J Mol Sci 2009;10:1314–45
- Arrigo A-P, Suhan JP, Welch WJ. Dynamic changes in the structure and intracellular locale of the mammalian low-molecular-weight heat shock protein. Mol Cell Biol 1988;8:5059–71
- Paul C, Simon S, Gibert B, Virot S, Manero F, Arrigo AP. Dynamic processes that reflect anti-apoptotic strategies set up by HspB1 (Hsp27). Exp Cell Res 2010;316:1535–52
- Arrigo A-P. Structure-functions of HspB1 (Hsp27). Methods Mol Biol 2011;787:105–19
- Bhat SP, Nagineni CN. AlphaB subunit of lens-specific protein a-cristallin is present in other ocular and non-ocular tissues. Bioch Biophys Res Commun 1989;158:319–25
- Srinivasan A, Nagineni C, Bhat S. alpha A-crystallin is expressed in non-ocular tissues. J Biol Chem 1992;267:23337–41
- Mymrikov EV, Seit-Nebi AS, Gusev NB. Large potentials of small heat shock proteins. Physiol Rev 2011;91:1123–59
- Arrigo A-P. Anti-apoptotic, tumorigenic and metastatic potential of Hsp27 (HspB1) and alphaB-crystallin (HspB5): Emerging targets for the development of new anti-cancer therapeutic strategies. In: Calderwood S, Sherman M, Ciocca D, eds. Heat Shock Proteins in Cancer. New York: Springer-Verlag, 2007, pp. 73--92
- Aloy MT, Hadchity E, Bionda C, Diaz-Latoud C, Claude L, Rousson R, et al. Protective role of Hsp27 protein against gamma radiation-induced apoptosis and radiosensitization effects of Hsp27 gene silencing in different human tumor cells. Int J Radiat Oncol Biol Phys 2008;70:543–53
- Arrigo A-P, Simon S. Beneficial and deleterious, the dual role of small stress proteins in human diseases: implications for therapeutic strategies. In: Simon S, Arrigo A-P, eds. Small Stress Proteins and Human Diseases. New York: Nova Sciences, 2010, pp. 457--76
- Gibert B, Eckel B, Gonin V, Goldschneider D, Fombonne J, Deux B, et al. Targeting heat shock protein 27 (HspB1) interferes with bone metastasis and tumour formation in vivo. Br J Cancer 2012;107:63–70
- Arrigo A-P, Gibert B. HspB1 dynamic phospho-oligomeric structure dependent interactome as cancer therapeutic target. Curr Mol Med 2012;12:1151–63
- Arrigo A-P. Pathology-dependent effects linked to small heat shock proteins expression. Scientifica 2012;2012:Article ID 185641. doi.org/10.6064/2012/185641
- Ciocca DR, Arrigo A-P, Calderwood SK. Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: An update. Arch Toxicol 2013;87:19–48
- Arrigo A-P. In search of the molecular mechanism by which small stress proteins counteract apoptosis during cellular differentiation. J Cell Biochem 2005;94:241–6
- Wilhelmus MM, Boelens WC, Otte-Holler I, Kamps B, Kusters B, Maat-Schieman ML, et al. Small heat shock protein HspB8: Its distribution in Alzheimer’s disease brains and its inhibition of amyloid-beta protein aggregation and cerebrovascular amyloid-beta toxicity. Acta Neuropathol 2006;111:139–49
- Sun X, Fontaine JM, Hoppe AD, Carra S, DeGuzman C, Martin JL, et al. Abnormal interaction of motor neuropathy-associated mutant HspB8 (Hsp22) forms with the RNA helicase Ddx20 (gemin3). Cell Stress Chaperones 2010;15:567–82
- Vicart P, Caron A, Guicheney P, Li Z, Prevost MC, Faure A, et al. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet 1998;20:92–5
- Arrigo A-P, Simon S. Expression and functions of heat shock proteins in the normal and pathological Mammalian eye. Curr Mol Med 2010;10:776–93
- Gibert B, Eckel B, Fasquelle L, Moulin M, Bouhallier F, Gonin V, et al. Knock down of heat shock protein 27 (HspB1) induces degradation of several putative client proteins. PLoS One 2012;7:e29719
- Echeverria PC, Bernthaler A, Dupuis P, Mayer B, Picard D. An interaction network predicted from public data as a discovery tool: Application to the Hsp90 molecular chaperone machine. PLoS One 2011;6:e26044
- Zantema A, Jong ED, Lardenoije R, Eb AJVD. The expression of heat shock protein hsp27 and a complexed 22-kiloDalton protein is inversely correlated with oncogenicity of adenovirus transformed cells. J Virol 1989;63:3368–75
- Groenen P, Merck K, de Jong W, Bloemendal H. Structure and modifications of the junior chaperone alpha-crystallin. From lens transparency to molecular pathology. Eur J Biochem 1994;225:1–19
- Fu L, Liang JJ. Enhanced stability of alpha B-crystallin in the presence of small heat shock protein Hsp27. Biochem Biophys Res Commun 2003;302:710–14
- Fontaine JM, Sun X, Benndorf R, Welsh MJ. Interactions of Hsp22 (HspB8) with Hsp20, alphaB-crystallin, and HspB3. Biochem Biophys Res Commun 2005;337:1006–11
- Sreelakshmi Y, Sharma KK. The interaction between alphaA- and alphaB-crystallin is sequence-specific. Mol Vis 2006;12:581–7
- Srinivas PN, Reddy PY, Reddy GB. Significance of alpha-crystallin heteropolymer with a 3:1 alphaA/alphaB ratio: Chaperone-like activity, structure and hydrophobicity. Biochem J 2008;414:453–60
- Skouri-Panet F, Michiel M, Ferard C, Duprat E, Finet S. Structural and functional specificity of small heat shock protein HspB1 and HspB4, two cellular partners of HspB5: Role of the in vitro hetero-complex formation in chaperone activity. Biochimie 2012;94:975–84
- Mymrikov EV, Seit-Nebi AS, Gusev NB. Heterooligomeric complexes of human small heat shock proteins. Cell Stress Chaperones 2012;17:157–69
- Arrigo A-P, Welch W. Characterization and purification of the small 28,000-Dalton mammalian heat shock protein. J Biol Chem 1987;262:15359–69
- Franklin TB, Krueger-Naug AM, Clarke DB, Arrigo AP, Currie RW. The role of heat shock proteins Hsp70 and Hsp27 in cellular protection of the central nervous system. Int J Hyperthermia 2005;21:379–92
- Evgrafov OV, Mersiyanova I, Irobi J, Van Den Bosch L, Dierick I, Leung CL, et al. Mutant small heat-shock protein 27 causes axonal Charcot-Marie-Tooth disease and distal hereditary motor neuropathy. Nat Genet 2004;36:602–6
- Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, et al. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell 1994;78:1027–37
- Blagosklonny MV. Hsp-90-associated oncoproteins: Multiple targets of geldanamycin and its analogs. Leukemia 2002;16:455–62
- Bukach OV, Glukhova AE, Seit-Nebi AS, Gusev NB. Heterooligomeric complexes formed by human small heat shock proteins HspB1 (Hsp27) and HspB6 (Hsp20). Biochim Biophys Acta 2009;1794:486–95
- Rothbard JB, Kurnellas MP, Brownell S, Adams CM, Su L, Axtell RC, et al. Therapeutic effects of systemic administration of chaperone alphaB-crystallin associated with binding proinflammatory plasma proteins. J Biol Chem 2012;287:9708–21
- Clark JI, Muchowski PJ. Small heat-shock proteins and their potential role in human disease. Curr Opin Struct Biol 2000;10:52–9
- Chen P, Ji W, Liu FY, Tang HZ, Fu S, Zhang X, et al. Alpha-crystallins and tumorigenesis. Curr Mol Med 2012;12:1164–73
- Saha S, Das KP. Relationship between chaperone activity and oligomeric size of recombinant human alphaA- and alphaB-crystallin: A tryptic digestion study. Proteins 2004;57:610–17
- Ghosh JG, Houck SA, Clark JI. Interactive sequences in the molecular chaperone, human alphaB crystallin modulate the fibrillation of amyloidogenic proteins. Int J Biochem Cell Biol 2008;40:954–67
- Kappe G, Verschuure P, Philipsen RL, Staalduinen AA, Van de Boogaart P, Boelens WC, et al. Characterization of two novel human small heat shock proteins: Protein kinase-related HspB8 and testis-specific HspB9. Biochim Biophys Acta 2001;1520:1–6
- Badri KR, Modem S, Gerard HC, Khan I, Bagchi M, Hudson AP, et al. Regulation of Sam68 activity by small heat shock protein 22. J Cell Biochem 2006;99:1353–62
- Roelofs MF, Boelens WC, Joosten LA, Abdollahi-Roodsaz S, Geurts J, Wunderink LU, et al. Identification of small heat shock protein B8 (HSP22) as a novel TLR4 ligand and potential involvement in the pathogenesis of rheumatoid arthritis. J Immunol 2006;176:7021–7
- Irobi J, Van Impe K, Seeman P, Jordanova A, Dierick I, Verpoorten N, et al. Hot-spot residue in small heat-shock protein 22 causes distal motor neuropathy. Nat Genet 2004;36:597–601
- Carra S, Brunsting JF, Lambert H, Landry J, Kampinga HH. HspB8 participates in protein quality control by a non-chaperone-like mechanism that requires eIF2{alpha} phosphorylation. J Biol Chem 2009;284:5523–32
- Crippa V, Sau D, Rusmini P, Boncoraglio A, Onesto E, Bolzoni E, et al. The small heat shock protein B8 (HspB8) promotes autophagic removal of misfolded proteins involved in amyotrophic lateral sclerosis (ALS). Hum Mol Genet 2010;19:3440–56
- Fuchs M, Poirier DJ, Seguin SJ, Lambert H, Carra S, Charette SJ, et al. Identification of the key structural motifs involved in HspB8/HspB6-Bag3 interaction. Biochem J 2010;425:245–55
- Nivon M, Abou-Samra M, Richet E, Guyot B, Arrigo A-P, Kretz-Remy C. NF-kappaB regulates protein quality control after heat stress through modulation of the BAG3-HspB8 complex. J Cell Sci 2012;125:1141–51
- Kato K, Shinohara H, Goto S, Inaguma Y, Morishita R, Asano T. Copurification of small heat shock protein with alphaB crystallin from human skeletal muscle. J Biol Chem 1992;267:7718–25
- Sun X, Fontaine JM, Rest JS, Shelden EA, Welsh MJ, Benndorf R. Interaction of human Hsp22 (HspB8) with other small heat shock proteins. J Biol Chem 2004;279:2394–402
- Shemetov AA, Seit-Nebi AS, Gusev NB. Phosphorylation of human small heat shock protein HspB8 (Hsp22) by ERK1 protein kinase. Mol Cell Biochem 2011;355:47–55
- Zantema A, Vries MV-D, Maasdam D, Bol S, Eb Avd. Heat shock protein 27 and alphaB-cristallin can form a complex, which dissociates by heat shock. J Biol Chem 1992;267:12936–41
- Diaz-Latoud C, Buache E, Javouhey E, Arrigo A-P. Substitution of the unique cysteine residue of murine hsp25 interferes with the protective activity of this stress protein through inhibition of dimer formation. Antioxid Redox Signal 2005;7:436–45
- Gibert B, Hadchity E, Czekalla A, Aloy MT, Colas P, Rodriguez-Lafrasse C, et al. Inhibition of heat shock protein 27 (HspB1) tumorigenic functions by peptide aptamers. Oncogene 2011;34:3672–81
- Heinrich JC, Tuukkanen A, Schroeder M, Fahrig T, Fahrig R. RP101 (brivudine) binds to heat shock protein Hsp27 (HspB1) and enhances survival in animals and pancreatic cancer patients. J Cancer Res Clin Oncol 2011;137:1349–61
- Gibert B, Simon S, Dimitrova V, Diaz-Latoud C, Arrigo A-P. Peptide Aptamers – Tools to negatively or positively modulate HspB1(27) function. Phil Trans Royal Soc B 2013;368:20120075
- Dall’Era MA, Oudes A, Martin DB, Liu AY. Hsp27 and Hsp70 interact with CD10 in C4-2 prostate cancer cells. Prostate 2007;67:714–21
- Al-Madhoun AS, Chen YX, Haidari L, Rayner K, Gerthoffer W, McBride H, et al. The interaction and cellular localization of HSP27 and ERbeta are modulated by 17beta-estradiol and HSP27 phosphorylation. Mol Cell Endocrinol 2007;270:33–42
- Zoubeidi A, Zardan A, Beraldi E, Fazli L, Sowery R, Rennie P, et al. Cooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity. Cancer Res 2007;67:10455–65
- Kang SH, Kang KW, Kim KH, Kwon B, Kim SK, Lee HY, et al. Upregulated Hsp27 in human breast cancer cells reduces Herceptin susceptibility by increasing Her2 protein stability. BMC Cancer 2008;8:286
- Wu Y, Liu J, Zhang Z, Huang H, Shen J, Zhang S, et al. Hsp27 regulates IL-1 stimulated IKK activation through interacting with TRAF6 and affecting its ubiquitination. Cell Signal 2009;21:143–50
- Charette SJ, Landry J. The interaction of Hsp27 with DAXX identifies a potential regulatory role of Hsp27 in Fas-induced apoptosis. Ann NY Acad Sci 2000;926:126–31
- Lee HJ, Lee YS. Repeated-dose toxicity of Hsp27-binding heptapeptide in mice. Drug Chem Toxicol 2010;33:284–90
- Patil SB, Pawar MD, Bitar KN. Direct association and translocation of PKC-alpha with calponin. Am J Physiol Gastrointest Liver Physiol 2004;286:G954–63
- Wu R, Kausar H, Johnson P, Montoya-Durango DE, Merchant M, Rane MJ. Hsp27 regulates Akt activation and polymorphonuclear leukocyte apoptosis by scaffolding MK2 to Akt signal complex. J Biol Chem 2007;282:21598–608
- Chebotareva NA, Makeeva VF, Bazhina SG, Eronina TB, Gusev NB, Kurganov BI. Interaction of Hsp27 with native phosphorylase kinase under crowding conditions. Macromol Biosci 2010;10:783–9
- Zoubeidi A, Zardan A, Wiedmann RM, Locke J, Beraldi E, Fazli L, et al. Hsp27 promotes insulin-like growth factor-I survival signaling in prostate cancer via p90Rsk-dependent phosphorylation and inactivation of BAD. Cancer Res 2010;70:2307–17
- Cayado-Gutierrez N, Moncalero VL, Rosales EM, Beron W, Salvatierra EE, Alvarez-Olmedo D, et al. Downregulation of Hsp27 (HspB1) in MCF-7 human breast cancer cells induces upregulation of PTEN. Cell Stress Chaperones 2013;18:243–9
- Rocchi P, Beraldi E, Ettinger S, Fazli L, Vessella RL, Nelson C, et al. Increased Hsp27 after androgen ablation facilitates androgen-independent progression in prostate cancer via signal transducers and activators of transcription 3-mediated suppression of apoptosis. Cancer Res 2005;65:11083–93
- Brunet Simioni M, De Thonel A, Hammann A, Joly AL, Bossis G, Fourmaux E, et al. Heat shock protein 27 is involved in SUMO-2/3 modification of heat shock factor 1 and thereby modulates the transcription factor activity. Oncogene 2009;28:3332–44
- de Thonel A, Vandekerckhove J, Lanneau D, Selvakumar S, Courtois G, Hazoume A, et al. Hsp27 controls GATA-1 protein level during erythroid cell differentiation. Blood 2010;116:85–96
- Wettstein G, Bellaye PS, Kolb M, Hammann A, Crestani B, Soler P, et al. Inhibition of Hsp27 blocks fibrosis development and EMT features by promoting Snail degradation. FASEB J 2013;27:1549–60
- Cuesta R, Laroia G, Schneider RJ. Chaperone Hsp27 inhibits translation during heat shock by binding eIF4G and facilitating dissociation of cap-initiation complexes. Genes Dev 2000;14:1460–70
- Andrieu C, Taieb D, Baylot V, Ettinger S, Soubeyran P, De-Thonel A, et al. Heat shock protein 27 confers resistance to androgen ablation and chemotherapy in prostate cancer cells through eIF4E. Oncogene 2010;29:1883–96
- Sinsimer KS, Gratacos FM, Knapinska AM, Lu J, Krause CD, Wierzbowski AV, et al. Chaperone Hsp27, a novel subunit of AUF1 protein complexes, functions in AU-rich element-mediated mRNA decay. Mol Cell Biol 2008;28:5223–37
- Knapinska AM, Gratacos FM, Krause CD, Hernandez K, Jensen AG, Bradley JJ, et al. Chaperone Hsp27 modulates AUF1 proteolysis and AU-rich element-mediated mRNA degradation. Mol Cell Biol 2011;31:1419–31
- Mounier N, Arrigo A-P. Actin cytoskeleton and small heat shock proteins: How do they interact? Cell Stress Chaperones 2002;7:167–76
- Hino M, Kurogi K, Okubo MA, Murata-Hori M, Hosoya H. Small heat shock protein 27 (Hsp27) associates with tubulin/microtubules in HeLa cells. Biochem Biophys Res Commun 2000;271:164–9
- Perng MD, Cairns L, van den IP, Prescott A, Hutcheson AM, Quinlan RA. Intermediate filament interactions can be altered by Hsp27 and alphaB-crystallin. J Cell Sci 1999;112:2099–112
- Bjorkdahl C, Sjogren MJ, Zhou X, Concha H, Avila J, Winblad B, et al. Small heat shock proteins Hsp27 or alphaB-crystallin and the protein components of neurofibrillary tangles: Tau and neurofilaments. J Neurosci Res 2008;86:1343–52
- Arany I, Clark JS, Reed DK, Ember I, Juncos LA. Cisplatin enhances interaction between p66Shc and HSP27: Its role in reorganization of the actin cytoskeleton in renal proximal tubule cells. Anticancer Res 2012;32:4759–63
- Fanelli MA, Montt-Guevara M, Diblasi AM, Gago FE, Tello O, Cuello-Carrion FD, et al. P-cadherin and beta-catenin are useful prognostic markers in breast cancer patients: Beta-catenin interacts with heat shock protein Hsp27. Cell Stress Chaperones 2008;13:207–20
- Rosenbaum EE, Brehm KS, Vasiljevic E, Liu CH, Hardie RC, Colley NJ. XPORT-dependent transport of TRP and rhodopsin. Neuron 2011;72:602–15
- Sun Y, Zhou M, Fu D, Xu B, Fang T, Ma Y, et al. Ubiquitination of heat shock protein 27 is mediated by its interaction with SMAD ubiquitination regulatory factor 2 in A549 cells. Exp Lung Res 2011;37:568–73
- Parcellier A, Schmitt E, Gurbuxani S, Seigneurin-Berny D, Pance A, Chantome A, et al. Hsp27 is a ubiquitin-binding protein involved in I-kappaBalpha proteasomal degradation. Mol Cell Biol 2003;23:5790–802
- O’Callaghan-Sunol C, Gabai VL, Sherman MY. Hsp27 modulates p53 signaling and suppresses cellular senescence. Cancer Res 2007;67:11779–88
- Zhu Y, Tassi L, Lane W, Mendelsohn ME. Specific binding of the transglutaminase, platelet factor XIII, to HSP27. J Biol Chem 1994;269:22379–84
- Cosentino C, Grieco D, Costanzo V. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J 2011;30:546–55
- Beresford PJ, Jaju M, Friedman RS, Yoon MJ, Lieberman J. A role for heat shock protein 27 in CTL-mediated cell death. J Immunol 1998;161:161–7
- Pandey P, Farber R, Nakazawa A, Kumar S, Bharti A, Nalin C, et al. Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene 2000;19:1975–81
- Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol 2000;2:645–52
- Hayashi N, Peacock JW, Beraldi E, Zoubeidi A, Gleave ME, Ong CJ. Hsp27 silencing coordinately inhibits proliferation and promotes Fas-induced apoptosis by regulating the PEA-15 molecular switch. Cell Death Differ 2012;19:990–1002
- Choi YW, Tan YJ, Lim SG, Hong W, Goh PY. Proteomic approach identifies Hsp27 as an interacting partner of the hepatitis C virus NS5A protein. Biochem Biophys Res Commun 2004;318:514–19
- Bruinsma IB, Bruggink KA, Kinast K, Versleijen AA, Segers-Nolten IM, Subramaniam V, et al. Inhibition of alpha-synuclein aggregation by small heat shock proteins. Proteins 2011;79:2956–67
- Nemes Z, Devreese B, Steinert PM, Van Beeumen J, Fesus L. Cross-linking of ubiquitin, Hsp27, Parkin, and alpha-synuclein by gamma-glutamyl-epsilon-lysine bonds in Alzheimer’s neurofibrillary tangles. FASEB J 2004;18:1135–7
- Robertson AL, Headey SJ, Saunders HM, Ecroyd H, Scanlon MJ, Carver JA, et al. Small heat-shock proteins interact with a flanking domain to suppress polyglutamine aggregation. Proc Natl Acad Sci USA 2010;107:10424–9
- Yerbury JJ, Gower D, Vanags L, Roberts K, Lee JA, Ecroyd H. The small heat shock proteins alphaB-crystallin and Hsp27 suppress SOD1 aggregation in vitro. Cell Stress Chaperones 2013;18:251–7
- Ackerley S, James PA, Kalli A, French S, Davies KE, Talbot K. A mutation in the small heat shock protein HSPB1 leading to distal hereditary motor neuronopathy disrupts neurofilament assembly and the axonal transport of specific cellular cargoes. Hum Mol Genet 2006;15:347–54
- Shimura H, Miura-Shimura Y, Kosik KS. Binding of tau to heat shock protein 27 leads to decreased concentration of hyperphosphorylated tau and enhanced cell survival. J Biol Chem 2004;279:17957–62
- Ehrnsperger M, Graber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J 1997;16:221–9
- Jia Y, Ransom RF, Shibanuma M, Liu C, Welsh MJ, Smoyer WE. Identification and characterization of hic-5/ARA55 as an hsp27 binding protein. J Biol Chem 2001;276:39911–18
- Liu C, Gilmont RR, Benndorf R, Welsh MJ. Identification and characterization of a novel protein from Sertoli cells, PASS1, that associates with mammalian small stress protein Hsp27. J Biol Chem 2000;275:18724–31
- Havasi A, Li Z, Wang Z, Martin JL, Botla V, Ruchalski K, et al. Hsp27 inhibits Bax activation and apoptosis via a phosphatidylinositol 3-kinase-dependent mechanism. J Biol Chem 2008;283:12305–13
- Preville X, Salvemini F, Giraud S, Chaufour S, Paul C, Stepien G, et al. Mammalian small stress proteins protect against oxidative stress through their ability to increase glucose-6-phosphate dehydrogenase activity and by maintaining optimal cellular detoxifying machinery. Exp Cell Res 1999;247:61–78
- Yi MJ, Park SH, Cho HN, Yong Chung H, Kim JI, Cho CK, et al. Heat-shock protein 25 (HspB1) regulates manganese superoxide dismutase through activation of Nfkb (NF-kappaB). Radiat Res 2002;158:641–9
- Marin-Vinader L, Shin C, Onnekink C, Manley JL, Lubsen NH. Hsp27 enhances recovery of splicing as well as rephosphorylation of SRp38 after heat shock. Mol Biol Cell 2006;17:886–94
- Mehlen P, Préville X, Kretz-Remy C, Arrigo A-P. Human hsp27, Drosophila hsp27 and human aB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these protein against TNFa-induced cell death. EMBO J 1996;15:2695–706
- Dodd SL, Hain B, Senf SM, Judge AR. Hsp27 inhibits IKKbeta-induced NF-kappaB activity and skeletal muscle atrophy. FASEB J 2009;23:3415–23
- Vos MJ, Kanon B, Kampinga HH. HspB7 is a SC35 speckle resident small heat shock protein. Biochim Biophys Acta 2009;1793:1343–53
- Alford KA, Glennie S, Turrell BR, Rawlinson L, Saklatvala J, Dean JL. HSP27 functions in inflammatory gene expression and TAK1-mediated signalling. J Biol Chem 2007;282:6232–41
- Tong SW, Yang YX, Hu HD, An X, Ye F, Ren H, et al. HSPB1 is an intracellular antiviral factor against hepatitis B virus. J Cell Biochem 2013;114:162–73
- Kerr BA, Byzova TV. AlphaB-crystallin: A novel VEGF chaperone. Blood 2010;115:3181–3
- Ghosh JG, Shenoy AK, Jr., Clark JI. Interactions between important regulatory proteins and human alphaB crystallin. Biochemistry 2007;46:6308–17
- Adhikari AS, Singh BN, Rao KS, Rao Ch M. alphaB-crystallin, a small heat shock protein, modulates NF-kappaB activity in a phosphorylation-dependent manner and protects muscle myoblasts from TNF-alpha induced cytotoxicity. Biochim Biophys Acta 2011;1813:1532–42
- Liu S, Li J, Tao Y, Xiao X. Small heat shock protein alphaB-crystallin binds to p53 to sequester its translocation to mitochondria during hydrogen peroxide-induced apoptosis. Biochem Biophys Res Commun 2007;354:109–14
- Lin DI, Barbash O, Kumar KG, Weber JD, Harper JW, Klein-Szanto AJ, et al. Phosphorylation-dependent ubiquitination of cyclin D1 by the SCF(FBX4-alphaB crystallin) complex. Mol Cell 2006;24:355–66
- Fu L, Liang JJ. Detection of protein–protein interactions among lens crystallins in a mammalian two-hybrid system assay. J Biol Chem 2002;277:4255–60
- Xi JH, Bai F, McGaha R, Andley UP. Alpha-crystallin expression affects microtubule assembly and prevents their aggregation. FASEB J 2006;20:846–57
- den Engelsman J, Gerrits D, de Jong WW, Robbins J, Kato K, Boelens WC. Nuclear import of alphaB-crystallin is phosphorylation-dependent and hampered by hyperphosphorylation of the myopathy-related mutant R120G. J Biol Chem 2005;280:37139–48
- Gangalum RK, Bhat SP. The small heat shock protein alphaB-crystallin is a Golgi associated membrane protein in the developing ocular lens. Invest Ophthalmol Vis Sci 2009;50:3283–90
- Mao YW, Liu JP, Xiang H, Li DW. Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ 2004;11:512–26
- Hu WF, Gong L, Cao Z, Ma H, Ji W, Deng M, et al. alphaA- and alphaB-crystallins interact with caspase-3 and Bax to guard mouse lens development. Curr Mol Med 2012;12:177–87
- Hook D, Harding J. Alpha-crystallin acting as a molecular chaperone protects catalase against steroid-induced inactivation. FEBS Lett 1996;382:281–4
- Shinder GA, Lacourse MC, Minotti S, Durham HD. Mutant Cu/Zn-superoxide dismutase proteins have altered solubility and interact with heat shock/stress proteins in models of amyotrophic lateral sclerosis. J Biol Chem 2001;276:12791–6
- Del Vecchio PJ, MacElroy KS, Rosser MP, Church RL. Association of alpha-crystallin with actin in cultured lens cells. Curr Eye Res 1984;3:1213–19
- Wang K, Spector A. alpha-crystallin stabilizes actin filaments and prevents cytochalasin-induced depolymerization in a phosphorylation-dependent manner. Eur J Biochem 1996;242:56–66
- Singh BN, Rao KS, Ramakrishna T, Rangaraj N, Rao Ch M. Association of alphaB-crystallin, a small heat shock protein, with actin: Role in modulating actin filament dynamics in vivo. J Mol Biol 2007;366:756–67
- Ghosh JG, Houck SA, Clark JI. Interactive sequences in the stress protein and molecular chaperone human alphaB crystallin recognize and modulate the assembly of filaments. Int J Biochem Cell Biol 2007;39:1804–15
- Ohto-Fujita E, Fujita Y, Atomi Y. Analysis of the alphaB-crystallin domain responsible for inhibiting tubulin aggregation. Cell Stress Chaperones 2007;12:163–71
- Ghosh JG, Houck SA, Clark JI. Interactive domains in the molecular chaperone human alphaB crystallin modulate microtubule assembly and disassembly. PLoS One 2007;2:e498
- Djabali K, de Nechaud B, Landon F, Portier MM. AlphaB-crystallin interacts with intermediate filaments in response to stress. J Cell Sci 1997;110:2759–69
- Djabali K, Piron G, de Nechaud B, Portier MM. alphaB-crystallin interacts with cytoplasmic intermediate filament bundles during mitosis. Exp Cell Res 1999;253:649–62
- Tang G, Perng MD, Wilk S, Quinlan R, Goldman JE. Oligomers of mutant glial fibrillary acidic protein (GFAP) Inhibit the proteasome system in alexander disease astrocytes, and the small heat shock protein alphaB-crystallin reverses the inhibition. J Biol Chem 2010;285:10527–37
- Muchowski PJ, Valdez MM, Clark JI. AlphaB-crystallin selectively targets intermediate filament proteins during thermal stress. Invest Ophthalmol Vis Sci 1999;40:951–8
- Barton KA, Hsu CD, Petrash JM. Interactions between small heat shock protein alpha-crystallin and galectin-related interfiber protein (GRIFIN) in the ocular lens. Biochemistry 2009;48:3956–66
- Thedieck C, Kalbacher H, Kratzer U, Lammers R, Stevanovic S, Klein G. AlphaB-crystallin is a cytoplasmic interaction partner of the kidney-specific cadherin-16. J Mol Biol 2008;378:145–53
- Devlin GL, Carver JA, Bottomley SP. The selective inhibition of serpin aggregation by the molecular chaperone, alpha-crystallin, indicates a nucleation-dependent specificity. J Biol Chem 2003;278:48644–50
- Sun G, Guo M, Shen A, Mei F, Peng X, Gong R, et al. Bovine PrPC directly interacts with alphaB-crystalline. FEBS Lett 2005;579:5419–24
- Hatters DM, Lindner RA, Carver JA, Howlett GJ. The molecular chaperone, alpha-crystallin, inhibits amyloid formation by apolipoprotein C-II. J Biol Chem 2001;276:33755–61
- Shammas SL, Waudby CA, Wang S, Buell AK, Knowles TP, Ecroyd H, et al. Binding of the molecular chaperone alphaB-crystallin to Abeta amyloid fibrils inhibits fibril elongation. Biophys J 2011;101:1681–9
- Inagaki N, Hayashi T, Arimura T, Koga Y, Takahashi M, Shibata H, et al. AlphaB-crystallin mutation in dilated cardiomyopathy. Biochem Biophys Res Commun 2006;342:379–86
- Spector A, Li LK, Augusteyn RC, Schneider A, Freund T. Alpha-crystallin: The isolation and characterization of distinct macromolecular fractions. Biochem J 1971;124:337–43
- Kamradt MC, Lu M, Werner ME, Kwan T, Chen F, Strohecker A, et al. The small heat shock protein alpha B-crystallin is a novel inhibitor of TRAIL-induced apoptosis that suppresses the activation of caspase-3. J Biol Chem 2005;280:11059–66
- Li DW, Liu JP, Mao YW, Xiang H, Wang J, Ma WY, et al. Calcium-activated RAF/MEK/ERK signaling pathway mediates p53-dependent apoptosis and is abrogated by alphaB-crystallin through inhibition of RAS activation. Mol Biol Cell 2005;16:4437–53
- Liu JP, Schlosser R, Ma WY, Dong Z, Feng H, Lui L, et al. Human alphaA- and alphaB-crystallins prevent UVA-induced apoptosis through regulation of PKCalpha, RAF/MEK/ERK and AKT signaling pathways. Exp Eye Res 2004;79:393–403
- Rajasekaran NS, Connell P, Christians ES, Yan LJ, Taylor RP, Orosz A, et al. Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell 2007;130:427–39
- van den Ljssel P, Wheelock R, Prescott A, Russell P, Quinlan RA. Nuclear speckle localisation of the small heat shock protein alphaB-crystallin and its inhibition by the R120G cardiomyopathy-linked mutation. Exp Cell Res 2003;287:249–61
- Havugimana PC, Hart GT, Nepusz T, Yang H, Turinsky AL, Li Z, et al. A census of human soluble protein complexes. Cell 2012;150:1068–81
- Carra S, Seguin SJ, Lambert H, Landry J. HspB8 chaperone activity toward poly(Q)-containing proteins depends on its association with Bag3, a stimulator of macroautophagy. J Biol Chem 2008;283:1437–44
- Fontaine JM, Sun X, Hoppe AD, Simon S, Vicart P, Welsh MJ, et al. Abnormal small heat shock protein interactions involving neuropathy-associated Hsp22 (HspB8) mutants. FASEB J 2006;20:2168–70
