Abstract
Purpose: A systematic review and meta-analysis was conducted to evaluate the outcome of controlled clinical trials in head and neck cancers (HNCs) using hyperthermia and radiotherapy versus radiotherapy alone. Material and methods: A total of 498 abstracts were screened from four databases and hand searched as per the PRISMA guidelines. Only two-arm studies treating HNCs with either radiotherapy alone, or hyperthermia and radiotherapy without concurrent chemotherapy or surgery were considered. The evaluated end point was complete response (CR). Results: Following a detailed screening of the titles, abstracts and full text papers, six articles fulfilling the above eligibility criteria were considered. In total 451 clinical cases from six studies were included in the meta-analysis. Five of six trials were randomised. The overall CR with radiotherapy alone was 39.6% (92/232) and varied between 31.3% and 46.9% across the six trials. With thermoradiotherapy, the overall CR reported was 62.5% (137/219), (range 33.9–83.3%). The odds ratio was 2.92 (95% CI: 1.58–5.42, p = 0.001); the risk ratio was 1.61 (95% CI: 1.32–1.97, p < 0.0001) and the risk difference was 0.25 (95% CI: 0.12–0.39, p < 0.0001), all in favour of combined treatment with hyperthermia and radiotherapy over radiotherapy alone. Acute and late grade III/IV toxicities were reported to be similar in both the groups. Conclusions: Hyperthermia along with radiotherapy enhances the likelihood of CR in HNCs by around 25% compared to radiotherapy alone with no significant additional acute and late morbidities. This level I evidence should justify the integration of hyperthermia into the multimodality therapy of HNCs.
Introduction
The management of head and neck cancers (HNCs) involves a judicious combination of radiotherapy (RT), surgery and chemotherapy (CT). In a bid to improve therapeutic outcome, RT has been used either as a definitive treatment with altered fractionation regimes, or in combination with concurrent CT or the epidermal growth factor receptor inhibitor cetuximab [Citation1–4]. As tumour hypoxia is known to confer radio resistance in HNCs, numerous clinical trials have been undertaken to explore sensitisation of the hypoxic cell fraction using normobaric oxygen and carbogen, hyperbaric oxygen or a wide range of pharmaceutical hypoxic cell sensitisers [Citation5]. A systematic review of 4805 patients from 32 randomised trials provided level IA evidence that hypoxic cell sensitisation along with RT can achieve significantly improved rates of loco-regional tumour control, disease-specific survival, and overall survival in HNCs [Citation5].
Hyperthermia (HT) at 39–45 °C, is considered to be one of the most potent radiosensitisers through inhibition of DNA damage repair, sensitisation of ‘S’ phase cells and hypoxic cell sensitisation, particularly of nutritionally deficient cells at low pH [Citation6–8]. Thermoradiobiologically, HT complements the modes of action of low-LET radiation (X- and gamma rays) which show limited cytotoxicity in hypoxic cells, radio-resistant ‘S’ phase cells and also on the repair of sublethal/potentially lethal radiation-induced DNA damage. This complementary action of HT with RT is reflected in the favourable outcomes from clinical trials across a wide range of malignancies [Citation7].
Another strong rationale for combining HT with RT is that it causes reoxygenation. These effects are long-lasting, affecting other RT treatment fractions, aside from the one given on the day of HT. Heat-induced reoxygenation has been demonstrated preclinically [Citation9] and clinically in patients with locally advanced breast cancer [Citation10], soft tissue sarcoma [Citation11], and in companion canine soft tissue sarcomas [Citation12,Citation13]. In all cases, improvement in oxygenation after HT was associated with better response to thermoradiotherapy (HTRT). Furthermore, HT demonstrates additive or synergistic effects with a number of chemotherapeutic agents and both thermochemotherapy (HTCT) and thermochemoradiotherapy (HTCTRT) have yielded encouraging results [Citation7].
Most authors report a better outcome with combined HT and RT in HNCs when compared to historical controls or prospective case control studies [Citation14–26]. Some studies were undertaken for specific disease sites, such as the nasopharynx or metastatic neck nodes, while others have included all head and neck tumour subsites. A few studies have reported their results along with other tumour sites (mixed tumour group) and the outcomes for HNCs were reported separately. However, these individual studies had a limited number of patients in the RT or HTRT treatment groups. These small sample sizes could result in some uncertainty in the interpretation of the study outcomes. Thus, a systematic review of the literature was undertaken and a meta-analysis performed to evaluate the efficacy of HTRT in terms of achieving complete response (CR) of the HNCs.
Materials and methods
Search strategy
The systematic review and meta-analysis was conducted as per the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines designed to improve the reporting of systematic reviews and meta-analysis [Citation27]. The 27-item checklist and four-phase flow chart deal with identification of the records searched, screening of the records searched, assessing the eligibility of the short-listed articles after going through the full text and finally including studies that are considered for both qualitative and quantitative synthesis (meta-analysis).
For the present systematic review and meta-analysis, four databases, namely PubMed, Embase, Scopus and the Cochrane library were considered and they were last searched on 14 June 2015 (). The MeSH terms used were ‘Head and Neck Neoplasms’ AND ‘Radiotherapy’ AND ‘Hyperthermia, Induced’. The search was not limited to any date or language. Additional papers were retrieved through a hand search.
Inclusion criteria
Only two-arm studies (both randomised and non-randomised) fulfilling the following criteria were included: (1) HNCs treated with local HT and RT (those using concurrent CT, interstitial brachytherapy and/or surgery were excluded), (2) treatment outcome in terms of CRs were reported and (3) full text articles in English were available.
Study selection
Following exclusion of duplicates, articles were screened based on their titles and abstracts. Topics unrelated to HNCs, in vitro thermoradiobiological studies, technical papers on HT instrumentation, thermal dose, reviews, case reports, use of concurrent CT with RT and/or HT, use of interstitial brachytherapy, nanotechnology, re-treatments and non-English articles were excluded as detailed in . Articles that had been updated in a later publication by the same author/s and those with mixed patient groups in which the outcomes for HNCs were not given separately were omitted.
Data extraction and quality assessment
The primary end point was CR at the end of treatment and was assessed by clinical examination or imaging studies. Details of the pre-treatment patient characteristics and RT and HT parameters were tabulated as per the information cited in the articles (). Although most of the studies reported CR in terms of number of patients (4/6), two studies expressed CR with respect to the number of lesions.
Table 1. Summary of the key patient and treatment characteristics along with the outcome of the head and neck studies (radiotherapy versus radiotherapy and hyperthermia) included in the meta-analysis.
Acute and late toxicities were checked in each of the studies. As these studies were reported over a period of 27 years (1987–2014), uniform toxicity scoring criteria could not be expected. The toxicity and scoring criteria when available are given in .
Critical appraisal
Based on the pre-defined study criteria, study quality was assessed according to the PRISMA guidelines [Citation27]. All possible factors relating to patient characteristics and treatment parameters that could have an impact on the outcome were evaluated. Only the studies that reported a CR for the patients treated with RT and HT, or where a CR could be calculated from the data presented in the articles were considered.
Statistical methods
The Comprehensive Meta-analysis Software package (version 3.0) was used to execute the meta-analysis [Citation28]. The descriptive statistical analysis was carried out using IBM SPSS version 21.0 [Citation29]. The odds ratio (OR), risk ratio (RR) and risk difference (RD) and numbers needed to treat (NNT) were calculated and the values expressed using a 95% confidence interval (CI) and the corresponding p values. Heterogeneity was assessed by the I2 statistic, which represents the estimated proportion of unexplained inter-study variance prior to pooling of the studies. A random effect model was used for all analyses. The potential publication bias was evaluated by funnel plots and rank correlation tests with Kendall’s tau [Citation30]. All p values are two-sided and considered statistically significant if less than 0.05.
Results
A total of 498 articles were identified through the search and were screened as detailed in . Following the exclusion of duplicates, 348 articles were screened based on their title and abstracts and 335 of these were omitted as they did not meet the inclusion criteria. Thirteen articles were shortlisted for full text review from which seven articles were further excluded as detailed in [Citation14–20]. Six articles involving 451 clinical cases, of which 232 were treated with RT alone and 219 by HTRT, were subjected to the meta-analysis () [Citation21–26].
Five of these six studies were randomised trials. One study was carried out exclusively in nasopharyngeal tumours [Citation21], while others were for all HNCs. Most of the patients included had locally advanced stage III or IV tumours. No concurrent CT or surgery was used. The RT dose varied from 32–80 Gy and was usually delivered at 1.8–2 Gy per fraction. Perez et al. [Citation24] delivered 32 Gy at 4 Gy per fraction twice a week, while Arcangeli et al. [Citation26] treated their patients with three fractions per day of 1.5–2 Gy each with an interfraction interval of 4 h.
Hyperthermia was delivered using microwaves (n = 2) or radiofrequency (n = 4) at 8–915 Mhz and the time intervals varied from immediate to 60 min (). In all but one study HT was applied after RT [Citation25]. Four trials used HT twice a week along with RT, while it was used once a week by Huilgol et al. [Citation22] and on alternate days by Arcangeli et al. [Citation26]. In most studies, a temperature of 42.5 °C was attained and maintained for 20–45 min. Single or multi-sensor invasive thermometry using thermistors or thermocouples was used in these studies. Wen et al. [Citation21] used thermometry probes attached to their applicator for tumour surface temperature measurements. The details of the HT and RT parameters for each study are stated in .
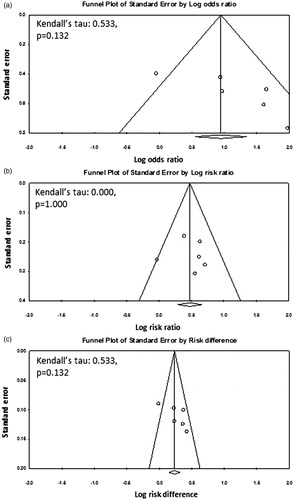
With RT alone, an overall CR of 39.6% (92/232) was reported and this varied between 31.3% and 46.9% across the six trials. The overall CR with HTRT was 62.5% (137/219), and ranged from 33.9% to 83.3%. The resultant OR was 2.92 (95% CI: 1.58–5.42, p = 0.001) with I2 of 55.38 (p = 0.047) (). The corresponding RR was 1.61 (95% CI: 1.32–1.97, p < 0.0001, I2 = 13.37, p = 0.329) and RD was 0.25 (95% CI: 0.12–0.39, p < 0.0001, I2 = 59.44, p = 0.031) in favour of combined treatment with HT and RT over RT alone (). The funnel plots and the Kendall’s tau for OR, RR and RD did not show any publication bias (). However, this should be interpreted with caution considering the limited number of studies available for this meta-analysis [Citation30].
Figure 2. Forest plots from the six individual two-arm studies depicting (a) the odds ratio, (b) risk ratio, (c) risk difference.
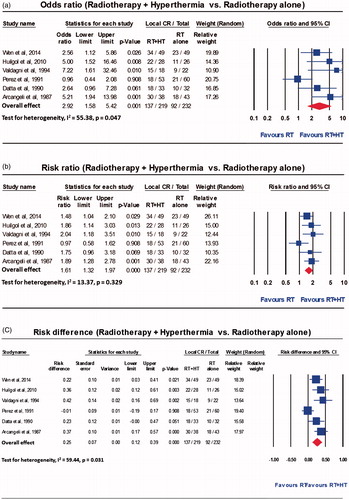
Figure 3. Funnel plots along with the Kendall’s tau and the p values for (a) odds ratio, (b) risk ratio and (c) risk difference for clinical trials with radiotherapy alone versus radiotherapy and hyperthermia.
The toxicity observed in these patients for each study is indicated in . It was not feasible to compute the toxicity profiles as the acute and late toxicities were not uniformly reported based on standard scoring criteria. However, none of the studies reported any significant increase in toxicities with HTRT compared to RT alone.
Discussion
Radiation therapy in HNCs is known to offer an advantage in terms of organ preservation and function. This has been feasible in early T1 and T2 tumours with an acceptable local control of 70–90%. However, RT alone is often inadequate for T3–T4 tumours and concurrent CT, biological therapies or altered fractionation strategies are required to improve treatment outcomes. These could be associated with increased toxicity depending on the treatment intensification [Citation1–3,Citation31]. The adoption of newer treatment techniques, such as intensity-modulated radiotherapy (IMRT) supported by image guidance, has shown that treatment-related morbidities for patients with HNCs can be reduced, resulting in an improved quality of life [Citation32].
Several groups reporting both single-arm and controlled clinical trials have advocated HT with RT, CT or both over the past four decades. This was based on the strong thermo-radiobiological basis of HT and its sensitising abilities to RT and CT [Citation5–9,Citation12]. However, with the technological advancements in HT treatment delivery, treatment planning and thermometry, HT can now be delivered with more certainty, thereby ensuring safer and more effective treatment without significant additional morbidity [Citation7].
Although some of the individual studies carried out in locally advanced HNCs have been promising, HT has still failed to gain popularity among the oncology community. This is perhaps due to a lack of adequate-sized randomised clinical trials, a dearth of HT equipment tailored to the head neck region, inadequate thermometry, and paucity of HT treatment planning software. The present systematic review and meta-analysis was undertaken to evaluate the outcomes of all previous trials using HTRT versus RT alone to seek a level I evidence of the effectiveness of HTRT in HNCs.
Several studies have shown that CR is a strong predictor of survival in HNCs [Citation33,Citation34]. In addition, Michiels et al. [Citation35] used individual patient data from 104 trials that included 22,744 irradiated patients to show that locoregional control and event-free survival correlated with overall survival in locally advanced HNCs. We therefore chose attainment of CR at the end of definitive treatment by RT or HTRT as the primary end point.
Five of the six trials reported the long-term survival outcomes using different end points (). It was evident that the HTRT patients fared better compared to RT alone in all studies. The longest survival figures reported by Valdagni et al. [Citation23] show that patients with HTRT had significantly better freedom from local relapse (HTRT versus RT: 68.6% versus 24.2%, p = 0.015) and overall survival (HTRT versus RT: 53.3% versus 0%, p = 0.02) at 5 years. The Radiation Therapy Oncology Group (RTOG) trial reported by Perez et al. [Citation24] did not report the survival outcomes separately for HNCs.
The OR, RR and RD in this meta-analysis all indicate that a significantly better outcome was achieved with HTRT as compared with RT alone. However, it was also noted that there was significant heterogeneity as indicated by the I2 statistics for OR (I2 = 55.38, p = 0.047) and RD (I2 = 59.44, p = 0.031) (). The forest plots suggest that this could be due to the outcomes reported by Perez et al. where no significant advantage was evident with HTRT over RT alone [Citation24].
To explore the cause of the heterogeneity, the computation of OR, RR and RD were repeated by excluding the RTOG trial reported by Perez et al. [Citation24]. This resulted in a revised OR of 3.69 (95% CI: 2.32–5.89, I2 = 0.000, p = 0.618), RR of 1.74 (95% CI: 1.43–2.13, I2 = 0.000, p = 0.847) and RD of 0.31 (95% CI: 0.21–0.41, I2 = 0.000, p = 0.656). The revised calculations show a significant fall in I2 values to 0.000, indicating no heterogeneity in the outcomes from the remaining five trials. This confirmed that the heterogeneity was solely due to the results from Perez et al. [Citation24]. However, the effect measures from the five studies remained similar to the original values. A closer perusal of the RTOG study from Perez et al. [Citation24] revealed that the phase III trial was for a mixed patient group of 307 patients, in whom 113 lesions pertained to HNCs. The authors stated that 50% of all the lesions in the trial had been previously treated with irradiation. No separate outcomes were available for those being re-irradiated. Moreover, 30% of the patients received less than 90% of the prescribed RT dose (32 Gy). Only 52% of the patients of the HTRT arm were treated as per the desired RT and HT dose schedules outlined in the protocol. Furthermore, only 56% of the lesions less than 3 cm in diameter and 36% of larger tumours received ‘adequate’ therapy. Thus, a negative outcome in patients subjected to HTRT in this study should be considered in the context of these mitigating factors. This paper highlights the need for proper patient selection, adequate heating techniques and patient compliance before a therapeutic intervention is judged not to be beneficial. Furthermore, on evaluating the reasons for the negative results, it became evident that there were also issues due to the lack of quality assurance in the delivery of HT [Citation36]. This eventually led to the establishment of RTOG guidelines for conduct of HTRT trials [Citation37].
Evidence from this meta-analysis indicates that HTRT can improve therapeutic outcomes compared to RT alone. The significant RD of 0.25 (increase in CR of at least 25%) could translate into better survival outcomes with HTRT. Particularly promising is that HT, unlike modalities such as CT or biotherapies, may not add to the morbidity of RT. As concurrent chemoradiotherapy (CTRT) is one of the accepted treatment approaches in locally advanced HNCs, it would also be worth exploring the integration of HT with CTRT, especially with cisplatin.
Cisplatin is an active component of most CTRT regimes in HNCs [Citation1]. Further, preclinical studies and various phase I/II clinical have shown that trimodality therapy with cisplatin and HTRT can achieve a synergistic effect which could translate into clinical efficacy [Citation38]. Two recent randomised trials in nasopharyngeal cancer compared HTRT versus RT alone. Cisplatin was used in both arms [Citation18,Citation19]. Kang et al. [Citation18] randomised 154 patients with stages N2 and N3 nasopharyngeal carcinoma to receive either CTRT (n = 78) or HTCTRT. They observed a significantly better CR and 5-year overall survival in patients treated with HTCTRT compared to CTRT alone (CR: 81.6% versus 62.8%, p < 0.05, 5-year overall survival 68.4% versus 50%, p < 0.05 for HTCTRT and CTRT respectively). Hua et al. [Citation19] randomised 180 patients with nasopharyngeal cancers (all stages) to either CTRT (n = 90) or HTCTRT (n = 90). Chemotherapy consisted of cisplatin and 5-FU. Patients with HTCTRT had a better CR rate (HTCTRT versus CTRT: 95.6% versus 81.1%, p = 0.003) and 5-year progression-free survival (HTCTRT versus CTRT: 72.7% versus 63.1%, p = 0.039). Acute and late morbidities were comparable in both groups. Thus, these studies showed that HTCTRT with cisplatin is safe, feasible and more effective than CTRT alone. Consequently, the addition of HT could be explored in other HNC subsites where cisplatin is an integral part of the CTRT treatment.
There has been considerable interest in the human papillomavirus (HPV) positive head and neck tumours and their enhanced sensitivity to radiation [Citation39–41]. Similar observations are also evident for cervical cancers [Citation42,Citation43]. A number of mechanisms have been proposed to explain the relative radiosensitivity of these HPV tumours [Citation44]. For example, HPV could cause a temporary down regulation of the p53 and pRb pathways. During the course of radiotherapy, a gradual reduction in HPV levels could result in the repression of E6 and E7 oncoproteins, resulting in restoration of the dormant p53 and pRb apoptotic pathways [Citation45]. Recently, it has also been shown that exposure of HPV positive cell lines to a temperature of 42 °C for 1 h resulted in E6 degradation, thereby preventing the formation of the E6-p53 complex. p53-dependent apoptosis and G2-phase arrest ensued [Citation46]. Thus, HT along with RT could result in a synergistic effect that would be particularly effective for the HPV positive HNCs. Moreover, HT has also been shown to be a strong immunomodulator, which could further enhance tumour response in HPV positive tumours [Citation7,Citation47].
HPV status was not reported in the six studies included in this meta-analysis and it was not possible to ascertain the effect of HTRT in HPV positive patients as it is increasingly becoming apparent that HPV-positive patients need less intensive treatment due to the increased radiosensitivity of these tumours [Citation48]. Based on the emerging preclinical evidence of synergy, HTRT should be evaluated as a less toxic therapy in future trials of HPV positive head and neck cancers.
As evident from this meta-analysis, a risk difference of 0.25 in favour of HTRT over RT alone results in a NNT figure of 4 with HTRT (). This has been achieved with neither the RT nor HT being carried out with state-of-the-art techniques. Most patients in these trials were irradiated using parallel opposed portals along with single or multiple point-based thermometry. However, with the availability of IMRT and image guidance, RT dose intensification with effective sparing of the organs at risk may result in an improved therapeutic ratio. Similarly, the recent developments in HT techniques in HNCs include special hyperthermia head and neck applicators [Citation49], feasibility of HT treatment planning [Citation50–53], non-invasive magnetic resonance-based thermometry [Citation54] and the use of integrated online complaint-adaptive steering of specific absorption rate (SAR) during real time HT sessions [Citation55]. All these could improve the delivery of HT and allay the initial scepticism regarding the efficacy and safety of HT.
With the strong clinical evidence that HTRT improves outcome without additional morbidity compared with RT alone in HNCs, HT should be integrated as a key therapeutic component in the multimodality therapy of these tumours in routine clinical practice. However, well-designed randomised trials are required to determine whether the therapeutic efficacy of HTRT could be further augmented using triple modality treatment in the management of locally advanced HNCs with acceptable morbidity. The trials could be designed to answer whether 1) HTRT is an effective treatment for HPV positive HNCs, and 2) HTRT can be considered an effective alternative to concurrent CTRT with a lower morbidity. This would require a three-arm phase III randomised trial in locally advanced HNCs with CTRT (as control arm) versus HTRT versus HTCTRT. HPV status could be incorporated as a stratification factor in the trial design and a subgroup analysis with an adequate sample size could be conducted to explore the outcomes according to HPV status. Such a trial would be paramount in defining the place of HT in the management of locally advanced HNCs.
Conclusion
The present systematic review and meta-analysis provides level I evidence of the efficacy of HTRT over RT alone in HNCs. With the current availability of sophisticated hardware and software for HT treatment delivery and monitoring, it is time to integrate HT as an essential component of the multimodality therapeutic approach, especially for locally advanced HNCs. Future randomised trials are needed to evaluate the addition of concurrent CT to HTRT and also to examine the role of HTRT in HPV positive tumours.
Acknowledgement
We gratefully acknowledge the guidance and critical inputs from Michael Borenstein at Biostat Inc., Englewood, NJ, USA, on the meta-analysis carried out in this study.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Kodaira T, Nishimura Y, Kagami Y, Ito Y, Shikama N, Ishikura S, et al. Definitive radiotherapy for head and neck squamous cell carcinoma: Update and perspectives on the basis of EBM. Jpn J Clin Oncol 2015;45:235–43
- Baujat B, Bourhis J, Blanchard P, Overgaard J, Ang KK, Saunders M, et al. Hyperfractionated or accelerated radiotherapy for head and neck cancer. Cochrane Database Syst Rev 2010;12:CD002026
- Pignon JP, le Maitre A, Maillard E, Bourhis J, Group M-NC. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009;92:4–14
- Levy AR, Johnston KM, Sambrook J, Donato B, Penrod JR, Corral M, et al. Indirect comparison of the efficacy of cetuximab and cisplatin in squamous cell carcinoma of the head and neck. Curr Med Res Opin 2011;27:2253–9
- Overgaard J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck – a systematic review and meta-analysis. Radiother Oncol 2011;100:22–32
- Overgaard J. The heat is (still) on – the past and future of hyperthermic radiation oncology. Radiother Oncol 2013;109:185–7
- Datta NR, Ordonez SG, Gaipl US, Paulides MM, Crezee H, Gellermann J, et al. Local hyperthermia combined with radiotherapy and/or chemotherapy: Recent advances and promises for the future. Cancer Treat Rev 2015;41:742–53
- Dewhirst MW, Vujaskovic Z, Jones E, Thrall D. Re-setting the biologic rationale for thermal therapy. Int J Hyperthermia 2005;21:779–90
- Song CW, Park H, Griffin RJ. Improvement of tumor oxygenation by mild hyperthermia. Radiat Res 2001;155:515–28
- Jones EL, Prosnitz LR, Dewhirst MW, Marcom PK, Hardenbergh PH, Marks LB, et al. Thermochemoradiotherapy improves oxygenation in locally advanced breast cancer. Clin Cancer Res 2004;10:4287–93
- Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Dodge RK, Charles HC, et al. Radiation therapy and hyperthermia improve the oxygenation of human soft tissue sarcomas. Cancer Res 1996;56:5347–50
- Thrall DE, Maccarini P, Stauffer P, Macfall J, Hauck M, Snyder S, et al. Thermal dose fractionation affects tumour physiological response. Int J Hyperthermia 2012;28:431–40
- Vujaskovic Z, Poulson JM, Gaskin AA, Thrall DE, Page RL, Charles HC, et al. Temperature-dependent changes in physiologic parameters of spontaneous canine soft tissue sarcomas after combined radiotherapy and hyperthermia treatment. Int J Radiat Oncol Biol Phys 2000;46:179–85
- Arcangeli G, Arcangeli G, Guerra A, Lovisolo G, Cividalli A, Marino C, et al. Tumour response to heat and radiation: Prognostic variables in the treatment of neck node metastases from head and neck cancer. Int J Hyperthermia 1985;1:207–17
- Valdagni R, Amichetti M, Pani G. Radical radiation alone versus radical radiation plus microwave hyperthermia for N3 (TNM-UICC) neck nodes: A prospective randomized clinical trial. Int J Radiat Oncol Biol Phys 1988;15:13–24
- Amichetti M, Romano M, Busana L, Bolner A, Fellin G, Pani G, et al. Hyperfractionated radiation in combination with local hyperthermia in the treatment of advanced squamous cell carcinoma of the head and neck: A phase I–II study. Radiother Oncol 1997;45:155–8
- Valdagni R, Kapp DS, Valdagni C. N3 (TNM-UICC) metastatic neck nodes managed by combined radiation therapy and hyperthermia: Clinical results and analysis of treatment parameters. Int J Hyperthermia 1986;2:189–200
- Kang M, Liu WQ, Qin YT, Wei ZX, Wang RS. Long-term efficacy of microwave hyperthermia combined with chemoradiotherapy in treatment of nasopharyngeal carcinoma with cervical lymph node metastases. Asian Pac J Cancer Prev 2013;14:7395–400
- Hua Y, Ma S, Fu Z, Hu Q, Wang L, Piao Y. Intracavity hyperthermia in nasopharyngeal cancer: A phase III clinical study. Int J Hyperthermia 2011;27:180–6
- Hiraki Y, Nakajo M, Miyaji N, Takeshita T, Churei H, Ogita M. Effectiveness of RF capacitive hyperthermia combined with radiotherapy for stages III and IV oro-hypopharyngeal cancers: A non-randomized comparison between thermoradiotherapy and radiotherapy. Int J Hyperthermia 1998;14:445–57
- Wen QL, He LJ, Ren PR, Chen CQ, Wu JB. Comparing radiotherapy with or without intracavitary hyperthermia in the treatment of primary nasopharyngeal carcinoma: A retrospective analysis. Tumori 2014;100:49–54
- Huilgol NG, Gupta S, Sridhar CR. Hyperthermia with radiation in the treatment of locally advanced head and neck cancer: A report of randomized trial. J Cancer Res Ther 2010;6:492–6
- Valdagni R, Amichetti M. Report of long-term follow-up in a randomized trial comparing radiation therapy and radiation therapy plus hyperthermia to metastatic lymph nodes in stage IV head and neck patients. Int J Radiat Oncol Biol Phys 1994;28:163–9
- Perez CA, Pajak T, Emami B, Hornback NB, Tupchong L, Rubin P. Randomized phase III study comparing irradiation and hyperthermia with irradiation alone in superficial measurable tumors. Final report by the Radiation Therapy Oncology Group. Am J Clin Oncol 1991;14:133–41
- Datta NR, Bose AK, Kapoor HK, Gupta S. Head and neck cancers: Results of thermoradiotherapy versus radiotherapy. Int J Hyperthermia 1990;6:479–86
- Arcangeli G, Benassi M, Cividalli A, Lovisolo GA, Mauro F. Radiotherapy and hyperthermia. Analysis of clinical results and identification of prognostic variables. Cancer 1987;60:950–6
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009;339:b2700
- Comprehensive Meta-analysis Software, version 3.0. Available from http://www.meta-analysis.com/index.php
- IBM SPSS software. Available from http://www-01.ibm.com/support/docview.wss?uid=swg21608060
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101
- Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 2010;11:21–8
- Marta GN, Silva V, de Andrade Carvalho H, de Arruda FF, Hanna SA, Gadia R, et al. Intensity-modulated radiation therapy for head and neck cancer: Systematic review and meta-analysis. Radiother Oncol 2014;110:9–15
- Suntharalingam M, Haas ML, van Echo DA, Haddad R, Jacobs MC, Levy S, et al. Predictors of response and survival after concurrent chemotherapy and radiation for locally advanced squamous cell carcinomas of the head and neck. Cancer 2001;91:548–54
- Hauswald H, Simon C, Hecht S, Debus J, Lindel K. Long-term outcome and patterns of failure in patients with advanced head and neck cancer. Radiat Oncol 2011;6:70
- Michiels S, Le Maitre A, Buyse M, Burzykowski T, Maillard E, Bogaerts J, et al. Surrogate endpoints for overall survival in locally advanced head and neck cancer: Meta-analyses of individual patient data. Lancet Oncol 2009;10:341–50
- Perez CA, Gillespie B, Pajak T, Hornback NB, Emami B, Rubin P. Quality assurance problems in clinical hyperthermia and their impact on therapeutic outcome: A report by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1989;16:551–8
- Dewhirst MW, Phillips TL, Samulski TV, Stauffer P, Shrivastava P, Paliwal B, et al. RTOG quality assurance guidelines for clinical trials using hyperthermia. Int J Radiat Oncol Biol Phys 1990;18:1249–59
- Bergs JW, Franken NA, Haveman J, Geijsen ED, Crezee J, van Bree C. Hyperthermia, cisplatin and radiation trimodality treatment: A promising cancer treatment? A review from preclinical studies to clinical application. Int J Hyperthermia 2007;23:329–41
- Lohaus F, Linge A, Tinhofer I, Budach V, Gkika E, Stuschke M, et al. HPV16 DNA status is a strong prognosticator of loco-regional control after postoperative radiochemotherapy of locally advanced oropharyngeal carcinoma: Results from a multicentre explorative study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Radiother Oncol 2014;113:317–23
- Pang E, Delic NC, Hong A, Zhang M, Rose BR, Lyons JG. Radiosensitization of oropharyngeal squamous cell carcinoma cells by human papillomavirus 16 oncoprotein E6 *I. Int J Radiat Oncol Biol Phys 2011;79:860–5
- Xie X, Piao L, Bullock BN, Smith A, Su T, Zhang M, et al. Targeting HPV16 E6-p300 interaction reactivates p53 and inhibits the tumorigenicity of HPV-positive head and neck squamous cell carcinoma. Oncogene 2014;33:1037–46
- Datta NR, Kumar P, Singh S, Gupta D, Srivastava A, Dhole TN. Does pretreatment human papillomavirus (HPV) titers predict radiation response and survival outcomes in cancer cervix? A pilot study. Gynecol Oncol 2006;103:100–5
- Kim JY, Park S, Nam BH, Roh JW, Lee CH, Kim YH, et al. Low initial human papilloma viral load implicates worse prognosis in patients with uterine cervical cancer treated with radiotherapy. J Clin Oncol 2009;27:5088–93
- Vozenin MC, Lord HK, Hartl D, Deutsch E. Unravelling the biology of human papillomavirus (HPV) related tumours to enhance their radiosensitivity. Cancer Treat Rev 2010;36:629–36
- Datta NR, Singh S, Kumar P, Gupta D. Human papillomavirus confers radiosensitivity in cancer cervix: A hypothesis toward a possible restoration of apoptotic pathways based on clinical outcomes. Future Oncol 2015;11:1363–71
- Oei AL, van Leeuwen CM, ten Cate R, Rodermond HM, Buist MR, Stalpers LJA, et al. Hyperthermia selectively targets human papillomavirus in cervical tumors via p53-dependent apoptosis. Cancer Res 2015 (in press)
- Schmidtner J, Distel LV, Ott OJ, Nkenke E, Sprung CN, Fietkau R, et al. Hyperthermia and irradiation of head and neck squamous cancer cells causes migratory profile changes of tumour infiltrating lymphocytes. Int J Hyperthermia 2009;25:347–54
- Masterson L, Moualed D, Liu ZW, Howard JE, Dwivedi RC, Tysome JR, et al. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma: A systematic review and meta-analysis of current clinical trials. Eur J Cancer 2014;50:2636–48
- Togni P, Rijnen Z, Numan WC, Verhaart RF, Bakker JF, van Rhoon GC, et al. Electromagnetic redesign of the HYPERcollar applicator: Toward improved deep local head-and-neck hyperthermia. Phys Med Biol 2013;58:5997–6009
- Paulides MM, Stauffer PR, Neufeld E, Maccarini PF, Kyriakou A, Canters RA, et al. Simulation techniques in hyperthermia treatment planning. Int J Hyperthermia 2013;29:346–57
- Verhaart RF, Fortunati V, Verduijn GM, van der Lugt A, van Walsum T, Veenland JF, et al. The relevance of MRI for patient modeling in head and neck hyperthermia treatment planning: A comparison of CT and CT-MRI based tissue segmentation on simulated temperature. Med Phys 2014;41:123302
- Verhaart RF, Fortunati V, Verduijn GM, van Walsum T, Veenland JF, Paulides MM. CT-based patient modeling for head and neck hyperthermia treatment planning: Manual versus automatic normal-tissue-segmentation. Radiother Oncol 2014;111:158–63
- Verhaart RF, Rijnen Z, Fortunati V, Verduijn GM, van Walsum T, Veenland JF, et al. Temperature simulations in hyperthermia treatment planning of the head and neck region: Rigorous optimization of tissue properties. Strahlenther Onkol 2014;190:1117–24
- Pichardo S, Kohler M, Lee J, Hynnyen K. In vivo optimisation study for multi-baseline MR-based thermometry in the context of hyperthermia using MR-guided high intensity focused ultrasound for head and neck applications. Int J Hyperthermia 2014;30:579–92
- Rijnen Z, Bakker JF, Canters RA, Togni P, Verduijn GM, Levendag PC, et al. Clinical integration of software tool VEDO for adaptive and quantitative application of phased array hyperthermia in the head and neck. Int J Hyperthermia 2013;29:S181–93)