Abstract
An ever-increasing body of literature affirms the physical and biological basis for sensitisation of tumours to conventional therapies such as chemotherapy and radiation therapy by mild temperature hyperthermia. This knowledge has fuelled the efforts to attain, maintain, measure and monitor temperature via technological advances. A relatively new entrant in the field of hyperthermia is nanotechnology which capitalises on locally injected or systemically administered nanoparticles that are activated by extrinsic energy sources to generate heat. This review describes the kinds of nanoparticles available for hyperthermia generation, their activation sources, their characteristics, and the unique opportunities and challenges with nanoparticle-mediated hyperthermia.
Introduction
The notion that heat can be used for therapeutic purposes has been described in the medical literature for centuries. Commonly, heat has been used as a means of ablating cancer cells similar to surgical removal of tumours. We define this as thermoablation, a modality that utilises lethal levels of heat to irreparably coagulate proteins and other biological molecules and induce cell death. A milder rise in temperature which does not cause cell death by itself may limit some of the collateral damage to normal tissues beyond the ablation zone and could still have therapeutic benefits. We define this as hyperthermia (HT) and nominally assign it a temperature range of 41–50 °C, with a temperature above 50 °C being considered thermoablation. It is noteworthy that the consequences of a rise in temperature are dependent on duration of HT, homogeneity of temperature in tissue, tissue type, and context of treatment. The induction of cell death via necrosis and/or apoptosis of cancer cells can occur even with temperature regimes as low as 42 °C maintained for more than 1 hour [Citation1]. HT causes a sustained improvement in blood flow preferentially within tumours (and not normal tissues) resulting in increased perfusion and oxygenation of (and delivery of chemotherapy to) the poorly vascularised hypoxic core of the tumour which is generally resistant to ionising radiation. Cells residing in this hypoxic core of tumours also tend to be more acidotic, a feature that makes them more sensitive to thermal damage but less sensitive to radiation therapy [Citation2]. Collectively, these phenomena lead to an altered extracellular microenvironment that is more sensitive to heat-induced cellular damage. HT-induced cellular damage is cell-cycle independent, in contrast to radiation therapy and many chemotherapy regimens that are more cytotoxic when cells are in specific phases of the cell cycle. Complementary cell-cycle sensitivity of HT and radiation therapy allows synergy between these two treatment modalities. HT also activates immunological responses to prime tumours for eradication by the host immune response by surface display of tumour antigens for activation of tumour-specific effector T cell responses. Beyond the physiological and microenvironmental levels, at the molecular level the targets of traditional thermal therapy are a diverse array of proteins including cytoskeletal structures, plasma membrane components, intracellular enzymes and signal transduction molecules, heat shock proteins that orchestrate the refolding of damaged proteins and proteins associated with DNA repair, apoptosis and necrosis [Citation3]. Taken together, the intent of HT is to minimally raise the temperature of the target tissue to alter its physiology and biology, and in practice, prime the tissue for a greater response to other therapeutic modalities such as chemotherapy or radiation therapy [Citation4].
Beginning with the early observations by Dr William Cooley in the 1890s that infections in cancer patients are associated with tumour regression and that injection of cocktails of attenuated bacterial cultures induces a fever and a significant anti-tumour effect, there has been a revival of interest in HT for cancer therapy [Citation5]. As a new wave of thermal therapy began to find its place in modern medicine, HT saw itself as an adjunct to standard cancer therapies rather than a stand-alone therapeutic modality. Numerous clinical trials have documented that mild temperature HT can be safely administered to patients and that clinical benefits may be realised in terms of improved local control, palliation of symptoms and even overall survival [Citation6]. Despite knowledge of the therapeutic benefits of HT in cancer treatment, HT is underutilised in the clinic for a variety of reasons including the invasive methods of generating HT, the difficulty in maintaining temperatures at desired levels, the lack of monitoring and modelling of HT, and the logistical hurdles with delivering and reimbursing this treatment. Development of alternative methods of HT has been galvanised by the recognition that the effectiveness of HT is not really the problem but that the practical challenge of effecting HT drives its poor acceptance and adoption in the clinic.
Conventional hyperthermia
A variety of techniques have been employed to achieve HT in the clinic in modern practice including electromagnetic radiation (such as laser, microwave and radiofrequency) and high-intensity focused ultrasound. Depending on the extent of the body’s exposure to heat, there are three types of HT conventionally employed in clinical practice – whole body, regional, and local HT. Whole body HT is achieved using hot water blankets and thermal chambers, whereas regional HT includes the perfusion of a tumour-bearing limb with part of the patient’s blood which is taken out and warmed ex vivo, and the perfusion of the peritoneum with a heated solution of anticancer agents in cases of peritoneal malignancies such as mesothelioma [Citation7]. These methods are not tumour-specific and are technically challenging to perform repeatedly with reproducible heating levels. The third type is local HT, which is tumour-specific. Luminal and interstitial HT techniques employ customised probes and applicators placed close to the tumour individually or as a grid to achieve relatively uniform heating of the tumour. Placement of external heating sources within tumours or metal antennas that are activated by an external energy source are generally invasive, challenging for deep-seated tumours, and often result in heat distributions across the tumour that are non-uniform. External heating techniques such as ultrasound and electromagnetic phased arrays are not invasive like luminal and interstitial implants but come with their own challenges with attaining and maintaining reproducible and uniform heating levels. While HT is a treatment modality that holds a lot of promise for cancer therapy, the methods of attaining, maintaining, monitoring and modelling it suffer from many inadequacies. Therefore, there remains a continuing need for newer methods of generating HT. Ideally, these would be tumour-focused, minimally invasive, and more uniform in generating HT.
Nanoparticles for hyperthermia
Nanoparticles are traditionally defined as materials with their longest dimension less than 100 nm, although particles up to 1 μm in size are also often lumped within this definition. This review focuses primarily on metallic nanoparticles. In the case of such particles, when compared to their bulk counterparts, metals at a nanoscale often demonstrate unique properties which can be exploited for therapeutic gain. This is often due to the much larger surface area to volume ratio of nanoparticles that allows elemental metal atoms at the surface to have greatest potential for interaction with surrounding molecules. Given that most components of the cellular machinery operate at this scale to maintain structure and function, there is the possibility that scaling down to this level affords an opportunity to interact, modulate and control such cellular and/or subcellular structure and function. Advances in fabrication and engineering of nanoparticles now allow an unprecedented level of precision in the design of particles with finely tuned surface properties that can be further modified for interaction with biological systems. It is within this context that nanoparticles are envisioned as conduits for generating HT [Citation8]. Nanoscale transducers can be engineered as specific absorbers of tuned electromagnetic radiation for efficient conversion of this energy to heat, which in turn is coupled and transmitted to the tissues the nanoparticles reside within [Citation9]. The most common energy sources used for generating heat using nanoparticles are light, and alternating magnetic fields; these are outlined under each section on individual types of nanoparticles. This is not meant to be an exhaustive review of all studies to date with formulations of nanoparticles capable of generating heat. Rather, this is more of a bird’s eye view of the landscape of nanoparticle-mediated HT and a perspective on the promises and the pitfalls of this technology.
Before describing the different nanoparticles that generate heat for biological applications, there are some unique properties of nanoparticles that warrant being highlighted. First, metallic nanoparticles, being excellent conductors of heat, efficiently transmit heat generated within them to adjacent tissues. Second, when administered intravenously, they can accumulate preferentially in tumours via the enhanced permeability and retention (EPR) effect, wherein particles smaller in size than a couple of hundred nanometres passively extravasate from leaky, chaotic and immature tumour blood vessels via large fenestrations (60–400 nm) in their vascular lining and are inefficiently cleared by an underdeveloped lymphatic drainage system [Citation10,Citation11]. Counterbalancing this fortuitous synergy between nanoparticle size and pore size of tumour vasculature but not normal vasculature is the barrier to tumour accumulation posed by innate scavengers of foreign objects in circulation. Circulating macrophages and resident reticuloendothelial cells in the spleen and liver recognise these particles as foreign, engulf them and clear them from circulation. Reducing the size of the particles to below 5.5 nm has been shown to aid renal clearance and avoid reticuloendothelial capture [Citation12]. An alternative method to evade capture is to maintain a near-neutral charge on the surface of particles so plasma proteins do not adsorb to their surface, activate the complement cascade (opsonisation), and trigger macrophage clearance [Citation13–16]. Another method employed is that of decorating the surface of particles with substances that render them some stealth properties. Commonly, the surface of nanoparticles is functionalised with hydrophilic and biocompatible materials which are protease-resistant, non-immunogenic and non-antigenic such as polyethylene glycol (PEG), dextran, or chitosan [Citation15,17–19]. The resulting increase in circulation time allows greater EPR-mediated passive accumulation in tumours. Third, over and above passive accumulation in tumours, nanoparticles can be decorated with targeting molecules such as tumour-homing peptides and antibodies that allow docking to cancer-specific antigens (‘active targeting’) to achieve even greater tumour accumulation and specificity, thereby reducing collateral thermal damage to adjacent normal tissues when activated via an external trigger [Citation20]. From a mechanistic standpoint, targeted delivery of nanoparticles to tumours reduces the side effects of treatment [Citation21]. Untargeted nanoparticles enter the cells through innate cellular mechanisms of endocytosis [Citation22] where the plasma membrane engulfs and encloses the nanoparticle; this pocket detaches from the membrane to form an endocytotic vesicle as it continues on its path from an early endosome to a late endosome and eventually an endolysosome [Citation23]. Internalised nanoparticles can sterically hinder cellular processes and cause cell stress, which manifests as lower proliferation rate [Citation21], induction of oxidative stress [Citation24], cytoskeleton disruption [Citation25], hindered differentiation [Citation26] and DNA damage [Citation27]. They can also cause autophagy and lysosomal dysfunctions and other effects, which can eventually trigger apoptosis or induce necrosis [Citation28].
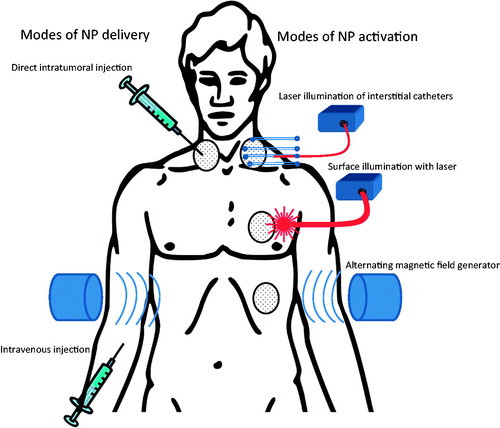
We will now highlight some of the types of nanoparticles typically used for hyperthermic treatment of cancers. The typical modes of nanoparticle delivery and activation are illustrated in .
Magnetic nanoparticles
When ferromagnetic material is magnetised to saturation (alignment of all magnetic domains) in one direction, it takes a magnetic field in the opposite direction to return it to its original state. Alternating magnetic fields (AMFs) magnetise and demagnetise these materials in opposite directions in a loop called a hysteresis loop. When these materials are scaled down to small nanoparticles, their magnetisation can fluctuate back and forth in direction randomly and rapidly with changes in temperature, and this results in measurement of magnetisation as zero (superparamagnetism) in the absence of an external magnetic field. However, in the presence of a magnetic field, these superparamagnetic nanoparticles behave like a paramagnet with a single magnetic domain (the sum of all the electron spins of all atoms in the nanoparticle aligned into a single giant magnetic moment) but with much greater magnetic susceptibility. When these superparamagnetic nanoparticles are then placed in a tuned AMF they can be resonantly excited to generate heat. The mechanism of conversion of AMF energy to heat by superparamagnetic nanoparticles can be explained by a number of theories. Néel relaxation causes energy to be released when the magnetic dipole of a particle flips between two stable orientations within a magnetic field that are separated by an energy barrier. Brownian relaxation invokes random collision with other particles and physical rotation of particles within an AMF. Hysteresis losses can occur in a magnetic material placed in an AMF. Anisotropic magnetic particles can generate frictional heating during physical rotation. While AMF can generate heat in nanoparticles in tissues, non-magnetic material in tissues can also get heated unintentionally due to dielectric losses in a low electrical conductivity material and eddy current losses in a high electrical conductivity material. Careful calibration of size, shape and coercivity of nanoparticles and tuning of the applied AMF frequency and amplitude can result in resonant excitation of just the nanoparticle and minimal off-target effects in normal tissues. The efficiency of conversion of absorbed AMF energy by a nanoparticle to heat is described as its specific absorption rate (SAR). In biological systems, the intracellular degradation and aggregation of nanoparticles can influence the preservation of SAR values. In general, superparamagnetic nanoparticles tend to be less than 20 nm and they resonate within magnetic fields ranging from 10 kHz–10 MHz, which can easily penetrate soft tissues and bones.
A notable attribute of magnetic HT is that AMF fields are typically directed towards the entire body rather than focused specifically on the tumour alone. This means that untargeted particles residing in normal organs can also be heated while the tumour is heated within the AMF. This makes treatment of tumours close to the liver or spleen challenging when nanoparticles are administered intravenously. An alternative approach is to inject the particles directly into the tumour to overcome this limitation of collateral heating of off-target tissue that has accumulated nanoparticles. This approach is associated with an inhomogeneous distribution of nanoparticles through the tissue for uniform heating. Nonetheless, direct injection affords the opportunity to administer sufficient quantities of nanoparticle into the tumour to generate HT. Other challenges with implementation of magnetic particle HT include the inability to accurately perform thermal dosimetry and the dependence of heating efficiency on thermal dose rate. Larger dose rates are achieved by applying large field amplitudes in a short time frame and result in a more sustained rise in temperature before heat is dissipated by thermal washout (blood coursing through vessels at body temperature and creating a heat sink) [Citation29]. Nonetheless, iron oxide particles that are nontoxic, non-immunogenic, biocompatible, stable, and possibly biodegradable can be generated by careful design.
Iron oxide nanoparticles
Iron oxide nanoparticles can be visualised on magnetic resonance imaging by their shortening of T1 (longitudinal relaxation – spin-lattice) relaxation time, which leads to a brighter signal on T1-weighted images and T2 (transversal relaxation – spin-spin) relaxation time, which leads to a darker signal on T2-weighted images than in the surrounding tissue. As noted previously, when placed in an AMF, they can generate heat. For clinical translation, their additional favourable attributes include their biocompatibility and the versatility of fabrication of particles with a wide range of diameters [Citation30]. They can be modified with appropriate coatings, including organic molecules such as dextran, dendrimers, chitosan, or aminosilanes or inorganic molecules such as gold to prevent aggregation and increase their circulatory half-life [Citation30]. A formulation of 12 nm Fe3O4 nanoparticles coated with aminosilane has been tested clinically by MagForce(Berlin, Germany), wherein ∼5 mL of the formulation is injected directly into recurrent glioblastomas in patients who are then subjected to twice-weekly treatment in a 100 kHz AMF while receiving 30 Gy of radiation therapy at 2 Gy/fraction. This magnetic fluid hyperthermia (MFH) holds great promise for thermotherapy of deep-seated tumours and has demonstrated a median overall survival of 13.4 months in a cohort of 59 glioblastoma patients [Citation31]. This is considerably longer than the typical median survival of about 6 months for this subset of patients. Importantly, this was achieved with minimal toxicity – reported toxicities included sweating, fever, tachycardia and convulsions [Citation31]. In the post-mortem analysis of glioblastoma patients on this phase II study who had received treatment with aminosilane-coated iron oxide nanoparticles, nanoparticles were localised to macrophages in areas of geographic tumour necrosis rather than the cancer cells themselves [Citation32]. The lack of dispersal beyond the areas of instillation argues for the need for multiple routes of instillation to cover the entire tumour, and visualisation (pre-treatment imaging) of adequate coverage of the tumour when nanoparticles are directly injected into the tumour. In contrast, when particles are injected intravenously, their biodistribution is highly dependent on the formulation used, the organ evaluated, and the time point of assessment. For instance, in one study, the maximum accumulation of Fe3O4 nanoparticles was in the lung and kidneys at 6 h, liver, brain, stomach and small intestine on day 1 post-administration and the heart and spleen on day 3 post-administration [Citation33]. The highest concentrations of nanoparticles were observed in the liver (1 day post-administration) and spleen (3 days post administration). A potential strategy to overcome the need for direct injection of magnetic nanoparticles into tumour tissue is to administer them intravenously and home them specifically to tumour cells or subcellular compartments of tumour cells to enhance treatment efficacy. One such proof-of-principle concept study is an in vitro assessment of iron oxide nanoparticles coated with crosslinked dextran and decorated with tumour-homing peptide CREKA that binds to fibrinogen in the extracellular matrix. AMF exposure of cells treated with these particles (generating HT) and cisplatin resulted in greater cytotoxicity than treatment with HT alone or cisplatin alone [Citation34] It remains to be seen whether this strategy will achieve HT in vivo with intravenous administration of the nanoparticle.
Super-paramagnetic iron oxide nanoparticles
As an extension of iron oxide nanoparticles, they can be synthesised as super-paramagnetic iron oxide nanoparticles (SPION)s. SPIONs randomly flip their magnetic dipole orientation in the absence of a magnetic field but align themselves along the magnetic field direction when placed in an external magnetic field. The resultant magnetic susceptibility is considerably higher than that of paramagnetic materials. AMF causes the particles to rapidly flip their magnetic polarity. However, there is some hysteretic loss involved in the flipping, which manifests as heat. Loading a tissue with SPIONs and then subjecting it to an AMF, generates heat. Several factors can influence the extent of this heating – the magnitude of the AMF, the size and characteristic of the SPIONs, the concentration of SPIONs in the tumour and the depth of the tumour within the body. One approach to embed large quantities of SPIONs into tumours via direct injection is to use hydrogels and organogels which entrap SPIONs within them and prevent distant migration. An exploratory study with thermosensitive chitosan and poloxamer polymers as hydrogels, alginate solution as an ionic crosslinker, and organogels derived from single-solvent or co-solvent exchange used as carriers of SPIONs noted that organogel formulations possessed the most favourable properties, i.e. high loading of SPIONs, efficient generation of heat, localisation within the centre of the tumour, and low toxicity [Citation35].
Similar to the recent studies with actively targeted iron oxide nanoparticles, SPIONs functionalised with anti-human epidermal growth factor receptor-2 aptamers have shown HT induction at 90-fold lower concentrations than untargeted SPIONs [Citation34]s. In another study, functionalisation of ultra-small paramagnetic iron oxide particles with cyclic arginine-glycine-aspartic peptides to target integrin αvβ3 and αvβ5 resulted in preferential uptake by breast cancer cells compared to unconjugated SPIONs as assessed by MR imaging, confocal microscopy and Prussian blue staining [Citation36].
Doped iron oxide
The presence of dopants in iron oxide nanoparticles has been shown to increase their specific absorption rate (SAR) and enhance efficiency of heat generation in an AMF [Citation37]. This can be further tuned by varying the size of the nanoparticles and the anisotropy of the crystal. For instance, manganese, cobalt, platinum, and nickel have been shown to significantly enhance the specific power loss (SPL) of SPIONs. However, they may also increase the oxidative instability and free radical-induced toxicity of such doped nanoparticles in biological systems. In a recent study, iron oxide doped with cobalt and mineralised within a ferritin protein cage decorated with PEG and α-melanocyte-stimulating hormone peptide has demonstrated greater magnetic anisotropy and thermal transduction efficiency than undoped samples, resulting in a strong reduction in melanoma cells in vitro [Citation38].
Even greater levels of efficiency of converting AMF energy to heat are attainable with core-shell nanoparticles with exchange-coupling of elements in a magnetically hard core with elements in a magnetically soft shell [Citation39]. However, even with a 10-fold increase in SPL with this formulation, generation of HT required direct injection of the nanoparticles into the tumour. Though not classical for doping of iron oxide with another element, intratumoural injection of yttrium aluminosilicate microspheres doped with iron oxide nanocrystals dispersed throughout the glass matrix resulted in localised therapeutic HT (≥ 43 °C) in rats with liver tumours subjected to AMF treatment [Citation40].
Gold nanoparticles
In contrast to magnetic nanoparticles that are activated in an alternating magnetic field to generate heat, gold nanoparticles are photothermally activatable. Resonance of surface plasmons of gold atoms on the surface of nanoparticles upon illumination with light at the resonance wavelength evinces unique properties of matter at a nanoscale, one of which is the efficient conversion of light energy to heat. The wavelength of greatest interest in clinical applications is the optical window where light penetrates deepest in biological tissues (with least absorption by water, melanin, oxyhaemoglobin, and deoxyhaemoglobin), i.e., the near infrared (NIR) end of the spectrum between 700 and 850 nm. Altering the size, shape and composition of gold nanoparticles allows for tuning of the resonance wavelength to the incident light [Citation41]. Accordingly, photothermally activatable gold nanoparticles have been fabricated with a surface layer of gold and an inner core that is hollow, made of dielectric material such as silica, or gold itself. Gold is considered biologically compatible because it is an inert noble metal that is thermally and chemically stable. It is also easy to conjugate biomolecules on to its surface (for PEGylation or decoration with tumour-homing peptides and antibodies). Lastly, gold has a long history of use in clinical practice (for treatment of rheumatoid arthritis).
Silica–gold nanoshells
Core-shell gold nanoparticles have a layer of gold surrounding a core made of dielectric material, most commonly silica due its biocompatibility. This metal–dielectric structure causes a red shift of the characteristic plasmon absorption spectrum of gold to the NIR region of the electromagnetic spectrum. The plasmon resonance frequency is tunable by altering the core to shell ratio. Silica–gold nanoshells (GNS) are usually 50–150 nm in diameter, stable in solution at low temperatures, and biologically non-reactive, particularly if coated with a layer of PEG. GNS measuring about 150 nm in diameter (120 nm silica core and 15 nm gold shell) are plasmon resonant at NIR wavelengths and highly efficient photothermal activators. Their size allows for EPR-mediated passive accumulation in tumours, albeit not deep penetration into tumours. Decorating their surface with covalent attachment of PEG that binds rigidly to the surface gold atoms renders them additional biocompatibility by minimising opsonisation, immune activation and sequestration in the liver and spleen, thereby increasing their blood circulation time [Citation42]. In preclinical biocompatibility, toxicity and biodistribution studies performed with these GNS prior to clinical use, there was no indication of toxicity for durations of up to 404 days [Citation43].
Initial studies used these GNS for thermoablation and demonstrated that tumour-bearing mice treated with intravenously administered GNS prior to NIR laser illumination had substantially longer survival durations than those treated with GNS and sham irradiation [Citation42]. Studies show that the increased temperatures generated by the GNS following NIR light absorption are sufficient to produce irreversible photothermal damage in subcutaneous tumours. Actively targeted gold nanoparticles to tumours in mice have demonstrated improvements in survival when compared to passively targeted nanoparticles [Citation44]. However, thermoablative monotherapy has the potential for collateral damage of adjacent normal tissues beyond the ablated tumour. In view of this concern and by extension of the observations on photothermal activation of GNS for cancer applications, a subsequent study noted that mild temperature HT with laser illumination of GNS passively accumulated in tumours results in sensitisation of tumours to radiation therapy. Murine tumours pre-treated with GNS when subjected to mild temperature HT (about 41 °C for 20 min) followed immediately by a single dose of radiation therapy (10 Gy with 125 kV X-rays) showed a tumour volume doubling time that was nearly twice that with radiation therapy alone [Citation45]. This was attributed to an initial increase in perfusion of tumours (thereby delivering more oxygen to the radioresistant hypoxic core of tumours) and a subsequent disruption of vasculature (due to the perivascular sequestration of the GNS) [Citation45]. Furthermore, a preferential sensitisation of radioresistant cancer stem-like cells was noted in follow-up studies [Citation46,Citation47]. The first of these studies noted that breast cancer xenografts decreased in volume after radiation and even more so after radiation and GNS-mediated HT, but the relative proportion of stem-like cells increased in the radiated group while they did not in the group treated with radiation and HT. A lower frequency of tumour formation was observed after transplantation of the cancer cells in limited dilutions from residual tumours following combined treatment as compared to analogous transplantation of cells from residual tumours following radiation therapy alone. This is presumably due to the localisation of stem-like cells in perivascular niches and the heat generated by GNS-mediated HT being most intense in this region where the GNS reside after passive extravasation from tumour vasculature [Citation46].
Tumour photothermal ablation has progressed significantly in the past few years with multiple on-going clinical trials based on a US FDA investigation device exemption (IDE) granted for their use as AuroShell® particles (Nanospectra Biosciences, Houston, TX). Pilot studies have been performed in prostate cancer patients to demonstrate passive accumulation in tumours upon intravenous administration [Citation48]. Understandably, the limited penetration of NIR light in biological tissues restricts applications of GNS to superficial tissues only, unless interstitial laser fibres are used for illumination of deep-seated tumours. A clinical trial of interstitial laser-based illumination for photothermal ablation of recurrent or refractory head and neck cancers is also on-going (clinicaltrials.gov NCT00848042).
Gold nanorods
Gold nanorods (GNR) are cylindrical solid gold nanoparticles that are also photothermally activatable. Altering the aspect ratio (ratio of GNR length to GNR diameter) changes plasmon resonance wavelength of the GNR. Tuning the longitudinal resonance to the NIR region by changing the aspect ratio facilitates the use of GNR for photothermal applications. Polarisation of spheres into cylindrical structures requires a surfactant during fabrication. Commonly, cetyl trimethyl ammonium bromide (CTAB) is used as the surfactant, but potential toxicity concerns with CTAB motivate attempts to remove it physically by centrifugation, filtration and dialysis or replace it with PEG [Citation49]. GNR are smaller (less than 50 nm typically) than GNS, have longer circulation times and are more efficient as photothermal activation agents [Citation50]. The strong extinction coefficient of GNR at the longitudinal surface plasmon resonance wavelength accounts for their potent photothermal activation properties, and this results in a six-fold faster heating per gram of gold with GNR than with GNS [Citation50,Citation51]. Photothermal ablation of tumours in nude mice after intravenous administration of PEGylated GNR resulted in the formation of a minor scar without tumour regrowth whereas tumours rapidly regrew after treatment with saline with or without laser illumination and PEGylated GNR without laser illumination [Citation50]. Following intravenous administration and GNR accumulation in the tumour, HT was localised to the tumour tissues with high spatial confinement as long as particles accumulate preferentially in tumour tissue compared to adjacent normal tissues [Citation52]. As noted in another study, the tumour accumulation and anti-tumour efficacy of photothermal therapy following intravenous administration of GNR were comparable to that following intratumoural administration of GNR [Citation53]. This suggests that intravenous administration is capable of achieving sufficient intratumoural accumulation to effect antitumour effects following photothermal activation without the need for direct intratumoural injection.
Hollow gold nanoshells
Hollow gold nanoshells (HGNS) comprising a hollow core with a thin outer gold shell are smaller in size (<100 nm) than silica-gold nanoshells while still being plasmon resonant at NIR wavelengths. Their synthesis involves beginning with a core–shell nanoparticle with a cobalt or silver core and a gold shell and then oxidising and sacrificing the core to leave behind a hollow centre. In turn, this hollow cavity can be used as a vehicle for drug delivery [Citation54]. The smaller size of these shells than GNS potentially allows greater extravasation from vasculature with smaller fenestrations. As with other nanostructures, functionalisation of their surface allows tumour-specific homing and delivery. Examples of this strategy include HGNS functionalised with epidermal growth factor receptor (EGFR) antibodies for targeting and ablating cells overexpressing EGFR [Citation55] and HGNS functionalised with α-melanocyte-stimulating hormone analogues for ablation of subcutaneous murine melanoma xenografts in vivo [Citation56]. One concern raised with these shells is that of in vivo instability of the hollow structure and that the residual silver or cobalt in the hollow cavity could result in standalone cytotoxicity since it does not stay contained within the HGNS [Citation57]. One potential advantage of residual cobalt present in the metallic shell is that it broadens the plasmon resonance peak of cobalt HGNS, allowing tuning of the plasmon peak without changing nanoparticle diameter [Citation58].
Gold nanomatryoshkas
Nanomatryoshka are gold nanoparticles that maintain the photothermal activation properties of GNS but are smaller in size. They comprise a gold core coupled to a thin silica shell with a gold epilayer around them; the resultant multilayered particle relies on plasmon hybridisation which involves strong coupling between the plasmons of the gold core and the shell to make them intensely photothermally activatable. As with GNS, the plasmon resonance is tunable to NIR wavelengths by altering the relative thicknesses of the core, the shell and the epilayer. Synthesised in this fashion, nanomatryoshkas smaller than 100 nm have a low-energy plasmon subradiant mode at 783 nm and a high-energy superradiant plasmon mode at 560 nm, thereby allowing greater clinical applicability [Citation59]. Initial results with these gold nanomatryoshkas suggest that they accumulate passively in orthotopic xenografts five times more efficiently than GNS and are more effective for photothermal therapy than GNS [Citation59]. These promising results argue for a role for nanomatryoshkas alongside GNS in clinical translational applications.
Hybrid nanoshells
Hybrid gold nanoshells preserve the desirable optical properties of NIR localised surface plasmon resonance of GNS and can be synthesised in size regimes between 10 and 100 nm. These particles have varied, functional (non-plasmonic) core components ranging from ‘hard’ semiconductor quantum dots to superparamagnetic nanoparticles to ‘soft’ liposomes made using poly-l-histidine as a template to direct Au deposition [Citation60]. These particles are generally engineered with the intent of lending additional functionality to GNS or nanomatryoshkas either for imaging (with magnetic resonance imaging compatible agents or with fluorescent molecules) or therapy (with SPIONs for AMF activation).
Carbon nanotubes
Carbon nanotubes (CNT) are one-dimensional nanomaterials composed of sheets of honeycomb-like lattice of carbon atoms (graphene) rolled in the shape of a tube hundreds of nanometres to microns long but only a few nanometers in diameter. Single-walled carbon nanotubes (SWCNT) have a single roll of the graphene sheet whereas multi-walled carbon nanotubes (MWCNT) are comprised of such tubes concentrically stacked within each other. CNT absorb incident energy over a broad frequency spectrum including visible light, NIR light, and even radiofrequency waves; the extinction coefficient of such absorption is considerably higher than that of natural chromophores such as melanin, haemoglobin, and water. Upon activation by electromagnetic waves, electronic transitions within the nanoparticle are activated; the relaxation that follows results in amplified vibrational modes within the carbon lattice. Tunable properties include the arrangement of carbon atoms in the wall of the cylinder for potent thermal conductivity, alterations in length and diameter, number of concentric tubes and dopants within or outside the cylinder. The tunable electrical, thermal and spectroscopic properties of these CNT make them highly versatile conduits for multiple biological applications including the generation of HT.
Direct injection of SWCNT into head and neck squamous cell carcinoma tumour xenografts in mice followed by NIR laser illumination for 3 min resulted in complete durable eradication of tumours, albeit with an eschar formed on the skin due to thermal injury [Citation61]. The nanotubes were cleared from the tumours over the course of two months largely through renal and biliary excretion. MWCNT, with enhanced absorption cross-sections when compared to SWCNT, have shown potent anti-tumour activity when injected intratumourally in renal cancer xenografts in mice prior to exposure of these tumours to short pulses (30-s treatment) of low-power (3 W/cm2) laser illumination [Citation62]. The extent of tumour regression was dose-dependent and durable long-term tumour control was observed with the higher doses of MWCNT. Alternatively, these MWCNT can be activated by tuned radiofrequency waves, potentially at the risk of some off-target toxicity. A persisting concern with carbon nanotubes is the possibility of developing granulomas reminiscent of mesotheliomas arising from asbestos exposure when the mesothelial and pleural linings of mice are exposed [Citation63].
Potential advantages of nanoparticle-mediated hyperthermia
The previous sections have highlighted some of the methods of generating HT with nanoparticles and outlined how systemically administered or locally injected nanoparticles with a high absorption cross section for transduction of an extrinsic energy source to heat can deliver heat to the tumour while preventing thermal injury to surrounding healthy tissues. In this section, we underscore several potential advantages of this form of HT over both global and focal HT achieved without nanoparticles.
Tumour targeting
A unique attribute of nanoparticle-mediated HT is the potential for confinement of HT conformally to the tumour via active targeting of the nanoparticle to the tumour as a means of enhancing the tumour-to-normal-tissue ratio of nanoparticle accumulation. This is typically achieved by decoration of nanoparticles with peptides and antibodies that allow tumour-specific homing and accumulation. The benefit of active targeting extends beyond an increase in tumour accumulation to a change in the distribution of the nanoparticle within the tumour at the level of the cell and/or tissue, i.e. cellular internalisation or confinement to specific cells of the tumour microenvironment such as fibroblasts, endothelial cells and immune cells [Citation64]. In a classical approach, glycosylated antibodies can be directionally conjugated to gold nanoparticles using a heterobifunctional linker such as using hydrazide-PEG-dithiol to link the Fc region of the antibody to the gold surface [Citation65].
‘Inside-out’ hyperthermia
Mathematical and experimental studies and first principles of thermodynamics speak to the dissipation of heat from a source following a temperature gradient away from the source with a sharp fall-off of the temperature with increasing distance from the heat source. For all external heat sources the tumour-vascular interface is a heat sink where blood travelling through the tumour vasculature at body temperature quickly dissipates the heat generated by HT within the parenchyma of the tumour. In contrast, nanoparticles that extravasate from tumour vasculature after intravenous administration tend to reside in the perivascular space if they are larger (such as GNS) or have a gradient away from the vessel with the highest concentration being in the juxtavascular area. Therefore, HT-range temperatures within tumour parenchyma are generated by a heat source sitting at the vascular interface and radiating heat from within the tumour. The vascular interface is no longer a ‘cold spot’ in the heat (isotherm) map of the tumour but a ‘hotspot’. This has two important consequences. First, the lack of adequate heating of the vascular interface (the heat sink effect) and the resulting poor efficacy of treatment due to inadequate heating of this region is no longer a therapeutic challenge. Second, vascular endothelial damage or disruption may occur and synergise with tumour cell killing to increase anti-tumour efficacy. This reversal of heat loss compared to other forms of HT is a distinctive feature of nanoparticle-mediated HT that we refer to as ‘inside-out’ HT. On the flip side, it is possible that heat generated by perivascularly confined nanoparticles may not be conducted efficiently to cell populations farther from the vasculature that tend to be hypoxic and most likely to benefit from HT. While experimental data on direct measurement of heat generated with hypoxic and normoxic areas of tumours are hard to come by, at least one study suggests that contrast perfusion into (and by inference, oxygenation of) the hypoxic cores of tumours is improved immediately following GNS-mediated HT [Citation45].
Cancer stem cell sensitisation
In many cancers, cancer stem cells are seen as a source of tumour initiation, therapeutic resistance, and tumour metastases. Elimination of cancer stem cells has proven particularly challenging with traditional therapy. Some studies suggest that stem-like cells are sensitised to radiation therapy by GNS-mediated HT. In the first such study, treatment of animal models of breast cancer with radiation and HT resulted in a greater decrease in tumour size than treatment with radiation alone, but more interestingly, treatment with radiation increased the relative proportion of stem-like cells in the tumours whereas treatment with radiation and HT did not [Citation66]. Furthermore, when treated tumours were digested and transplanted into syngeneic mice in limiting dilutions, more cells were needed to regrow tumours from mice treated with radiation and HT than mice treated with radiation alone. This lends credence to the notion that stem-like cells were probably sensitised to radiation by HT. One hypothesis is that stem-like cells reside in the perivascular niche of tumours and nanoparticle-mediated HT creates a large rise in temperature within this region due to the ‘hotspot’ formed here, as noted above. An alternative explanation is provided by an in vitro study that noted that GNR-mediated HT selectively eliminated cancer stem cells in culture and attributed this to greater and faster uptake of GNR by these cells [Citation47]. In another study comparing MWCNT-mediated HT to water-bath HT in triple negative breast cancer, cancer stem cells and non-stem cells were equally sensitised to HT with MWCNT-mediated HT whereas cancer stem cells were resistant to water-bath HT [Citation67]. This study posited that the high surface temperature of MWCNT-mediated HT irreversibly permeabilises cell membranes and causes necrotic cell death whereas water-bath HT causes apoptotic cell death. Tumours derived from a breast cancer stem cell line were eliminated after treatment with laser-induced MWCNT-mediated HT.
Theranostics
The versatility of synthesis of nanoparticles of different compositions and functionalities allows fabrication of custom nanoparticles that can be visualised by contemporaneous imaging modalities and/or newly developed imaging modalities. This would allow detecting, sensing, imaging, targeting and treatment of tumours with the same nanoparticle. This dual capability of both diagnosis and therapy is referred to as theranostics.
An important consideration when using nanoparticles for cancer therapy is quantification and/or visualisation of particles within tumours. For instance, magnetic nanoparticles can be visualised on magnetic resonance imaging as areas of signal void when high enough concentrations of iron oxide nanoparticles are present and this can be used for visualising particles as well as for treatment planning and thermal dosimetry [Citation68]. With direct injection of magnetic nanoparticles, imaging and dosimetry are readily doable. Gold nanoparticles are detectable by computed tomography when large quantities of gold are present but not readily visualisable with concentrations achieved from intravenous administration. Alternative techniques to detect gold nanoparticles in tumours include diffuse optical spectroscopy where changes in the optical absorption and scattering properties of tissue in the presence of plasmonic gold nanoparticles can provide accurate quantification of gold content. One such study validated these measurements in phantoms and tumour xenografts in mice by neutron activation analysis [Citation69]. However, diffuse optical spectroscopy does not provide spatial information on the distribution of gold nanoparticles within tumours. Narrowband imaging capitalises on the strong absorption of gold nanoparticles at their plasmon resonant wavelength and distinguishes between blood and NIR-resonant nanoparticles by imaging in narrow wavelength bands in the visible and NIR, respectively [Citation70]. This provides accurate spatial and geographical information on the heterogeneous distribution of gold nanoparticles within tumours but is restricted to shallow depths of penetration of light. Another technique to discriminate gold nanoparticles and carbon nanotubes within tissues is photoacoustic imaging, where incident optical energy from a laser source is absorbed by plasmon resonant gold nanoparticles leading to acoustic emissions that are detected by an array of ultrasound transducers [Citation71]. In addition to visualising native HT-generating nanoparticles by these techniques, nanoparticles can be readily functionalised to aid visualisation by magnetic resonance imaging (by doping them with SPIONs or gadolinium), fluorescence imaging (by doping them with fluorescent dyes), and positron emission tomography (by radiolabelling them with radioactive elements). Visualisation of spatial distribution of nanoparticles within tumours opens up the possibility of modelling HT generated by these particles and inverse treatment planning. For instance, gold nanoshell-mediated HT can be computationally modelled using the diffusion approximation of light transport theory for light distribution of lasers in tissue and a modified bioheat equation to quantify temperature rise from photothermal activation [Citation72]. In principle, this approach can handle heterogeneity of nanoparticle distributions within tumours and provides information about the contribution of each heat source to the global increase in temperature. Alternatively, using the Green’s function method to modify the heat equation, the change in optical properties of tissue when laden with gold nanoparticles can be used to quantify heat generated when illuminated with a laser [Citation73]. Similarly, knowledge of the location of magnetic nanoparticles within a tumour (from CT or MRI scans) can be used to predict heat generated using the bioheat equation and to generate a treatment plan [Citation74]. As with all models, the heat transfer in biological systems is an index of conduction loss (a physical parameter) and perfusion (a parameter defined more by physiology). Heterogeneity of heating within tumours and the effect of heat itself on perfusion (the physiological parameter, where the body regulates local temperature by increasing blood flow to dissipate heat) make modelling and treatment planning challenging. Nonetheless, visualisation of nanoparticles allows prediction of heat generated by them, at least to a first approximation, and non-invasive measurement with MR thermometry would provide validation and confirmation of the modelling.
Drug delivery
Nanoparticle formulations that generate HT can often be custom synthesised to deliver drug payloads. Such formulations allow externally-triggered thermally-mediated release of payloads such as peptides, antibodies, toxins, and oligonucleotides within the confines of the tumour alone. This circumvents the undesirable biodistribution of drug within healthy organs of the body when administered intravenously and avoids off-target accumulation and dose-limiting toxicity. For instance, HGNS have been loaded with doxorubicin such that the spatial and temporal coupling of thermal injury and drug-induced toxicity will increase the efficacy of nanoparticle-mediated thermal therapy [Citation75]. In another approach, when a 33-nm polyethylene glycol-coated colloidal gold nanoparticle with an incorporated TNF-alpha payload was used to treat mice bearing SCK mammary carcinomas, dramatic increases in tumour growth delay were observed when the formulation was combined with HT [Citation76]. Conceivably, using a nanoparticle that generates HT instead of the gold colloid would combine drug delivery and nanoparticle-mediated HT into a single construct. Similar to delivery of drugs, oligonucleotide delivery for replacement therapy or gene silencing can be achieved using plasmon resonant nanoparticles decorated on their surface with the oligonucleotide where light triggers HT generation and release of the oligonucleotide [Citation77]. An elegant example of siRNA delivery into cells in vitro is the recent demonstration that a TAT-peptide coated plasmonic HGNS with dense siRNA loading can efficiently enter an undifferentiated human embryonic stem cell and deliver siRNA to the cytosol upon NIR illumination [Citation78]. Lastly, the HT-generating nanoparticle can be encapsulated within a traditional drug-delivery nanoparticle platform such as a liposome or a polymer such as chitosan, dextran, polylactide, or poly(DL-lactide-co-glycolide).
Immunomodulation
Normal body temperature is regulated by neurovascular homeostatic mechanisms, which assist in heat dissipation during exposure to warm environments and heat generating exercises. Fever is a ‘cardinal sign’ of infection and inflammation in both endotherms and ectotherms. Owing to the prominence of fever in infectious or inflammatory environments, the immune consequences of fever have been studied extensively. Reduced IL-2 production from co-stimulation of CD4+ T cells via CD28 ligation has been reported during fever [Citation79]. Macrophages, the body’s scavengers of cellular debris, rapidly respond to various ‘alarm’ signals generated from inflammatory sites by releasing pro-inflammatory mediators [Citation80]. Raising the core temperature to 39.5 °C with mild systemic heat treatment significantly enhances the serum TNF-α and IL-6 concentration, and modulates the macrophage function in BALB/c mice challenged with lipopolysaccharide [Citation81]. This thermally enhanced production of pro-inflammatory cytokine can produce synergistic effects with conventional cancer therapy, can be beneficial in eliminating pathogens, and can hasten the resolution of inflammation [Citation82]. Furthermore, integrated actions of these immune phagocytes aids in eliminating pathogens, repairing damaged tissues, initiating an adaptive immune response, and most importantly, restoring tissue homeostasis [Citation83]. As demonstrated by the use of IL-12 in combination with fever-range whole body HT in BALB/c mice, additional immunomodulation beyond fever-range HT can also exert potent anti-tumour effects [Citation84].
As with differences noted between traditional HT and nanoparticle-mediated HT in terms of location of hotspots of temperature within tumours, sensitisation of cancer stem cells and induction of necrosis versus apoptosis, it remains to be determined whether nanoparticle-mediated HT might generate unique signatures of immunomodulation that can be exploited for therapeutic benefit. While tumour-associated antigen presentation to activate effector T cells is a hallmark of traditional HT, nanoparticle-mediated ‘inside-out’ HT could not only expose additional endothelial antigens from angiogenic tumour vasculature but also increase permeability of vasculature (or vascular disruption) to allow greater penetration of effector T cells into the tumour microenvironment. Similarly, nanoparticles internalised into cells may expose a different array of tumour neoantigens after HT generation than when HT is generated extrinsically. Lastly, enormous interest has recently been focused on mobilising the immune system against cancer and designing agents which stimulate complementary cancer-killing mechanisms [Citation85]. Nanoparticle-based drug delivery systems hold great promise in the evolving arena of cancer immunotherapy [Citation86]. A new delivery system for cisplatin describing platinum prodrug-modified PEGylated phospholipid micelle encapsulating biocompatible iron oxide nanoparticles has recently been reported. This nanoconstruct was functionalised with polyinosinic-polycytidylic acid (a double-stranded RNA analogue widely used as an adjuvant in clinical trials of cancer immunotherapy) and demonstrated significant enhancement of cytotoxicity in tumour cells and concomitantly activated dendritic cells in vitro [Citation87]. On the flip side, nanoparticles may generate immune effects that are deleterious as well. Given the concerns with carbon nanotube toxicity, a recent study noted that functionalised CNT do not adversely affect the viability of the immunocompetent cells (B cells and T cells) and macrophages in vitro and stimulated macrophages to secrete pro-inflammatory cytokines [Citation88]. Similarly, plasma proteins that adsorb on to gold nanoparticles and form a protein corona around them can influence opsonisation, complement activation, immune cell uptake and clearance, and thereby alter biodistribution. A recent study noted that the molecular weight of the PEG coating on such particles dictates the amount of protein binding within the corona but only slightly alters the composition of the protein corona [Citation89]. Furthermore, the presence of specific proteins within the corona per se does not imply that the protein is activated or altered in function; only specific functional assays such as coagulation and complement activation assays can determine this.
Persisting challenges with nanoparticle-ediated hyperthermia
Biocompatibility and scale-up
Although the development of nanoparticle-based HT systems has been heralded as an important biomedical breakthrough, concerns about their biocompatibility need to be addressed individually for each formulation and each application. As described before, concerns have been raised about the toxicity of CNT when organ surfaces such as peritoneum or pleura are exposed to them [Citation63]. Also, the CTAB used during synthesis of GNR can be toxic if present in large quantities or if dissociated from the GNR in vivo. A recent report on the biocompatibility of GNS highlights the typical battery of tests conducted to confirm biocompatibility and safety even though the bulk metal (gold) is considered safe [Citation43]. These include cytotoxicity, pyrogenicity, genotoxicity, in vitro haemolysis, intra-cutaneous reactivity, acute systemic toxicity in the mouse after single and multiple injections, and long-term toxicity in rodents and dogs monitored for over a year. Maintaining consistency in physicochemical characteristics between batches of formulations and upon scale-up from small batches to large batches are also pre-requisites for clinical translation. In addition to the standalone toxicity of the nanoparticle, the toxicity of excipients, toxicity of functionalisation moieties, and preservation of association between the core particle and its epilayers or functionalisation moieties also needs to be ascertained. The Nanotechnology Characterization Laboratory at the US National Cancer Institute assists investigators from academic institutions and industry with some of the biocompatibility testing of promising nanoparticles being considered for clinical translation. Cataloguing their experience with characterising over 250 different nanomaterials from over 75 different investigators, this group outlined some common mistakes and oversights in nanomaterial formulation in a monograph that serves as a valuable reference material for all investigators pursuing nanoparticle formulations for eventual clinical use [Citation90]. Another issue with scale-up, not in terms of manufacturing of the particles but with extending findings from rodents to humans, is that of thermoregulation in rodents being different from that of large animals and humans. Humans can readily dissipate heat by evaporation and cutaneous vasomotor alterations; rodents compensate for greater heat loss due to a higher surface-area to volume ratio of their bodies by maintaining a high metabolic rate to keep body temperature constant. Consequently, the physiological responses to hyperthermia are more robust and vigorous in rodents and extrapolation to humans can be problematic. Similarly, appropriate animal models for HT studies may be orthotopic, syngeneic or genetically engineered mouse models rather than subcutaneous xenograft models. These models are less sensitive to cutaneous thermoregulatory and homeostatic responses to HT, have greater fidelity to true tumours in humans, and in the case of syngeneic and genetically engineered mouse models, have a full complement of immune functions similar to that encountered in humans.
Heterogeneity of biodistribution
When injected into the systemic circulation, nanoparticles are distributed heterogeneously within the tumour and within the body in organs and tissues. This is dictated by shape, surface charge, hydrodynamic diameter, extent, the length and branching of the polyethylene glycol surface coating often used to confer ‘stealth’ properties for immune evasion from the reticuloendothelial system, and surface functionality of the nanoparticle [Citation91]. As noted previously, spherical nanoparticles with a zwitterionic surface charge and hydrodynamic diameter of approximately 5.5 nm are cleared by the kidneys and not entrapped within the reticuloendothelial system whereas larger nanoparticles of approximately 20 nm are captured by the reticuloendothelial macrophages [Citation45,Citation92]. Irrespective of the size of particles, when administered intravenously, there is a gradient away from the vasculature, and even tumour-specific targeting does not overcome this. Much larger particles remain in the perivascular space and do not penetrate deep into tumour parenchyma [Citation45,Citation92]. This potentially provides a therapeutic advantage due to focal vascular disruption following laser treatment and HT as noted in the radiosensitisation study with gold nanoshells described earlier [Citation45].
Efficiency of Extrinsic Energy Transduction
Large local concentrations of magnetic nanoparticles are required in a tumour for generation of heat when placed within an AMF field. This is typically not achievable with intravenous administration. Consequently, all magnetic nanoparticle-mediated HT applications to date have relied on intratumoural injection which is both invasive and non-uniform. Nevertheless, the extent of coverage of the tumour is readily visualised by MRI and deep-seated tumours can be accessed for intratumoural injection and AMF activation. On the other hand, photothermal activation of plasmon-resonant gold nanoparticles is achievable with lower concentrations of nanoparticles in tumour readily attainable via intravenous administration of the nanoparticle. In addition, light can be focused directly on the tumour and surrounding tissues with lower (off-target) accumulation of the nanoparticle can be shielded by collimation of the incident laser beam. Such focusing of AMF is not possible currently. Despite the high efficiency of transduction of extrinsic energy to heat using plasmon resonant nanoparticles, the greatest limitation of this approach is the restricted depth of penetration of incident light. Consequently, this would be a viable therapeutic strategy only for superficial tumours, the operative bed, endoscopically accessible tumours and/or tumours that can be infiltrated with light-emitting catheters.
Conclusions
HT has a long history of clinical use as a potent sensitiser of cancer to traditional therapies such as radiation therapy and chemotherapy. Nonetheless, adoption of HT in clinical practice in the modern era has been hindered by the challenges associated with achieving, maintaining, and monitoring HT. Recent advances in our understanding of fundamental properties of nanoparticles provide a framework for the use of nanoparticles as conduits for generating HT. This review outlines some of the types of nanoparticles that can be used for HT generation, their unique attributes that can be exploited for distinct gain in nanoparticle-mediated HT applications, and challenges faced with incorporation of nanoparticles into clinical workflow routines. Critical prerequisites for advancement of such nanoparticle-based HT strategies are the identification and validation of optimal nanoconstructs, methodologies for thermal activation using extrinsic energy sources, tumour-specificity, image-guidance, and thermal dosimetry. One such formulation that has made inroads into clinical practice is the use of iron oxide nanoparticles directly injected into tumours under image-guidance prior to exposure to an AMF source. While its therapeutic efficacy is being validated in larger randomised trials, similar approaches with other nanoparticles warrant being evaluated in coming years. Different techniques of nanoparticle-mediated HT will have different clinical scenarios where they will find optimal utility and these will need to be defined as clinical translational efforts are advanced. Conceivably, with successful demonstration of clinical efficacy of nanoparticle-mediated HT combined with standard-of-care cancer therapies, this technology may provide a boost to on-going efforts to integrate HT into the workflow in routine clinical practice.
Declaration of interest
This work was funded in part by institutional support from Morehouse School of Medicine and U54 CA118638 (to P.K.), University of Dammam (to A.A.) and National Institutes of Health (1R01CA155446) and the John E. and Dorothy J. Harris Endowed Professorship (to S.K.). The authors alone are responsible for the content and writing of the paper.
References
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 1984;10:787–800
- Bass H, Moore JL, Coakley WT. Lethality in mammalian cells due to hyperthermia under oxic and hypoxic conditions. Int J Radiat Biol Relat Stud Phys Chem Med 1978;33:57–67
- Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43:33–56
- Chatterjee DK, Wolfe T, Lee J, Brown AP, Singh PK, Bhattarai SR, et al. Convergence of nanotechnology with radiation therapy-insights and implications for clinical translation. Transl Cancer Res 2013;2:256–68
- Hoption Cann SA, van Netten JP, van Netten C. Dr William Coley and tumour regression: A place in history or in the future. Postgrad Med J 2003;79:672–80
- Datta NR, Ordonez SG, Gaipl US, Paulides MM, Crezee H, Gellermann J, et al. Local hyperthermia combined with radiotherapy and/or chemotherapy: Recent advances and promises for the future. Cancer Treat Rev 2015;41:742–53
- Feldman AL, Libutti SK, Pingpank JF, Bartlett DL, Beresnev TH, Mavroukakis SM, et al. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol 2003;21:4560–7
- Chatterjee DK, Diagaradjane P, Krishnan S. Nanoparticle-mediated hyperthermia in cancer therapy. Ther Deliv 2011;2:1001–14
- Krishnan S, Diagaradjane P, Cho SH. Nanoparticle-mediated thermal therapy: Evolving strategies for prostate cancer therapy. Int J Hyperthermia 2010;26:775–89
- Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul 2001;41:189–207
- Maeda H. Tumor-selective delivery of macromolecular drugs via the EPR effect: Background and future prospects. Bioconjug Chem 2010;21:797–802
- Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, et al. Renal clearance of quantum dots. Nat Biotechnol 2007;25:1165–70
- Elsabahy M, Wooley KL. Design of polymeric nanoparticles for biomedical delivery applications. Chem Soc Rev. 2012;41(7): 2545–2561
- Arvizo RR, Miranda OR, Moyano DF, Walden CA, Giri K, Bhattacharya R, Robertson JD, Rotello VM, Reid JM, Mukherjee P. Modulating pharmacokinetics, tumor uptake and biodistribution by engineered nanoparticles. PLoS One. 2011;6(9):e24374. doi: 10.1371/journal.pone.0024374. Epub 2011 Sep 13
- Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–51. doi: 10.1038/nbt.3330
- Xiao K, Li Y, Luo J, Lee JS, Xiao W, Gonik AM, Agarwal R, Lam KS. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials. 2011;32(13): 3435–3446
- Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, Tamarkin L. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004;11(3):169–83
- Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2(3):214–21
- Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268(1):235–7
- Cherukuri P, Glazer ES, Curleya SA. Targeted hyperthermia using metal nanoparticles. Adv Drug Deliv Rev 2010;62:339–45
- Wang M, Thanou M. Targeting nanoparticles to cancer. Pharmacol Res 2010;62:90–9
- Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. J Control Release 2010;145:182–95
- Pryor PR, Luzio JP. Delivery of endocytosed membrane proteins to the lysosome. Biochim Biophys Acta 2009;1793:615–24
- Manke A, Wang L, Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed Res Int 2013;2013:942916
- Xu F, Piett C, Farkas S, Qazzaz M, Syed NI. Silver nanoparticles (AgNPs) cause degeneration of cytoskeleton and disrupt synaptic machinery of cultured cortical neurons. Mol Brain 2013;6:29
- Panariti A, Miserocchi G, Rivolta I. The effect of nanoparticle uptake on cellular behavior: Disrupting or enabling functions? Nanotechnol Sci Appl 2012;5:87–100
- Singh N, Manshian B, Jenkins GJ, Griffiths SM, Williams PM, Maffeis TG, et al. NanoGenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials 2009;30:3891–914
- Elsaesser A, Howard CV. Toxicology of nanoparticles. Adv Drug Deliv Rev 2012;64:129–37
- Dennis CL, Jackson AJ, Borchers JA, Ivkov R, Foreman AR, Hoopes PJ, et al. The influence of magnetic and physiological behaviour on the effectiveness of iron oxide nanoparticles for hyperthermia. J Phys D Appl Phys 2008;41;134020:1–5
- Cassim SM, Giustini AJ, Baker I, Hoopes PJ. Development of novel magnetic nanoparticles for hyperthermia cancer therapy. Proc SPIE Int Soc Opt Eng 2011;7901:790115
- Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neuro-Oncol 2011;103:317–24
- van Landeghem FK, Maier-Hauff K, Jordan A, Hoffmann KT, Gneveckow U, Scholz R, et al. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials 2009;30:52–7
- Wang J, Chen Y, Chen B, Ding J, Xia G, Gao C, et al. Pharmacokinetic parameters and tissue distribution of magnetic Fe(3)O(4) nanoparticles in mice. Int J Nanomed 2010;5:861–6
- Kruse AM, Meenach SA, Anderson KW, Hilt JZ. Synthesis and characterization of CREKA-conjugated iron oxide nanoparticles for hyperthermia applications. Acta Biomater 2014;10:2622–9
- Le Renard PE, Jordan O, Faes A, Petri-Fink A, Hofmann H, Rufenacht D, et al. The in vivo performance of magnetic particle-loaded injectable, in situ gelling, carriers for the delivery of local hyperthermia. Biomaterials 2010;31:691–705
- Zheng SW, Huang M, Hong RY, Deng SM, Cheng LF, Gao B, et al. RGD-conjugated iron oxide magnetic nanoparticles for magnetic resonance imaging contrast enhancement and hyperthermia. J Biomater Appl 2014;28:1051–9
- Drake P, Cho HJ, Shih PS, Kao CH, Lee KF, Kuo CH, et al. Gd-doped iron-oxide nanoparticles for tumour therapy via magnetic field hyperthermia. J Mater Chem 2007;17:4914–18
- Fantechi E, Innocenti C, Zanardelli M, Fittipaldi M, Falvo E, Carbo M, et al. A smart platform for hyperthermia application in cancer treatment: Cobalt-doped ferrite nanoparticles mineralized in human ferritin cages. ACS Nano 2014;8:4705–19
- Lee JH, Jang JT, Choi JS, Moon SH, Noh SH, Kim JW, et al. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat Nanotechnol 2011;6:418–22
- Gordon AC, Lewandowski RJ, Salem R, Day DE, Omary RA, Larson AC. Localized hyperthermia with iron oxide-doped yttrium microparticles: Steps toward image-guided thermoradiotherapy in liver cancer. J Vasc Interv Radiol 2014;25:397–404
- Kennedy LC, Bickford LR, Lewinski NA, Coughlin AJ, Hu Y, Day ES, et al. A new era for cancer treatment: Gold-nanoparticle-mediated thermal therapies. Small 2011;7:169–83
- O’Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett 2004;209:171–6
- Gad SC, Sharp KL, Montgomery C, Payne JD, Goodrich GP. Evaluation of the toxicity of intravenous delivery of auroshell particles (gold-silica nanoshells). Int J Toxicol 2012;31:584–94
- Cheng FY, Chen CT, Yeh CS. Comparative efficiencies of photothermal destruction of malignant cells using antibody-coated silica@Au nanoshells, hollow Au/Ag nanospheres and Au nanorods. Nanotechnology 2009;20:425104
- Diagaradjane P, Shetty A, Wang JC, Elliott AM, Schwartz J, Shentu S, et al. Modulation of in vivo tumor radiation response via gold nanoshell-mediated vascular-focused hyperthermia: Characterizing an integrated antihypoxic and localized vascular disrupting targeting strategy. Nano Lett 2008;8:1492–500
- Atkinson RL, Zhang M, Diagaradjane P, Peddibhotla S, Contreras A, Hilsenbeck SG, et al. Thermal enhancement with optically activated gold nanoshells sensitizes breast cancer stem cells to radiation therapy. Sci Transl Med 2010;2:55ra79
- Xu Y, Wang J, Li X, Liu Y, Dai L, Wu X, et al. Selective inhibition of breast cancer stem cells by gold nanorods mediated plasmonic hyperthermia. Biomaterials 2014;35:4667–77
- Stern JM, Solomonov VV, Sazykina E, Schwartz JA, Gad SC, Goodrich GP. Initial evaluation of the safety of nanoshell-directed photothermal therapy in the treatment of prostate disease. Int J Toxicol 2015
- Mirska D, Schirmer K, Funari SS, Langner A, Dobner B, Brezesinski G. Biophysical and biochemical properties of a binary lipid mixture for DNA transfection. Colloids Surf B Biointerfaces 2005;40:51–9
- von Maltzahn G, Park JH, Agrawal A, Bandaru NK, Das SK, Sailor MJ, et al. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res 2009;69:3892–900
- Niidome T, Akiyama Y, Yamagata M, Kawano T, Mori T, Niidome Y, et al. Poly(ethylene glycol)-modified gold nanorods as a photothermal nanodevice for hyperthermia. J Biomater Sci Polym Ed 2009;20:1203–15
- Jang B, Kim YS, Choi Y. Effects of gold nanorod concentration on the depth-related temperature increase during hyperthermic ablation. Small 2011;7:265–70
- El-Sayed MA, Shabaka AA, El-Shabrawy OA, Yassin NA, Mahmoud SS, El-Shenawy SM, et al. Tissue distribution and efficacy of gold nanorods coupled with laser induced photoplasmonic therapy in ehrlich carcinoma solid tumor model. PLoS One 2013;8:e76207
- You J, Zhang G, Li C. Exceptionally high payload of doxorubicin in hollow gold nanospheres for near-infrared light-triggered drug release. ACS Nano 2010;4:1033–41
- Melancon MP, Lu W, Yang Z, Zhang R, Cheng Z, Elliot AM, et al. In vitro and in vivo targeting of hollow gold nanoshells directed at epidermal growth factor receptor for photothermal ablation therapy. Mol Cancer Ther 2008;7:1730–9
- Lu W, Xiong C, Zhang G, Huang Q, Zhang R, Zhang JZ, et al. Targeted photothermal ablation of murine melanomas with melanocyte-stimulating hormone analog-conjugated hollow gold nanospheres. Clin Cancer Res 2009;15:876–86
- Goodman AM, Cao Y, Urban C, Neumann O, Ayala-Orozco C, Knight MW, et al. The surprising in vivo instability of near-IR-absorbing hollow Au-Ag nanoshells. ACS Nano 2014;8:3222–31
- Thibodeaux CA, Kulkarni V, Chang WS, Neumann O, Cao Y, Brinson B, et al. Impurity-induced plasmon damping in individual cobalt-doped hollow Au nanoshells. J Phys Chem B 2014;118:14056–61
- Ayala-Orozco C, Urban C, Knight MW, Urban AS, Neumann O, Bishnoi SW, et al. Au Nanomatryoshkas as efficient near-infrared photothermal transducers for cancer treatment: Benchmarking against nanoshells. ACS Nano 2014;8:6372–81
- Jin Y. Multifunctional compact hybrid Au nanoshells: A new generation of nanoplasmonic probes for biosensing, imaging, and controlled release. Acc Chem Res 2014;47:138–48
- Huang N, Wang H, Zhao J, Lui H, Korbelik M, Zeng H. Single-wall carbon nanotubes assisted photothermal cancer therapy: Animal study with a murine model of squamous cell carcinoma. Lasers Surg Med 2010;42:638–48
- Burke A, Ding X, Singh R, Kraft RA, Levi-Polyachenko N, Rylander MN, et al. Long-term survival following a single treatment of kidney tumors with multiwalled carbon nanotubes and near-infrared radiation. Proc Natl Acad Sci USA 2009;106:12897–902
- Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WA, Seaton A, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol 2008;3:423–8
- Huang X, Peng X, Wang Y, Wang Y, Shin DM, El-Sayed MA, et al. A reexamination of active and passive tumor targeting by using rod-shaped gold nanocrystals and covalently conjugated peptide ligands. ACS Nano 2010;4:5887–96
- Kumar S, Aaron J, Sokolov K. Directional conjugation of antibodies to nanoparticles for synthesis of multiplexed optical contrast agents with both delivery and targeting moieties. Nat Protoc 2008;3:314–20
- Burke AR, Singh RN, Carroll DL, Torti FM, Torti SV. targeting cancer stem cells with nanoparticle-enabled therapies. J Mol Biomark Diagn 2012;Suppl8: PMC3875221
- Burke AR, Singh RN, Carroll DL, Wood JC, D'Agostino RB Jr., Ajayan PM, et al. The resistance of breast cancer stem cells to conventional hyperthermia and their sensitivity to nanoparticle-mediated photothermal therapy. Biomaterials 2012;33:2961–70
- Johannsen M, Gneveckow U, Taymoorian K, Thiesen B, Waldofner N, Scholz R, et al. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: Results of a prospective phase I trial. Int J Hyperthermia 2007;23:315–23
- Zaman RT, Diagaradjane P, Wang J, Swartz J, Gill-Sharp K, Rajaram N, et al. In vivo detection of gold nanoshells in tumors using diffuse optical spectroscopy. IEEE J Sel Top Quant Elec 2007;13:1715–20
- Puvanakrishnan P, Park J, Diagaradjane P, Schwartz JA, Coleman CL, Gill-Sharp KL, et al. Near-infrared narrow-band imaging of gold/silica nanoshells in tumors. J Biomed Opt 2009;14:024044
- De la Zerda A, Zavaleta C, Keren S, Vaithilingam S, Bodapati S, Liu Z, et al. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat Nanotechnol 2008;3:557–62
- Cheong SK, Krishnan S, Cho SH. Modeling of plasmonic heating from individual gold nanoshells for near-infrared laser-induced thermal therapy. Med Phys 2009;36:4664–71
- Elliott A, Schwartz J, Wang J, Shetty A, Hazle J, Stafford JR. Analytical solution to heat equation with magnetic resonance experimental verification for nanoshell enhanced thermal therapy. Lasers Surg Med 2008;40:660–5
- Maier-Hauff K, Rothe R, Scholz R, Gneveckow U, Wust P, Thiesen B, et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: Results of a feasibility study on patients with glioblastoma multiforme. J Neuro-Oncol 2007;81:53–60
- Lee HJ, Liu Y, Zhao J, Zhou M, Bouchard RR, Mitcham T, et al. In vitro and in vivo mapping of drug release after laser ablation thermal therapy with doxorubicin-loaded hollow gold nanoshells using fluorescence and photoacoustic imaging. J Control Release 2013;172:152–8
- Visaria RK, Griffin RJ, Williams BW, Ebbini ES, Paciotti GF, Song CW, et al. Enhancement of tumor thermal therapy using gold nanoparticle-assisted tumor necrosis factor-alpha delivery. Mol Cancer Ther 2006;5:1014–20
- Huschka R, Barhoumi A, Liu Q, Roth JA, Ji L, Halas NJ. Gene silencing by gold nanoshell-mediated delivery and laser-triggered release of antisense oligonucleotide and siRNA. ACS Nano 2012;6:7681–91
- Huang X, Hu Q, Braun GB, Pallaoro A, Morales DP, Zasadzinski J, et al. Light-activated RNA interference in human embryonic stem cells. Biomaterials 2015;63:70–9
- Zynda ER, Grimm MJ, Yuan M, Zhong L, Mace TA, Capitano M, et al. A role for the thermal environment in defining co-stimulation requirements for CD4(+) T cell activation. Cell Cycle 2015;14:2340–54
- Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr drug targets Inflamm Allergy 2005;4:281–6
- Ostberg JR, Taylor SL, Baumann H, Repasky EA. Regulatory effects of fever-range whole-body hyperthermia on the LPS-induced acute inflammatory response. J Leukoc Biol 2000;68:815–20
- Steiner AA, Oliveira DL, Roberts JL, Petersen SR, Romanovsky AA. Nicotine administration and withdrawal affect survival in systemic inflammation models. J Appl Physiol (1985) 2008;105:1028–34
- Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol 2010;10:427–39
- Pritchard MT, Wolf SF, Kraybill WF, Repasky EA. The anti-tumor effect of interleukin-12 is enhanced by mild (fever-range) thermal therapy. Immunol Invest 2005;34:361–80
- Page DB, Postow MA, Callahan MK, Allison JP, Wolchok JD. Immune modulation in cancer with antibodies. Annu Rev Med 2014;65:185–202
- Tong R, Langer R. Nanomedicines targeting the tumor microenvironment. Cancer J 2015;21:314–21
- Hernandez-Gil J, Cobaleda-Siles M, Zabaleta A, Salassa L, Calvo J, Mareque-Rivas JC. An iron oxide nanocarrier loaded with a Pt(IV) prodrug and immunostimulatory dsRNA for combining complementary cancer killing effects. Adv Healthc Mater 2015;4:1034–42
- Dumortier H, Lacotte S, Pastorin G, Marega R, Wu W, Bonifazi D, et al. Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano Lett 2006;6:1522–8
- Dobrovolskaia MA, Neun BW, Man S, Ye X, Hansen M, Patri AK, et al. Protein corona composition does not accurately predict hematocompatibility of colloidal gold nanoparticles. Nanomedicine 2014;10:1453–63
- Crist RM, Grossman JH, Patri AK, Stern ST, Dobrovolskaia MA, Adiseshaiah PP, et al. Common pitfalls in nanotechnology: Lessons learned from NCI's Nanotechnology Characterization Laboratory. Integr Biol (Camb) 2013;5:66–73
- Champion JA, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm Res 2009;26:244–9
- Diagaradjane P, Orenstein-Cardona JM, Colon-Casasnovas NE, Deorukhkar A, Shentu S, Kuno N, et al. Imaging epidermal growth factor receptor expression in vivo: pharmacokinetic and biodistribution characterization of a bioconjugated quantum dot nanoprobe. Clin Cancer Res 2008;14:731–41