Abstract
With the introduction of new biologically based imaging possibilities, a higher degree of individualisation and adaptation of radiotherapy will be possible. Better knowledge of the biology of the target and its sub-volumes will enable dose prescriptions tailored to the individual patients, tissues and sub-volumes. Repeated imaging during the course of treatment will in addition enable adaptation of the treatment to cope with anatomical, as well as biological changes of the patient and of the target tissues. To translate these bright future perspectives into significant improvements in clinical outcome, advanced tools to tailor the physical dose distributions are needed. The most conformal radiotherapy technique known to mankind and clinically available today is proton therapy; in particular Intensity Modulated Proton Therapy (IMPT) with active spot scanning can not only tailor the dose to the desired target, but also effectively avoid sensitive structures in the proximity of the target to a degree far better than other conformal techniques such as Intensity Modulated Radiotherapy with photons (IMRT). The development of IMPT is now mature enough for clinical introduction on a broad scale. Proton therapy is still more expensive than conventional radiotherapy, but with the present rapid increase in the number of proton facilities worldwide and new initiatives to improve efficiency, the difference in affordability will continue to decrease and in comparison with the benefits, soon diminish even further. Contrary to what is sometimes claimed, the demands for better physical dose distributions and better avoidance of non-target tissue, has never been higher. Prolonged expected survival in many groups of patients emphasises the need to reduce late toxicities. The success of concomitant systemic therapies, with their tendency to cause higher morbidity stresses even further the increased need for subtle dose-sculpting methodologies and tools. There is no contradiction between striving for better physical dose distributions and a more biologically based approach. On the contrary, physical dose distributions are the tools to which achieve a treatment that can meet the biological demands.
Optimisation in the context of radiotherapy is usually understood as the means to find a treatment that gives the highest probability for cure, or at least local tumour control, while still maintaining the probability of severe acute and late toxicities at an acceptable, i.e. low, level.
The clinical outcome is related to many parameters connecting the biological response of tissue to ionising radiation, some of the most important being dose level, fractionation pattern, dose distribution and type of radiation. In addition should be added individual differences in radiation sensitivity and a variety of medical and genetic factors. Better knowledge of these latter factors will open the way to a higher degree of individualisation of treatments [Citation1].
However, in the typical optimisation process of today's radiotherapy, few of the above factors are taken into account in the optimisation process.
Prescribed dose. Although it is often postulated that the goal of radiotherapy is to obtain as high a dose as possible to the target volume, while keeping the dose to healthy tissue at a minimum, the prescribed dose to an individual patient is, with few exceptions, based on the maximum tolerable dose to the particular population of patients to which this individual belongs, rather than to the level of risk for the individual patient herself.
Fractionation pattern. The effect of different fractionation patterns is a complex matter which would require detailed knowledge of the biology of the target and non-target tissues involved in the treatment. Even in cases where these biological parameters, such as the alpha/beta ratio, are claimed to be well understood, fractionation is seldom a parameter that is considered in the optimisation process for individual patients.
Type of radiation. Linear Energy Transfer (LET) and hence Relative Biological Effect (RBE) and maybe even Oxygen Enhancement Ratio (OER), differ significantly between, e.g. carbon ions and x-rays. Since the possible clinical advantages of high-LET radiation are yet not well explored and, even more importantly, since high-LET beams are not widely available, the type (or modality) of the radiation is not a parameter used in the optimisation for the treatment of individual patients. Choice between different photon energies, or even between electrons and photons, is part of the dose distribution optimisation process and not directly related to biology.
Dose distribution. This is the single parameter that today is widely used in the efforts to achieve an optimal radiation treatment for individual patients. In traditional treatment planning, experienced planners try to find the most advantageous combination of gantry angles, collimator setting, wedges, number of portals and more. With the introduction of IMRT, computer aided tools became available and have increased the possibilities and much more conformal dose distributions can be achieved.
A better knowledge of the individual biological characteristics of each patient, each type of tissue and even sub-volumes of tumours and surrounding tissues, will open the way to an increased individualisation of the treatment [Citation2], but is strictly speaking not a part of the optimisation process in the above sense, just as little as type of disease is.
So, at the end of the day, optimisation of radiotherapy at present all boils down to an optimisation of the dose distribution.
Biological optimisation
One could argue that since killing cells with radiation is an entirely biological process, the optimisation should be based on biological parameters, rather than a physical dose distribution. This is, of course, a misconception, since the dose distribution is the tool with which we can achieve the best clinical outcome. The aim is, and has always been, to obtain a dose distribution that, based on the clinical experience and biological knowledge available, will result in the best clinical outcome for the patient. The fact that accurate biological knowledge on an individual patient level is rarely known does not change the fundamental fact that prescription doses as well as acceptable doses to healthy tissues, are based on the clinical and biological knowledge at hand.
With the introduction of IMRT and computer aided optimisation, the need to translate the biological knowledge into a few numerical indices increased. Typically the biological knowledge and clinical experience is transferred to the computer as points in dose-volume histograms for certain defined volumes and structures. A widely used source for such numbers is the paper from Emami et al. [Citation3] and probably the QUANTEC data will play an important role in the near future [Citation4].
A further step towards a more biology based nomenclature is the introduction of concepts like Normal Tissue Complication Probability (NTCP) and Tumour Control Probability (TCP) [Citation5,Citation6]. By stating what an acceptable probability of a particular type of complication would be, this risk can be balanced against the probability to eradicate the tumour or even cure the patient and the link from dose distribution and clinical reality can be visualised in a way that may encourage a discussion on how to balance risk vs. cure on a patient-specific level. Equivalent Uniform Dose (EUD) and Generalised EUD (gEUD) are other concepts for biological optimisation with special application where the doses are heterogeneous [Citation7,Citation8].
Conventional wisdom tells us, and current paradigm within radiotherapy is, to prescribe and deliver a uniform dose to the target tissue. This is, however, optimal only when the tumour radiosensitivity is uniform and is hence applied when the radiosensitivity is assumed to be uniform. On the contrary, a non-uniform radiosensitivity would demand a non-uniform prescription and delivery of the dose [Citation9–11].
Increased knowledge of the biology of the target, e.g. by means of functional MR and PET, paves the way towards non-homogenous prescription and delivery of the dose to the target volume, sometimes referred to as dose-painting-by-numbers [Citation12,Citation13]. Once the biological information is there, the demands for tools to deliver the correct dose levels to the correct locations will increase.
So, biological optimisation of radiotherapy requires accurate, individual and detailed knowledge of the biology of the target and non-target tissues. But, at the end of the day, once this information is available, the optimisation all boils down to an optimisation of the dose distribution.
Individualisation
It would be tempting to state that all the new biological information we hope to obtain at an individual patient level in the future will enable individualised dose prescription and dose delivery. Although not incorrect, we must not forget that a lot can be done already with the knowledge at hand today. Most of the parameters described above, which can be modulated and optimised, are typically chosen to fit a certain population of patients. Anatomical 3D imaging reveals huge differences in the location of target tissues, as well as critical structures and their interrelation, between different individuals of the same patient group. By exploring the best dose distributions achievable in each patient, it will become evident that different individuals may have very different tolerance prescription doses. These differences may become even more evident once detailed biological information is available, but already from geometrical/ anatomical information generally available today, different individuals are indeed different, even if tolerance doses are extracted from population based information. Attempts have been made to prescribe doses to an interval, rather than to a fixed level, to enable dose escalation in patients who are identified as being at low risk for severe side effects, and to lower the dose to patients where, e.g. the location of risk organs makes a higher prescription dose hazardous, e.g. in the proposed IDEAL and ISTART trials [Citation14,Citation15]. However, this quite obvious approach to improve and individualise radiotherapy has not found its way to clinical application on a broader scale.
Adaptation
The general understanding of the concept of adaptation in the context of radiotherapy is to adapt the treatment plan, or treatment delivery, to more accurately take temporal changes into account. These changes may be tumour shrinkage during the course of treatment, weight loss or different biological patterns such as hypoxia and revascularisation. The main reasons why an adaptive approach is still somewhat unusual in practical radiotherapy, is partly due to lack of correct and sufficiently frequent biological information, but also due practical considerations such as human resources for re-planning and quality control (QC) issues [Citation16]. It is still a widespread opinion that measurements in phantoms need to be done to verify the planned dose delivery on an individual patient level. However, to make real adaptation a clinical reality, it is an absolute demand to find software-based alternatives to the expensive, inefficient and tedious idea of patient-specific QC measurements.
The role of protons
From what has been said above, the search for an optimal dose distribution is still a key issue in modern radiotherapy. These demands have not lessened with the introduction of more conformal treatment modalities, such as IMRT and Intensity Modulated Arc Therapy (IMAT). On the contrary, the more we get to know about what an ideal dose distribution should (and could) look like, the more obvious it is that our traditional tools do not present the final answer.
The most conformal radiotherapy technique known to mankind and clinically available today is proton therapy, in particular Intensity Modulated Proton Therapy (IMPT) with active spot scanning [Citation17], only challenged by brachytherapy under some very special conditions. (Conformation should in this context not only be understood as conformation of the high dose volume to the clinical target volume, but equally important avoidance of the healthy structures outside the target volume.)
Protons exhibit a higher LET than MV-photons, resulting in a slight increase of the RBE. The LET is increasing when the protons are slowing down with the result that the LET and hence, RBE, is at its highest level just before the protons come to rest. Although well known and possible to take into account [Citation18], protons are in general assumed to have a constant RBE of 1.1, and the important difference of protons relative to conventional radiation qualities is the relative dose distribution, rather than biological differences.
With IMPT, the dose distribution can be nicely tailored around the target volume, almost irrespectively of its shape. However, there are other much more affordable treatment modalities, doing equally well, as long as dose conformation to the target is the issue. Numerous treatment planning studies have compared IMPT, IMRT and IMAT confirming the fact that the substantial difference lies in volumes outside the target volume [Citation19–24]. While IMRT and other conformal photon-based techniques redistribute the dose within the patient, IMPT is actually able to decrease the imparted energy to the patient and in some situations even eliminate the dose to certain tissues, without compromising the target coverage.
As a consequence of the substantial and rapid technological development and general improvement of radiotherapy, patient survival has increased significantly, and for some groups life expectancy after treatment has improved dramatically. Combined with longer follow-up, improved cancer registries and end-result programs, long-term morbidity, in particular secondary radiation-induced cancers, has become increasingly obvious and important [Citation25].
In particular this is true for pediatric cases and in the treatment of young patients, e.g. for Hodgkin's lymphoma, where in some cases radiation-induced malignancies cause more deaths than the primary cancers [Citation26].
It has been shown that the cumulative incidence of secondary cancer induction can be as high as 20% of young patients treated with radiation [Citation26]. In a comparative treatment planning study of childhood medulloblastoma, an estimated life time risk of secondary cancers of 30% for IMRT was found [Citation27].
Also for adults an increase of secondary malignancies can be expected. In a study [Citation28] 51 500 patents who hade undergone radiotherapy for prostate cancer was compared to 70 500 similar patients who had received surgery for the same diagnosis. Despite the relatively high age of these patients (71 years) and a low mean survival time (4.3 years), there was a 6% increase in the incidence of solid tumours in the radiotherapy group and the relative risk increase to 15% after five years and to 34% for patients surviving ten years or more.
In connection with the introduction of IMRT, the consequences of the increased integral dose, compared to conventional radiotherapy in the treatment of head and neck patients was calculated [Citation29]. An eight-fold increase in the risk for secondary malignancies due to an increased over all dose leakage in the case of IMRT as compared to conventional treatments was found. However, probably the most important aspect lays in future perspectives on the use of drugs in combination with radiotherapy. A combination of chemotherapy and radiotherapy, in particular in concurrent applications, has been shown to be effective and has resulted in lower recurrence rates as well as improved survival in several tumour sites [Citation30].
Over the years the statement that radiotherapy will soon become obsolete, has been disseminated. Other modalities such as chemotherapy, monoclonal antibodies, targeted drugs or gene therapy will take over cancer therapy and make the perpetual striving for radiotherapy with better conformation redundant. In a letter to the editor, Schulz and Kagan [Citation31] states that even with the invention of an imaginary super machine that could produce any dose distribution, the impact on cancer mortality would be minimal. What was not foreseen by these authors was the success of combined therapy, and the need for better dose conformation that this technique demands.
Concurrent drug administration not only increases the radiosensitivity of the tumour clonogens, but also increases the toxicity significantly. Several studies have been forced to exclude a significant number of patients, or even close prematurely due to excessive toxicity [Citation32–35]. Although not enough is known on how the combined treatment affects the toxicity patterns, and in particular not for newer targeted biologic agents, it is widely recognised that the pattern of morbidity changes and the amount of toxicity increases, in some cases dramatically.
In the papers from Wang et al. [Citation36,Citation37] it has been shown, for example, that doses as low as 5 Gy have a significant impact on toxicity in the treatment of NSCLC with concurrent chemotherapy. In fact V5 was the single most significant factor associated with treatment-related pneumonitis.
With increased clinical experience with IMRT, it is not unlikely that large volumes of low doses might be shown to be more harmful than we have previously believed, in particular with combined chemoradiotherapy.
Consequently the future success of combined treatments also boils down to a matter of better dose distributions, at least to a large extent.
A couple of illustrative examples
To illustrate the overwhelming impact on the dose reduction to non-involved tissue that can realistically be achieved with proton therapy, two examples are given.
The first case is a 59-year-old woman with a T2N2M0 anal cancer. The prescribed dose was 60 CGy (Cobalt Gy equivalent) to the primary tumour and an inguinal lymph node on the right side with verified involvement and 46 CGy to the rest of the elective volume. In addition to the radiotherapy, the patient received chemotherapy.
Two plans were created, i) a plan with IMPT (a) and as a comparison ii) a plan with Tomotherapy Hi Art (). Tomotherapy was used because it was believed that it represented the state of the art in photon-based IMRT, but similar results would have been expected also for other IMRT modalities.
Figure 1. Treatment plans for a T2N2M0 anal cancer. The prescribed dose was 60 CGy to the primary tumour and an inguinal lymph node on the right side with verified involvement and 46 CGy to the rest of the elective volume. shows the dose distribution obtained with IMPT and the dose distribution with Tomotherapy Hi Art.
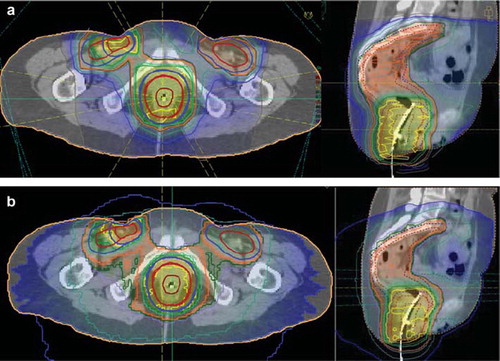
With both modalities a very good conformation to the target volumes was obtained (), and reasonable avoidance of the most critical organs was achieved, e.g. the optimisation criteria set by the clinicians were met. However, in the total amount of energy delivered to the patient, a dramatic difference can be seen (). Whereas the tomotherapy plan delivered an integral dose of 404 Joule to the patient for the whole course of treatment, the IMPT could be delivered with only 228 Joule (integral dose is here to be understood as the average dose to a certain volume, e.g. the whole body, times the volume, assuming a water equivalent density of all tissues). Reasonable in this comparison is of course to exclude the integral dose to the PTV, which was about 90 Joule in both cases (since the dose description and target definitions were exactly the same and the dose conformality was similar). That leaves us with 312 Joule to non-involved tissue in the case of tomotherapy and only 138 Joule for the IMPT plan; a reduction by 56% ().
Figure 2. Dose Volume Histogram for the IMPT proton plan and the Tomotherapy plan for a) the union of the planning target volumes and b) for the total volume, including the PTVs.
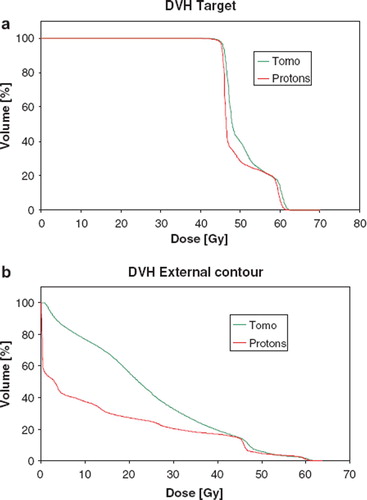
Table I. Comparison between the doses (in Cobalt equivalent Gy) and integral doses (I.D. in Joule) for the pelvis patient. The integral dose to the non-involved tissues was reduced by 56% with the IMPT plan compared to the Tomotherapy plan.
It might be expected that the difference will be at its maximum for large, deeply situated tumours with highly irregular shape, just as the in the case above. To illustrate that the effect of dose reduction can be at least as dramatic for other cases, a second example is given.
The second case is a 45-year-old male with a T1N2cM0 floor-of-mouth tumour. The primary PTV was prescribed to a dose of 68 CGy and the nodes to 54 CGy (). Two plans were done, i) a plan with IMPT () and as a comparison ii) a plan with IMRT ().
Figure 3. Treatment plans for a T1N2cM0 floor of mouth tumour. The primary PTV was prescribed to a dose of 68 CGy and the nodes to 54 CGy Figure 3a shows the dose distribution obtained with IMPT and Figure 3b the dose distribution with IMRT.
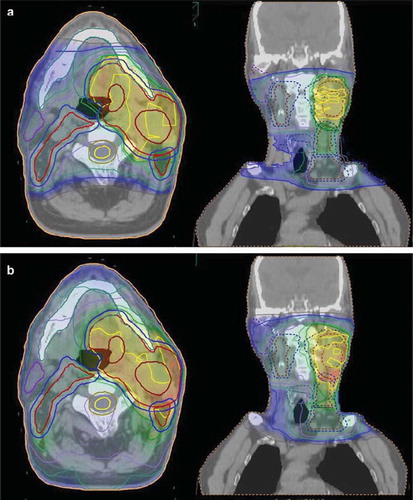
Just as in the above case, the dose conformation to the target volumes was excellent with both techniques, but a significant decrease, 65%, in the integral dose to non-involved tissue was obtained with the proton plan compared to the IMRT plan ().
Table II. Comparison between the doses (in Cobalt equivalent Gy) and integral doses (I.D. in Joule) for the Head & Neck patient. The integral dose to the non-involved tissues was reduced by 65% with the IMPT plan compared to the Tomotherapy plan.
Discussion
The striving to achieve better dose distributions has traditionally been focused on tailoring the high-dose volumes to the target tissues and less attention has been given to the reduction of doses outside the target tissues, at least as long as the optimisation criteria for organs at risk have been met. Today's tools for intensity modulated photon treatment have been developed to a level few could have imagined just a couple of decades ago and few would believe that equally important and significant improvements are still to come. That is, however, only as long as the dose conformation to the target tissues is the focus.
In the above examples it was shown that far less than 25% of the energy imparted to the patient actually reaches the intended target volumes. This unwanted dose can be significantly reduced by opting for protons instead of photons. In the examples shown, the reduction of the unwanted dose burden was 56% and 65%, respectively. The figures may differ from case to case, but the above examples are by no means extreme.
The clinical implication on integral dose and the importance of reducing it is yet not a solved issue. In a perspective of radiation protection it is difficult, not to say unintelligible, that the ambitions to chase every single mSv or even μSv, for the general public or patients undergoing x-ray examinations, never reached the cancer patient populations. In the context of reducing the (unwanted) population dose, the introduction of proton therapy would by far be the most cost effective measure calculated per eliminated man Sv.
Looking into the risk of secondary cancer induction, a linear relation between integral dose and risk has traditionally been applied in the low dose range. At higher doses it has been assumed that the risk per Gy drops at doses above some 5 Gy due to cell killing and diminishes at even higher doses [Citation38]. This assumption is contradicted by clinical findings where secondary solid tumours predominantly seems to appear in, or close to, the high dose volumes and no significant fall off with energy is noted [Citation39–41]. In more recent works [Citation42,Citation43], new models are applied that very convincing make a case for the re-instatement of integral dose as the key parameter for estimating second cancer induction.
In the above calculations only doses as calculated by the treatment planning systems are taken into account. This means for example that the neutron contamination of the proton beam is not taken into account, or the collimator leakage in the case of IMRT. This is however believed to be a minor problem; in particular in the case of neutron contamination since this contribution is minimal with spot scanning technique, compared to traditional passive scattering technique, and in general, since the total integral dose from these sources are small compared to the contribution from the high dose volumes or in-field volumes.
Further, the assumption is made that the same concept for margin design can be used irrespectively of treatment modality. Since range uncertainties play an important role in particle therapy, this assumption of equal margins, e.g. IMPT and IMRT can be questioned.
Conclusions
The importance of good dose distributions is a key issue, not only as a way to conform the dose distribution to the target volumes, but increasingly also to reduce the doses to non-involved tissues.
The best dose distributions, in the target as well as outside, are achieved by proton therapy delivered by Intensity Modulated spot-scanned proton beams.
Acknowledgements
The author is grateful to Mrs Ingrid Kristensen, University Hospital of Skåne, Sweden, for preparing all the treatment plans and to Prof. Alan E Nahum, Clatterbridge Centre for Oncology, Wirral, UK, for valuable discussions and input.
Declaration of interest: The author report no conflicts of interest. The author alone are responsible for the content and writing of the paper.
References
- Grau C, Muren LP, Høyer M, Lindegaard J, Overgaard J. Image-guided adaptive radiotherapy – integration of biology and technology to improve clinical outcome. Acta Oncol 2008;47:1182–5.
- Daşu A. Treatment planning optimisation based on imaging tumour proliferation and cell density. Acta Oncol 2008; 47:1221–8.
- Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, . Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21: 109–22.
- Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, . Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys 2010;76(3 Suppl):S10–9.
- Kutcher GJ. Quantitative plan evaluation: TCP/NTCP models. Front Radiat Ther Oncol 1996;29:67–80.
- Baumann M, Petersen C, Krause M. TCP and NTCP in preclinical and clinical research in Europe. Rays 2005;30: 121–6.
- Niemierko A. Reporting and analyzing dose distributions: A concept of equivalent uniform dose. Med Phys 1997;24:103–10.
- Wang JZ, Mayr NA, Yuh WT. Behind EUD. Acta Oncol 2008;47:971–2.
- Levin-Plotnik D, Hamilton RJ. Optimization of tumour control probability for heterogeneous tumours in fractionated radiotherapy treatment protocols. Phys Med Biol 2004; 49:407–24.
- Webb S. Optimum parameters in a model for tumour control probability including interpatient heterogeneity. Phys Med Biol 1994;39:1895–914.
- Webb S, Nahum AE. A model for calculating tumour control probability in radiotherapy including the effects of inhomogeneous distributions of dose and clonogenic cell density. Phys Med Biol 1993;38:653–66.
- Hall EJ. Dose-painting by numbers: A feasible approach? Lancet Oncol 2005;6:66.
- Bentzen SM. Theragnostic imaging for radiation oncology: Dose-painting by numbers. Lancet Oncol 2005;6:12–7.
- Fenwick JD, Nahum AE, Malik ZI, Eswar CV, Hatton MQ, Laurencejj VM, . Escalation and intensification of radiotherapy for stage III non-small cell lung cancer: Opportunities for treatment improvement. Clin Oncol 2009; 21:343–60.
- van Baardwijk A, Wanders S, Boersma L, Borger J, Ollers M, Dingemans AM, . Mature results of an individualized radiation dose prescription study based on normal tissue constraints in stages I to III non-small-cell lung cancer. J Clin Oncol 2010;28:1380–6.
- Miles EA, Clark CH, Urbano TG, Bidmead M, Dearnaley DP, Harrington KJ, . The impact of introducing intensity modulated radiotherapy into routine clinical practice. Radiother Oncol 2005;77:241–6.
- Lomax A. Intensity modulation methods for proton radiotherapy. Phys Med Biol 1999;44:185–205.
- Tilly N, Johansson J, Isacsson U, Medin J, Blomquist E, Grusell E, . The influence of RBE variations in a clinical proton treatment plan for a hypopharynx cancer. Phys Med Biol 2005;50:2765–77.
- Weber DC, Wang H, Cozzi L, Dipasquale G, Khan HG, Ratib O, . RapidArc, intensity modulated photon and proton techniques for recurrent prostate cancer in previously irradiated patients: A treatment planning comparison study. Radiat Oncol 2009;4:34.
- Zhang X, Li Y, Pan X, Xiaoqiang L, Mohan R, Komaki R, . Intensity-modulated proton therapy reduces the dose to normal tissue compared with intensity-modulated radiation therapy or passive scattering proton therapy and enables individualized radical radiotherapy for extensive stage IIIb non-small-cell lung cancer: A virtual clinical study. Int J Radiat Oncol Biol Phys 2010;77:357–66.
- Ares C, Khan S, Macartain AM, Heuberger J, Goitein G, Gruber G, . Postoperative proton radiotherapy for localized and locoregional breast cancer: Potential for clinically relevant improvements? Int J Radiat Oncol Biol Phys 2010; 76:685–97.
- Dowdell SJ, Metcalfe PE, Morales JE, Jackson M, Rosenfeld AB. A comparison of proton therapy and IMRT treatment plans for prostate radiotherapy. Australas Phys Eng Sci Med 2008;31:325–31.
- Muzik J, Soukup M, Alber M. Comparison of fixed-beam IMRT, helical tomotherapy, and IMPT for selected cases. Med Phys 2008;35:1580–92.
- Glimelius B, Ask A, Bjelkengren G, Björk-Eriksson T, Blomquist E, Johansson B, . Number of patients potentially eligible for proton therapy. Acta Oncol 2005;44: 836–49.
- Rijkee AG, Zoetelief J, Raaijmakers CP, Van Der Marck SC, Van Der Zee W. Assessment of induction of secondary tumours due to various radiotherapy modalities. Rad Prot Dosim 2006;118:219–26.
- Tubiana M. Can we reduce the incidence of second primary malignancies occurring after radiotherapy? A critical review. Radiother Oncol 2009;91:4–15.
- Mu X, Björk-Eriksson T, Nill S, Oelfke U, Johansson KA, Gagliardi G, . Does electron and proton therapy reduce the risk of radiation induced cancer after spinal irradiation for childhood medulloblastoma? A comparative treatment planning study. Acta Oncol 2005;44:554–62.
- Brenner DJ, Curtis RE, Hall EJ, Ron E. Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery. Cancer 2000;88:398–406.
- Verellen D, Vanhavere H. Risk assessment of radiation-induced malignancies based on whole-body equivalent dose estimates for IMRT treatment in the head and neck region. Radiother Oncol 1999;53:199–203.
- Verheij M, Vens C, van Triest B. Novel therapeutics in combination with radiotherapy to improve cancer treatment: Rationale, mechanisms of action and clinical perspective. Drug Resist Updat 2010;13:29–43.
- Schulz RJ, Kagan AR. More precisely defined dose distributions are unlikely to affect cancer mortality. Med Phys 2003;30:276.
- Blanco R, Solé J, Montesinos J, Mesía C, Algara M, Terrassa J, . ACROSS. Induction chemotherapy with cisplatin and gemcitabine followed by concurrent chemoradiation with twice-weekly gemcitabine in unresectable stage III non-small cell lung cancer: Final results of a phase II study. Lung Cancer 2008;62:62–71.
- Socinski MA, Blackstock AW, Bogart JA, Wang X, Munley M, Rosenman J, . Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol 2008;26:2457–63.
- Pfister DG, Su YB, Kraus DH, Wolden SL, Lis E, Aliff TB, . Concurrent cetuximab, cisplatin and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: A pilot phase II study of a new combined-modality paradigm. J Clin Oncol 2006;24:1072–8.
- Arrieta O, Gallardo-Rincón D, Villarreal-Garza C, Michel RM, Astorga-Ramos AM, Martínez-Barrera L, . High frequency of radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with concurrent radiotherapy and gemcitabine after induction with gemcitabine and carboplatin. J Thorac Oncol 2009;4:845–52.
- Wang S, Liao Z, Wei X, Liu HH, Tucker SL, Hu CS, . Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non-small-cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT). Int J Radiat Oncol Biol Phys 2006;66: 1399–407.
- Wang SL, Liao Z, Vaporciyan AA, Tucker SL, Liu H, Wei X, . Investigation of clinical and dosimetric factors associated with postoperative pulmonary complications in esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys 2006;64:692–9.
- Daşu A, Toma-Daşu I, Olofsson J, Karlsson M. The use of risk estimation models for the induction of secondary cancers following radiotherapy. Acta Oncol 2005;44:339–47.
- van Leeuwen FE, Klokman WJ, Stovall M, Dahler EC, van't Veer MB, Noordijk EM, . Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin's disease. J Natl Cancer Inst 2003;95:971–80.
- Travis LB, Hill D, Dores GM, Gospodarowicz M, van Leeuwen FE, Holowaty E, . Cumulative absolute breast cancer risk for young women treated for Hodgkin lymphoma. J Natl Cancer Inst 2005;97:1428–37.
- Gilbert ES, Stovall M, Gospodarowicz M, Van Leeuwen FE, Andersson M, Glimelius B, . Lung cancer after treatment for Hodgkin's disease: Focus on radiation effects. Radiat Res 2003;159:161–73.
- Sachs RK, Brenner DJ. Solid tumor risks after high doses of ionizing radiation. Proc Natl Acad Sci USA. 2005;102: 13040–5.
- Schneider U, Kaser-Hotz B. Radiation risk estimates after radiotherapy: Application of the organ equivalent dose concept to plateau dose-response relationships. Radiat Environ Biophys 2005;44:235–9.
