Abstract
Purpose. Metabolic response assessment is often used as a surrogate of local failure and survival. Early identification of patients with residual metabolic activity is essential as this enables selection of patients who could potentially benefit from additional therapy. We report on the development of a pre-treatment prediction model for metabolic response using patient, tumor and treatment factors. Methods. One hundred and one patients with inoperable NSCLC (stage I-IV), treated with 3D conformal radical (chemo)-radiotherapy were retrospectively included in this study. All patients received a pre and post-radiotherapy fluorodeoxyglucose positron emission tomography-computed tomography FDG-PET-CT scan. The electronic medical record system and the medical patient charts were reviewed to obtain demographic, clinical, tumor and treatment data. Primary outcome measure was examined using a metabolic response assessment on a post-radiotherapy FDG-PET-CT scan. Radiotherapy was delivered in fractions of 1.8 Gy, twice a day, with a median prescribed dose of 60 Gy. Results. Overall survival was worse in patients with residual metabolic active areas compared with the patients with a complete metabolic response (p=0.0001). In univariate analysis, three variables were significantly associated with residual disease: larger primary gross tumor volume (GTVprimary, p=0.002), higher pre-treatment maximum standardized uptake value (SUVmax, p=0.0005) in the primary tumor and shorter overall treatment time (OTT, p=0.046). A multivariate model including GTVprimary, SUVmax, equivalent radiation dose at 2 Gy corrected for time (EQD2, T) and OTT yielded an area under the curve assessed by the leave-one-out cross validation of 0.71 (95% CI, 0.65–0.76). Conclusion. Our results confirmed the validity of metabolic response assessment as a surrogate of survival. We developed a multivariate model that is able to identify patients at risk of residual disease. These patients may benefit from an individualized and more adequate therapeutic approach, thereby improving local control and survival.
Lung cancer is an important cause of cancer-related deaths worldwide [Citation1]. In 2008, lung cancer was the most common cause of death from cancer with an estimate of 342 000 deaths in Europe [Citation1]. Non-small cell lung cancer (NSCLC) accounts for at least 80% of all lung cancer cases [Citation2]. The majority of these NSCLC patients present advanced-stage disease (stage III and IV), which are considered inoperable [Citation3]. For these patients, the combination of radiotherapy and chemotherapy shows improved treatment outcome [Citation4,Citation5], however local tumor failure is still observed in approximately 70% of patients [Citation6]. Therefore early identification of patients with a high risk of local treatment failure is important, as these patients may potentially benefit from additional therapy. One method of investigating local treatment failure, is assessing metabolic response within the primary tumor after treatment with 18Fluorodeoxyglucose (FDG) positron emission tomography (PET) imaging [Citation7]. Several studies indicated that patients with metabolically active residual masses after treatment have a poorer prognosis compared to patients without residual metabolic activity [Citation8,Citation9]. Although, other studies have shown that FDG uptake before treatment is prognostic for residual metabolic activity within the tumor [Citation9–11], other pre-treatment clinical factors were not investigated for their prognostic capability. Therefore, we hypothesize that also other pre-treatment factors, including demographic, tumor and treatment characteristics, can have prognostic value for predicting metabolic response after treatment. In the present study we examined the association between commonly used prognostic factors in NSCLC patients and metabolic response after treatment in a univariate and multivariate analysis.
Materials and methods
Patient characteristics
The electronic medical record system and the medical patient charts were retrospectively reviewed to obtain demographic, clinical, tumor and treatment data. One hundred and one patients (40 women and 61 men) with inoperable non-small cell lung cancer (NSCLC), stage I-IV, were included in this study. Their age ranged from 43 to 86 years (mean: 65.6 years). All patients were treated with curative intent at MAASTRO Clinic with sequential chemoradiotherapy (82 patients) or with radical radiotherapy alone (19 patients) between December 2004 and September 2007. All patients received a pre and post-treatment FDG-PET-CT scan. For patients receiving sequential chemo-radiotherapy the pre-treatment scan was performed after chemotherapy. The average time interval between the last radiotherapy and the second FDG-PET-CT scan was 99 days (range: 49–184 days). No treatment was given between the end of radiotherapy and the post-treatment scan.
FDG-PET-CT Imaging
Pre and post-treatment FDG-PET-CT scans were performed using a Siemens Biograph (Siemens, Knoxville, TN). All patients were instructed to fast at least six hours before the intravenous administration of FDG (Tyco Health Care, Amsterdam, The Netherlands), followed by physiologic saline (10 ml). The total injected activity of FDG was dependent on the patient weight: (weight*4) + 20 Mbq. After a period of 45 minutes, during which the patient was encouraged to rest, PET and CT imaging were performed [Citation12].
Treatment characteristics
The radiotherapy treatment was delivered in fractions of 1.8 Gy, twice a day, with a mean lung dose (MLD) restricted to 19 Gy and a maximal allowed total tumor dose (TTD) of 79.2 Gy [Citation12]. Patients with stage III disease, who where physically fit enough received sequential chemo-radiotherapy, consisting of three courses of gemcitabine in combination with cisplatin or carboplatin, followed by radiotherapy as described for stage I/II. No concurrent chemo-radiotherapy was given. The biologic equivalent dose was used as indication of the intensity of chest RT delivered to the tumor and was calculated using the quadratic model [Citation13] and corrected for overall treatment time.
Metabolic response
Metabolic response was assessed for all patients with a FGD-PET-CT scan after treatment. Residual disease was defined as residual metabolic activity within the primary tumor, i.e. areas with FDG uptake higher than in the aortic arch (SUV > SUVAORTA) [Citation7,Citation8]. If there was no activity within the tumor, patients were defined as with a complete metabolic response [Citation10]. Survival data were obtained by reviewing the Dutch Communal Data register. Survival time was defined as the date from the start of radiotherapy until the date of death or last follow-up. Survival status could not be retrieved for one patient.
Statistical analysis
All data are expressed as means ± SD. Because the distribution of the continuous variables was rather skewed, the Mann-Whitney U test was used to determine statistical differences between the patients with and without residual disease. For categorical variables the χ2 test was used. Differences were considered to be significant when the p-value was lower than 0.05. The area under the curve (AUC) of the receiver operating characteristic (ROC), a plot of the true positive rate (correctly classified positive samples) and false positive rate (incorrectly classified negative samples) was used to analyze the association between the variables and residual disease in univariate analysis using a proximal-support vector machine (p-SVM) [Citation14]. A p-SVM was also used to build a multivariate prediction model, using metabolic residual disease as outcome measure. Combinatorial feature selection was performed to obtain an optimal subset of features. The set of variables with the highest AUC of the ROC curve was included in the multivariate predictive model. The Kaplan-Meier method was used to estimate survival probabilities and statistical differences were assessed using the log-rank test. Data were considered right-censored if patients were alive at the time of last follow-up. All the analyses were performed in Matlab 2008b (The MathWorks Inc, Natick, MA, USA) and SPSS (Version 15.0 for Windows, Chicago, IL).
Results
Patients characteristics
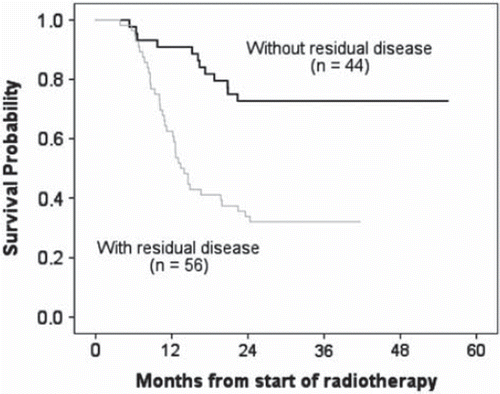
To assess the power of clinical parameters for the prediction of metabolic response, commonly known prognostic factors were collected before treatment and correlated with metabolic response after treatment. A total of 101 NSCLC patients were included in this analysis, of which 56 (55%) patients showed persistent residual FDG uptake on the post-radiotherapy CT-PET scan and 45 (45%) patients had a complete metabolic response (CMR) indicating no residual FDG uptake within the tumor post-radiotherapy. Patient, tumor and treatment characteristics for both groups are listed in . The median follow-up duration was 23.9 months (range: 3.8–55.5 months). The patients with residual active areas post-treatment had a significantly worse survival (median survival: 13.4 months) compared to patients with a complete metabolic response (median survival not reached) (; 95% CI, 38.9–49.8 months, p=0.0001). The hazard ratio for death for patients with residual areas compared to individuals without was 3.701 (95% confidence interval: 1.92 to 7.13; p=0.0001 by the log-rank test, two-sided).
Table I. Patient characteristics and their association with post-RT outcome in univariate analysis. Comparison of groups with residual disease and with complete metabolic response.
Univariate analysis
To assess the association between patient, tumor and treatment characteristics with post-radiotherapy outcome, a univariate analysis was performed. The area under the ROC curve of a univariate model for each parameter was estimated. These results are summarized in . The volume of the primary tumor (GTVprimary), maximum FDG uptake and OTT had the highest predictive power, while other commonly used predictors such as FEV1, WHO-performance status or clinical stage showed a low predictive ability. GTVprimary was significantly higher for patients with residual areas than for patients with a complete metabolic response (103 cm3 ± 126.13 cm3 vs. 48.3 cm3 ± 55.5 cm3, p = 0.008). Similarly, the maximum FDG uptake on the pre-RT scan was significantly higher for patients with residual disease compared to patients with a complete metabolic response (10.5 ± 5 vs. 7.7 ± 5.2, p = 0.007). The overall treatment time (OTT) was longer for patients with a complete metabolic response in comparison with patients with residual disease (27 ± 6 days vs. 24 ± 5 days, p = 0.013).
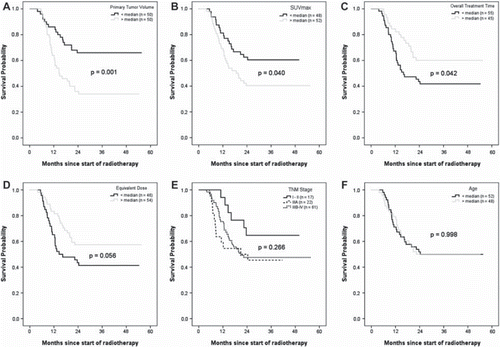
Kaplan-Meier survival curves for subgroups determined by the median for selected variables are shown in . Survival was significantly higher for patients with a tumor volume smaller than the median (GTVprimary= 46.6 cm3) (p=0.001). In patients with a SUVmax higher than the median (SUVmax = 8.4) in the pre-treatment scan, survival was significantly shorter, compared to patients with a SUVmax lower than the median (p=0.040). Significant differences in survival were also observed for OTT, with a more prolonged survival for patients with a treatment time longer than the median of 25 days (p=0.042). Survival differences in patients stratified according to TNM stage, were statistically not significant (p=0.266). The same result was obtained for age. Older patients did not have different survival compared to younger patients (p=0.998). Higher equivalent radiation dose was associated with better survival, however the difference was not statistically significant (p=0.056).
Figure 2. Survival among patients with advanced NSCLC for selected variables. For continuous variables, the cut-off value to stratify the patients was defined at the variable median. Shown are Kaplan-Meier curves for GTVprimary, SUVmax, OTT, EQD2, T, TNM stage and age. In panel E, patients with stage I and II were grouped together due to the small number of cases. Stage IV (1 patient) was grouped with Stage IIIB.
Multivariate analysis
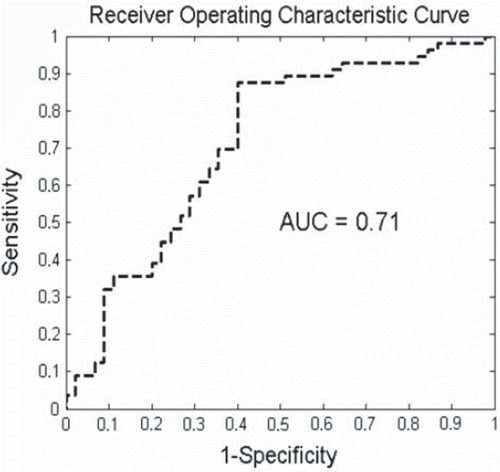
For the multivariate analysis, all the available variables were subjected to a combinatorial feature selection procedure. The combination with the highest AUC assessed by the leave-one-out cross validation approach was selected for the multivariate model. The variables included in the final multivariate p-SVM model were GTVprimary, maximum standardized FDG uptake, OTT and equivalent dose corrected for treatment time (EQD2, T). Addition of other parameters to this model did not improve its performance. The area under the curve of the final predictive model was 0.71 (95% CI, 0.65–0.76; ). The variables included in the multivariate model showed also a significant association with the post-radiotherapy outcome in univariate analysis.
Discussion
In this study we investigated the relationship of clinical parameters, including demographic, tumor and treatment characteristics, with metabolic response post-treatment. Our primary endpoint was defined as residual metabolic disease on a post-treatment PET-CT scan. Previous studies have shown that patients with residual metabolically active areas after treatment have a poorer prognosis compared with patients without [Citation10,Citation15,Citation16]. In agreement with these studies, also our results showed that patients with residual disease had a significantly worse survival (p=0.0001), compared to patients with a complete metabolic response, thus supporting the importance of our primary endpoint as surrogate for survival.
Previous studies examined the value of pre-treatment FDG-PET alone to determine treatment response after radiotherapy [Citation17] and chemotherapy [Citation18]. In our study, we explored not only the prognostic capability of FDG-PET but also the additional value of other clinico-pathological prognostic factors. Some of them, i.e. age, gender, tumor size, WHO performance status have been included in predictive models for survival in NSCLC patients [Citation19–21]. In a retrospective study with a large patient population of NSCLC patients (stage I and II) which received resection with curative intent, Agarwal et al., reported that age and gender, tumor volume and type of surgery were important for the prediction of survival [Citation22]. However, we did not find a significant association between age and metabolic response. Similarly, other studies have shown a relation between female gender and a favorable outcome [Citation23]. We did not find a significant difference based on gender between responders and non-responders.
WHO performance status and FEV1, have been cited as predictors of survival [Citation19,Citation21], in which worse performance status and impaired lung function measurements are associated with shorter survival. We could not identify an association between these parameters and the post-treatment outcome. Although the tumor-node-metastasis (TNM) staging system is an important tool to estimate prognosis and choose the best treatment modality, several studies have reported that TNM has a poor predictive capability for survival in NSCLC patients [Citation24]. In our cohort, the majority of patients were diagnosed with stage IIIA (22%) and IIIB (61%) disease. Therefore, stage was not a good predictor for residual disease, as differences in stage between the responding and the non-responding groups were not observed. Great interest has been given to the use of FDG-PET as a tool for tumor detection, staging and particularly for response assessment after radical radiotherapy or chemo-radiation [Citation25,Citation26]. The maximum FDG uptake in the primary tumor measured on a pre-treatment scan has consistently been shown as an important prognostic factor for survival in NSCLC [Citation15,Citation18,Citation25]. Our results showed that patients with residual metabolically active areas had a significantly higher FDG uptake on the pre-treatment scan, compared to patients with a complete metabolic response. A high pre-treatment FDG uptake within the primary tumor was also significantly associated with worse survival (p=0.040). Furthermore, the SUVmax showed a good predictive capability in univariate analysis.
Tumor volume also emerged as one of the most important predictors of residual disease. Our results are consistent with recently published studies, which have identified tumor size as an important prognostic factor of survival [Citation27]. Here we confirmed the predictive capability of tumor size in assessment of metabolic response. This might indicate that specially for larger tumors, an effective dose could not be reached due to the dose constraints of the current protocol. The total tumor load (GTVprimary + GTVnodal) showed a strong association with the post-treatment outcome (). This association is due to the primary tumor volume, and perhaps enhanced by the addition of secondary volumes, however GTVnodal alone did not show a predictive capability. A similar result was obtained for the number of positive lymph node stations on a pre-treatment PET-CT scan. Although the number of PLNSs is an important risk and staging factor for non-surgical patients [Citation28], and has been included in multivariate models for survival in NSCLC, we did not find an added prognostic value for residual disease, perhaps because the outcome was defined in the primary tumor.
Despite an overall difference of two days, overall treatment time was significantly higher for patients with complete metabolic response in comparison with patients with residual disease. OTT was also significantly associated with the outcome in univariate analysis. It is generally accepted that a short treatment time should be chosen, to minimize the effect of accelerated repopulation [Citation29]. The fact that we observe a longer treatment time in patients with a positive outcome is because those patients received a higher dose. Higher total treatment dose has been associated with improved local tumor control and better survival [Citation27,Citation30]. In the present study, the prescribed total dose was not different for patients with a complete response compared to patients with residual disease (p=0.809).
Several predictive models of survival have been published for NSCLC patients, reporting different values of the area under the ROC as performance measurement, ranging from 0.65 to 0.86. These models were developed on populations that underwent different treatment modalities such as surgery [Citation31], chemotherapy [Citation28], radiotherapy or a combination [Citation32] and consisted of patients with different tumor and patient characteristics. Thus, application of those models to different scenarios is still subject of research. Here we presented a multivariate model for prediction of residual disease. The final model consisted on tumor volume, overall treatment time, SUVmax and equivalent dose corrected for treatment time. This model yielded an AUC of 0.71 (95% CI, 0.65–0.76). This may have clinical relevance for patients identified at risk of treatment failure that may benefit from additional therapy. We were not able to analyze potential prognostic variables such as molecular markers or imaging surrogates [Citation33–35] that may improve the ability of the presented model to predict the post-treatment failure. The lack of an external cohort to validate the presented model and confirm our results is an important limitation to our study. Our results may require validation according to the treatment modality to avoid possible confounding effects associated with multiple treatment modalities.
In conclusion, our results demonstrated that patients who do not respond to radiotherapy can be identified early in the course of their treatment. To our knowledge, this is the first study that examines different clinico-pathological predictors of residual disease. We identified important prognostic factors of residual disease and developed a multivariate model that identified patients at risk of treatment failure. Furthermore, we confirmed the validity of residual disease as a surrogate of survival. Our results could assist clinicians in the treatment decision-making process and in stratification of patients for clinical trials.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer 2010;46:765–81.
- Brawner EJ, Patrick Nana-Sinkam S, Jett JR. Lung cancer screening in 2008: A review and update. Respir Med CME 2008;1:2–9.
- Scott WJ, Howington J, Feigenberg S, Movsas B, Pisters K. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines, 2nd ed. Chest 2007;132:S234–S42.
- Le Chevalier T, Arriagada R, Quoix E, Ruffie P, Martin M, Tarayre M, . Radiotherapy alone versus combined chemotherapy and radiotherapy in nonresectable non-small-cell lung cancer: First analysis of a randomized trial in 353 patients. J Natl Cancer Inst 1991;83:417–23.
- Florin Sirzén EK, Sörenson S, Cavallin-Ståhl E. A systematic overview of radiation therapy effects in non-small cell lung cancer. Acta Oncol 2003;42:493–515.
- Zatloukal P, Petruzelka L, Zemanova M, Havel L, Janku F, Judas L, . Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: A randomized study. Lung Cancer 2004;46:87–98.
- Aerts HJ, van Baardwijk AA, Petit SF, Offermann C, Loon J, Houben R, . Identification of residual metabolic-active areas within individual NSCLC tumours using a pre-radiotherapy (18)Fluorodeoxyglucose-PET-CT scan. Radiother Oncol 2009;91:386–92.
- Aerts HJ, Bosmans G, van Baardwijk AA, Dekker AL, Oellers MC, Lambin P, . Stability of 18F-deoxyglucose uptake locations within tumor during radiotherapy for NSCLC: A prospective study. Int J Radiat Oncol Biol Phys 2008;71: 1402–7.
- Weber WA, Figlin R. Monitoring cancer treatment with PET/CT: Does it make a difference? J Nucl Med 2007;48 (Suppl 1):S36–S44.
- Mac Manus MP, Hicks RJ, Matthews JP, Wirth A, Rischin D, Ball DL. Metabolic (FDG-PET) response after radical radiotherapy/chemoradiotherapy for non-small cell lung cancer correlates with patterns of failure. Lung Cancer 2005;49:95–108.
- Hoekstra CJ, Stroobants SG, Smit EF, Vansteenkiste J, van Tinteren H, Postmus PE, . Prognostic relevance of response evaluation using [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography in patients with locally advanced non-small-cell lung cancer. J Clin Oncol 2005;23:8362–70.
- van Baardwijk A, Wanders S, Boersma L, Borger J, Ollers M, Dingemans AM, . Mature results of an individualized radiation dose prescription study based on normal tissue constraints in stages I to III non-small-cell lung cancer. J Clin Oncol 2010;28:1380–6.
- Steel G. Basic clinical radiobiology, 3rd. London: Hodder Arnold Publications; 2002.
- Fung GM, Mangasarian OL. Multicategory proximal support vector machine classifiers. Mach Learn 2005;59:77–97.
- Decoster L, Schallier D, Everaert H, Nieboer K, Meysman M, Neyns B, . Complete metabolic tumour response, assessed by 18-fluorodeoxyglucose positron emission tomography (18FDG-PET), after induction chemotherapy predicts a favourable outcome in patients with locally advanced non-small cell lung cancer (NSCLC). Lung Cancer 2008;62: 55–61.
- Cerfolio RJ, Bryant AS, Winokur TS, Ohja B, Bartolucci AA. Repeat FDG-PET after neoadjuvant therapy is a predictor of pathologic response in patients with non-small cell lung cancer. Ann Thorac Surg 2004;78:1903–9; Discussion 1909.
- Erdi YE, Macapinlac H, Rosenzweig KE, Humm JL, Larson SM, Erdi AK, . Use of PET to monitor the response of lung cancer to radiation treatment. Eur J Nucl Med 2000; 27:861–6.
- Vansteenkiste JF, Stroobants SG, De Leyn PR, Dupont PJ, Verbeken EK. Potential use of FDG-PET scan after induction chemotherapy in surgically staged IIIa-N2 non-small-cell lung cancer: A prospective pilot study. The Leuven Lung Cancer Group. Ann Oncol 1998;9:1193–8.
- Brundage MD, Davies D, Mackillop WJ. Prognostic factors in non-small cell lung cancer: A decade of progress. Chest 2002;122:1037–57.
- Dehing-Oberije C, De Ruysscher D, van der Weide H, Hochstenbag M, Bootsma G, Geraedts W, . Tumor volume combined with number of positive lymph node stations is a more important prognostic factor than TNM stage for survival of non-small-cell lung cancer patients treated with (chemo)radiotherapy. Int J Radiat Oncol Biol Phys 2008;70:1039–44.
- Solan MJ, Werner-Wasik M. Prognostic factors in non-small cell lung cancer. Semin Surg Oncol 2003;21:64–73.
- Agarwal M, Brahmanday G, Chmielewski GW, Welsh RJ, Ravikrishnan KP. Age, tumor size, type of surgery, and gender predict survival in early stage (stage I and II) non-small cell lung cancer after surgical resection. Lung Cancer 2010;68:398–402.
- Fu JB, Kau TY, Severson RK, Kalemkerian GP. Lung cancer in women: Analysis of the national Surveillance, Epidemiology, and End Results database. Chest 2005;127:768–77.
- Ball D, Smith J, Wirth A, Mac Manus M. Failure of T stage to predict survival in patients with non-small-cell lung cancer treated by radiotherapy with or without concomitant chemotherapy. Int J Radiat Oncol Biol Phys 2002;54:1007–13.
- Hoang JK, Hoagland LF, Coleman RE, Coan AD, Herndon JE, 2nd, Patz EF, Jr. Prognostic value of fluorine-18 fluorodeoxyglucose positron emission tomography imaging in patients with advanced-stage non-small-cell lung carcinoma. J Clin Oncol 2008;26:1459–64.
- Forssell-Aronsson E, Kjellén E, Mattsson S, Hellström M, Swedish Cancer Society Investigation Group T. Medical imaging for improved tumour characterization, delineation and treatment verification. Acta Oncol 2002;41: 604–14.
- Kong FM, Ten Haken RK, Schipper MJ, Sullivan MA, Chen M, Lopez C, . High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: Long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys 2005;63:324–33.
- Dehing-Oberije C, Yu S, De Ruysscher D, Meersschout S, Van Beek K, Lievens Y, . Development and external validation of prognostic model for 2-year survival of non-small-cell lung cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys 2009;74:355–62.
- Saunders M, Dische S, Barrett A, Harvey A, Griffiths G, Palmar M. Continuous, hyperfractionated, accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small cell lung cancer: Mature data from the randomised multicentre trial. CHART Steering committee. Radiother Oncol 1999;52:137–48.
- Henning Willers FW, Bünemann H, Heilmann H-P. High-dose radiation therapy alone for inoperable non-small cell lung cancer: Experience with prolonged overall treatment times. Acta Oncol 1998;37:101–5.
- Mandrekar SJ, Schild SE, Hillman SL, Allen KL, Marks RS, Mailliard JA, . A prognostic model for advanced stage nonsmall cell lung cancer. Pooled analysis of North Central Cancer Treatment Group trials. Cancer 2006;107:781–92.
- Blanchon F, Grivaux M, Asselain B, Lebas FX, Orlando JP, Piquet J, . 4-year mortality in patients with non-small-cell lung cancer: Development and validation of a prognostic index. Lancet Oncol 2006;7:829–36.
- Shepherd FA, Rosell R. Weighing tumor biology in treatment decisions for patients with non-small cell lung cancer. J Thorac Oncol 2007;2(Suppl 2):S68–S76.
- Naqa IE, Deasy JO, Mu Y, Huang E, Hope AJ, Lindsay PE, . Datamining approaches for modeling tumor control probability. Acta Oncol Epub 2010 Mar 2.
- Encan T, Hannisdal E. Blood analyses as prognostic factors in primary lung cancer. Acta Oncol 1990;29:151–4.