Abstract
Purpose. To study the interaction between radiation dose distribution and hypofractionated radiotherapy with respect to the risk of radiation pneumonitis (RP) estimated from normal tissue complication probability (NTCP) models. Material and methods. Eighteen non-small cell lung cancer patients previously treated with helical tomotherapy were selected. For each patient a 3D-conformal plan (3D-CRT) plan was produced in addition to the delivered plan. The standard fractionation schedule was set to 60 Gy in 30 fractions. Iso-efficacy comparisons with hypofractionation were performed by changing the fractionation and the physical prescription dose while keeping the equivalent tumor dose in 2 Gy fractions constant. The risk of developing RP after radiotherapy was estimated using the Mean Equivalent Lung Dose in 2-Gy fractions (MELD2) NTCP model with α/β=4 Gy for the residual lung. Overall treatment time was kept constant. Results. The mean risk of clinical RP after standard fractionation was 7.6% for Tomotherapy (range: 2.8–15.9%) and 9.2% for 3D-CRT (range 3.2–20.2%). Changing to 20 fractions, the Tomotherapy plans became slightly less toxic if the tumor α/β ratio, (α/β)T, was 7 Gy (mean RP risk 7.5%, range 2.8–16%) while the 3D-CRT plans became marginally more toxic (mean RP risk 9.8%, range 3.2–21%). If (α/β)T was 13 Gy, the mean estimated risk of RP is 7.9% for Tomotherapy (range: 2.8–17%) and 10% for 3D-CRT (range 3.2–22%). Conclusion. Modern highly conformal dose distributions are radiobiologically more forgiving with respect to hypofractionation, even for a normal tissue endpoint where α/β is lower than for the tumor in question.
Hypofractionated radiotherapy, i.e. the delivery of radiation therapy with a dose per fraction exceeding 2.2 Gy [Citation1], attracts renewed interest in curative radiation therapy. In part, this is stimulated by the outcome of large randomized controlled trials, showing a smaller than conventionally expected differential between the fractionation sensitivity, quantified by the α/β ratio of the linear-quadratic model, of some tumor histologies and typical late side effects [Citation2–4]. A further stimulus comes from advances in treatment planning and delivery that allows whole or partial avoidance of incidental irradiation of critical normal structures around the target volume. It has been shown, that organs at risk with a marked volume effect may allow the use of hypofractionation even when the tumor α/β ratio (α/β)T is larger than that of the critical normal-tissue endpoint, (α/β)NT. Intuitively, this is because the decrease in physical dose required to maintain iso-effective schedules for tumor control will offset the theoretical disadvantage of hypofractionation in normal-tissue voxels exposed to a low percentage of the target volume dose [Citation5,Citation6]. Most recently, in a study independent of ours, Jin et al. demonstrated that for sufficiently high prescription doses, hypofractionation would tend to decrease the damaged volume of the lung as predicted by a local threshold dose model [Citation6].
Radiation therapy for non-small cell lung cancer (NSCLC) is an interesting clinical scenario where the target volume is surrounded by a relatively sensitive structure, the normal lung. Radical radiotherapy, alone or combined with chemotherapy [Citation7] is the primary therapy in patients who are medically or technically inoperable. Radiotherapy dose for patients with NSCLC is limited by the sensitivity of the normal lung to radiation, especially if large volumes of the lung are exposed [Citation8]. Several trials have shown a dose response relationship for the tumor [Citation9–11], so dose escalation can improve tumor control. Unfortunately, very high doses may be required to reduce the local recurrence rate considerably [Citation11] and despite recent advances, local recurrence remains an important cause for failure [Citation12].
Hypofractionation is attractive in terms of both logistics and patient convenience and may allow dose escalation/treatment acceleration without the increase in cost associated with accelerated hyperfractionation [Citation13]. Consequently, several hypofractionated regimens have been used in the treatment of lung cancer [Citation14].
Accelerated radiotherapy has been shown to improve tumor control in NSCLC [Citation15]. However, the current study assumes a constant overall treatment time in the schedules being compared in order to isolate the interaction between dose distribution and hypofractionation schedule from the effect of acceleration. The risk of radiation pneumonitis after hypofractionated therapy is compared between a modern conformal technique and a standard 3D-CRT technique using the mean lung dose model published in the recent QUANTEC study [Citation8].
Material and methods
Eighteen patients with NSCLC previously treated at the University of Wisconsin with helical tomotherapy were selected for this study [Citation14]. For all patients, planning CT images were acquired with a dedicated GE Discovery LightSpeed™ CT scanner and the treatment plans were optimized to deliver the prescription dose to at least 95% of the target volume while simultaneously meeting dose-volume constraints placed on the residual healthy lung, esophagus, and spinal cord. For each of the 18 patients, a conventional 3D-CRT plan was made in addition to the delivered plan for the purpose of this study.
The effect of hypofractionation was modeled separately from a possible effect of overall treatment time by assuming a varying number of fractions given in a fixed overall time. Hence, if more than 2 Gy/fractions was used, the number of treatment days per week was decreased. This approach served a double purpose: 1) it avoided the need to incorporate repopulation and repair parameter estimates, and the uncertainties associated with these and 2) it isolated the fraction size dependence of the dose-distribution effects.
The equivalent dose in 2-Gy fractions is defined as
where D is the total physical dose, N is the number of fractions and α/β is the ratio of the linear and quadratic parameters in the linear quadratic model and depends on the normal tissue or tumor endpoint of interest. For radiation pneumonitis α/β has been estimated at 4.0 ± 0.9 Gy [Citation16]. In the following α/β=4 Gy was used for RP and α/β=10 Gy was assumed for tumor control unless otherwise noted.
In the simulations, we kept EQD2 constant at 60 Gy for the isodose line encompassing 95% of the target. This was done by renormalizing the plan, such that the physical dose given to 95% of the PTV was equal to the dose, D’, fulfilling
This renormalization of the physical dose carried over to the normal tissue dose and was incorporated into the model by calculating the equivalent dose in 2 Gy fractions to the organ at risk (OAR), EQD2OAR, for each voxel of the dose matrix in the normal tissue,
Here d denotes the physical dose of the voxel after renormalization. For the part of the OAR receiving the full prescribed dose D’, EQD2OAR will be increased if the number of fractions is decreased. It has been shown, however, that low dose voxels are spared owing to the decrease in physical dose [Citation6]. In this study, we investigate how this will affect the fractionation sensitivity of highly conformal intensity modulated rotational therapy plans exhibiting extensive low dose baths, as compared with more traditional 3D-CRT techniques.
We assessed the toxicity of the plans using the mean lung dose model of NTCP with the modeling parameters taken from estimates recently published in the QUANTEC (Quantitative Analysis of Normal Tissue Effects in the Clinic) overview [Citation8]. The mean lung dose model is equivalent to the Lyman model with a dose reduction parameter n = 1 [Citation17,Citation18]. The complication probability was then calculated on the basis of the MLD through a logistic dose-response curve [Citation19],
with parameters b0=–3.87 and b1=0.126 Gy–1 defining the slope and position of the sigmoidal dose-response curve [Citation8]. Because the QUANTEC fit was based on low-medium grade pneumonitis (grade 1–3, various scales), the NTCP estimates presented here will be valid for low-medium grade RP as well.
Throughout this study, we used the Mean Equivalent Lung Dose in 2 Gy fractions (MELD2), i.e. the mean of EQD2 over the residual lung, defined as both sides of the lung minus the PTV. Furthermore, the physical dose-volume histogram was converted into a EQD2-volume histogram (EQD2VH) by converting the physical dose in each bin of the DVH into an EQD2, We used purpose-written Matlab routines as well as the CERR package [Citation20] for the specific calculations of NTCP. After calculating a mean risk of RP, we estimated its confidence interval by bootstrap resampling: drawing 5 000 samples of 18 random patients from the cohort (allowing each patient to be selected more than once) and calculating the mean risk of RP. The 68% confidence interval of the mean corresponds to the 16th to the 84th percentile of the distribution of the 5000 sample means.
Results
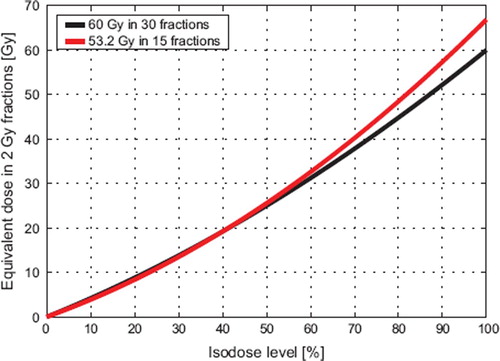
explores the dependence of EQD2 on the physical isodose level in the plan for normal fractionation and a hypofractionated plan using 15 fractions. For isodose levels above 40%, EQD2 is larger with the hypofractionated plan than with normal fractionation. For isodose levels below 40%, EQD2 is decreased slightly by hypofractionation. The isodose level of break even, defined as the isodose line where EQD2 is unchanged with hypofractionation, is dependent upon the ratio of α/β for both normal tissue and target. Break even will occur at higher isodose levels if (α/β)NT is increased or (α/β)T is decreased. In , we used α/β = 4 Gy for the residual lung and α/β = 10 Gy for the tumor.
Figure 1. Equvalent dose in 2 Gy fractions (EQD2) for the lung (α/β = 4 Gy) versus isodose level for 30- and 15-fraction schedules and a prescription dose of 60 Gy with standard fractionation. The physical dose is adjusted to iso-effect in the hypofractionated case by ensuring that the equivalent dose to the target remains constant assuming α/β = 10 Gy. The overall effect of the decrease in physical dose and the increase in fraction size is a rise in EQD2 for isodose levels above 40%. In contrast, lung exposed to less than 40% of the prescription dose will experience a minor decrease in EQD2 with hypofractionation.
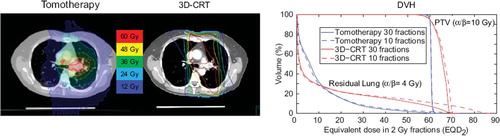
The dose distributions for Tomotherapy and 3D-CRT in a single patient, which is representative of the population, is shown in along with the corresponding EQD2VH at 10 and 30 fractions. The DVH confirms that hypofractionation causes EQD2OAR to increase for large-dose voxels of the OAR and decrease for low-dose voxels. A more pronounced difference was seen between the standard and hypofractionated DVH with 3D-CRT than with tomotherapy, as more of the lung was exposed to high doses. The effect of hypofractionation on the residual lung DVH in the tomotherapy case was modest.
Figure 2. An illustrative case with a tomotherapy and a 3D-CRT plan and the corresponding dose volume histograms corrected for fractionation by using α/β = 4 Gy for the residual lung and α/β = 10 Gy for the tumor.
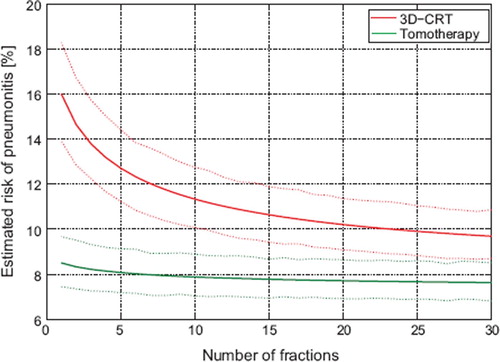
The mean estimated risk of pneumonitis of the 3D-CRT and tomotherapy plans for various dose-fractionation schedules is shown in . The figure shows that the risk of RP increased as the number of fractions decreased for the 3D-CRT plan. In contrast, the tomotherapy plans showed limited sensitivity to fraction size with an absolute change of RP risk of well below 1% when reducing the number of fractions from 30 to 15.
Figure 3. Mean risk of RP with the 3D-CRT and the tomotherapy plans for the 18 patients for different fractionation schedules. The solid lines represent the mean estimated risk of RP, while the dotted lines are the 68% confidence interval of the mean as estimated by bootstrap resampling. A more pronounced fractionation sensitivity is seen for 3D-CRT plans than for the tomotherapy plans.
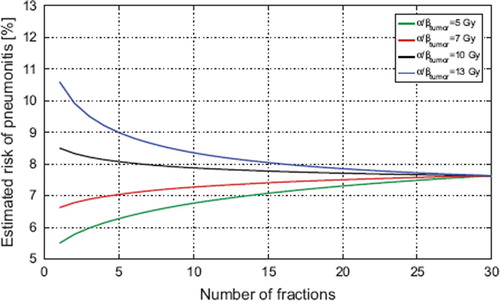
The result of depends on the fractionation sensitivity of the tumor and normal tissue. shows a sensitivity analysis on (α/β)T by displaying the estimated risk of RP versus number of fractions for the tomotherapy plans using α/βt = 5,7,10 and 13 Gy, respectively. It should be noted, that for (α/β)T ≤ 7Gy, hypofractionation with tomotherapy reduces the mean risk of RP in our study population.
Figure 4. A sensitivity analysis of the dependence on the tumor fractionation sensitivity for the tomotherapy plans. The risk of RP increases as the tumor fractionation sensitivity decreases because the physical dose required for iso-effect increases with tumor α/β. As long as only moderate hypofractionation is applied, however, the change in RP is much less than the interpatient variation and the uncertainty in the model. For a 20-fraction schedule the difference in estimated risk of RP between α/β = 7 Gy and α/β = 13 Gy is less than 0.4%.
Discussion
For a constant tumor control probability, hypofractionation is expected under the linear-quadratic model to be more toxic than standard or hyperfractionation when the traditional values of α/β are applied and repopulation effects are neglected. The current interest in hypofractionation, for example prostate and breast cancer, is hence mainly driven by indications that the α/β values of these tumors may be considerably lower than previously assumed [Citation2,Citation21]. This study shows that even with standard values of α/β of 4 and 10 Gy for normal tissue and tumor, respectively, and without any acceleration of treatment, lung toxicity after hypofractionation is predicted to be comparable to that of standard fractionation using the MLD model of NTCP with clinically estimated parameters. This result is in agreement with the recently published study by Jin et al., where the relative damaged volume of the lung was compared between standard, long course radiotherapy schedules and typical stereotactic radiotherapy schedules [Citation6] for a different prescription doses and found that for a sufficiently high prescription dose, hypofractionation was favoured. Referring to , this can be understood because the 40% isodose line occurs at higher physical doses as the prescription dose is increased. When the 40% isodose level becomes higher than the threshold dose in a local damage model, hypofractionated schedules will be predicted to be less toxic as in the study by Jin et al. In this study, we show that the risk of RP with modern, highly conformal treatment plans is largely insensitive to hypofractionation when using the MLD model and the parameters derived in the recent review of QUANTEC to predict the incidence of RP. The interaction between dose distribution and fraction size is the result of the biological effect of the low dose bath associated with the most conformal techniques being relatively lower with hypofractionation. This can be seen from and as follows: The tomotherapy plan exposed a small volume of normal lung to more than 40% of the prescribed target dose, corresponding to the break-even point in . In this volume hypofractionation increases the biological effect. On the other hand, a substantial volume of the lung was exposed to doses below this level, and this volume benefited from hypofractionation through a reduction in EQD2 as shown in . As it turns out, the two effects were of similar magnitude and consequently the tomotherapy plan was largely insensitive to fractionation. The 3D-CRT plans were penalized more by hypofractionation due to the much larger volume exposed to doses above 40%.
With the cost of cancer therapy becoming an increasing concern and modern radiotherapy techniques becoming still more advanced and expensive, reducing the number of fractions may be a way to offset this cost with little or no estimated detriment to the therapeutic ratio. Furthermore, considering the dose response of lung tumors and the proven benefit from treatment acceleration [Citation9–11,Citation15], escalating the dose per fraction may be more attractive than increasing the number of fractions as a strategy for improving tumor control in the chest. Hypofractionation increases the accuracy requirements for treatment planning and delivery [Citation5], but in-room image-guidance, motion management and improved dose calculation algorithms provide an increased level of accuracy [Citation22,Citation23]. In this context it should be noted that estimates a reduction in the risk of RP when treating with a highly conformal technique in 15 fractions as compared to a standard 3D-CRT technique delivering 30 fractions. Hence, if an advanced treatment fraction can be delivered at less than twice the cost of a standard 3D-CRT fraction, the advanced technique may reduce the overall cost of therapy and at the same time increase the therapeutic ratio.
The exact quantitative results of this modeling study obviously depend on the parameters and the models applied. In terms of sensitivity to (α/β)T, shows that the results are robust for reasonable values of the tumor fractionation sensitivity. With respect to the parameters in the MLD model, we note that since the link function between MLD and the risk of RP is monotonic, the ranking of plans and fractionation schedules will be invariant when changing the parameters. Hence, the 15 fraction tomotherapy schedule will be less toxic than the standard fractionated 3D-CRT schedule regardless of the values of b0 and b1. Other models than the MLD model will give different predictions, but referring to the very modest difference in the hypo- and normal fractionated lung EQD2VH seen in Figure 2, it is difficult to imagine a reasonable model that will predict substantial increase in the risk of RP after hypofractionated therapy with tomotherapy. Finally we note that if the value of (α/β)T is lower than what was assumed above, hypofractionation is predicted to decrease lung toxicity compared to a standard fractionated plan, cf . This has potential implications for breast cancer treatment with IMRT as α/β for sub-clinical breast cancer is estimated to be low [Citation2].
In conclusion, we have shown that a hypofractionated schedule delivered with modern highly conformal radiotherapy results in only a very limited change in the predicted risk of RP compared with a standard fractionated scheduled estimated to be isoeffective with respect to tumor control. This prediction is based on a best current estimate of α/β from clinical data of RP at 4 Gy and assuming, conservatively, that (α/β)T for NSCLC is 10 Gy in accordance with current consensus. In the modeling presented here, the overall treatment time was kept constant, and as a result the estimates of therapeutic gain are conservative if hypofractionation is used to accelerate radiation therapy [Citation15]. A sensitivity analysis showed robustness of the results with respect to varying tumor fractionation sensitivity and model parameters.
Highly conformal delivery techniques, in particular helical or rotational delivery, give rise to dose distributions for which the relative disadvantage of moderate hypofractionation for normal tissue endpoints with (α/β)NT < (α/β)T is estimated to be minimal or non-existing. This allows utilization of the advantages of hypofractionation with respect to logistics and patient convenience. This result can be generalized to any dose-limiting organ with a parallel structure for which the mean dose to the organ is a reliable predictor of clinically manifest toxicity. Furthermore from a clinical/biological perspective, hypofractionation is an efficient method for treatment acceleration. Historical trials of hypofractionation, applying 3D-CRT techniques or even parallel opposing fields, may overestimate the relative toxicity of hypofractionated therapy when compared with modern treatment delivery.
Acknowledgements
ISV is supported by The Lundbeck Foundation Center for Interventional Research in Radiation Oncology (CIRRO) and The Danish Council for Strategic Research. SMB acknowledges support from the National Cancer Institute grant no. 2P30 CA 014520-34.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bentzen SM. Quantitative clinical radiobiology. Acta Oncol 1993;32:259–75.
- Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bentzen SM, . The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet 2008;371:1098–107.
- Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bliss JM, . The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet Oncol 2008;9:331–41.
- Lukka H, Hayter C, Julian JA, Warde P, Morris WJ, Gospodarowicz M, . Randomized trial comparing two fractionation schedules for patients with localized prostate cancer. J Clin Oncol 2005;23:6132–8.
- Bentzen SM. High-tech in radiation oncology: Should there be a ceiling? Int J Radiat Oncol Biol Phys 2004;58:320–30.
- Jin JY, Kong FM, Chetty IJ, Ajlouni M, Ryu S, Ten HR, . Impact of fraction size on lung radiation toxicity: Hypofractionation may be beneficial in dose escalation of radiotherapy for lung cancers. Int J Radiat Oncol Biol Phys 2010;76:782–8.
- Rowell NP, O'rourke NP. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev 2004:CD002140.
- Marks LB, Bentzen SM, Deasy JO, Kong FM, Bradley JD, Vogelius IS, . Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys 2010;76:S70–S76.
- Belderbos JS, Heemsbergen WD, De JK, Baas P, Lebesque JV. Final results of a Phase I/II dose escalation trial in non-small-cell lung cancer using three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys 2006;66:126–34.
- Bradley J. A review of radiation dose escalation trials for non-small cell lung cancer within the Radiation Therapy Oncology Group. Semin Oncol 2005;32(2 Suppl 3):S111–S113.
- Kong FM, Ten Haken RK, Schipper MJ, Sullivan MA, Chen M, Lopez C, . High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: Long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys 2005;63:324–33.
- Sause W, Kolesar P, Taylor S IV, Johnson D, Livingston R, Komaki R, . Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest 2000; 117:358–64.
- Fowler JF. Biological factors influencing optimum fractionation in radiation therapy. Acta Oncol 2001;40:712–7.
- Adkison JB, Khuntia D, Bentzen SM, Cannon GM, Tome WA, Jaradat H, . Dose escalated, hypofractionated radiotherapy using helical tomotherapy for inoperable non-small cell lung cancer: Preliminary results of a risk-stratified phase I dose escalation study. Technol Cancer Res Treat 2008;7:441–7.
- Saunders M, Dische S, Barrett A, Harvey A, Griffiths G, Palmar M. Continuous, hyperfractionated, accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small cell lung cancer: Mature data from the randomised multicentre trial. CHART Steering committee. Radiother Oncol 1999;52:137–48.
- Bentzen SM, Skoczylas JZ, Bernier J. Quantitative clinical radiobiology of early and late lung reactions. Int J Radiat Biol 2000;76:453–62.
- Jackson A, Kutcher GJ, Yorke ED. Probability of radiation-induced complications for normal tissues with parallel architecture subject to non-uniform irradiation. Med Phys 1993; 20:613–25.
- Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl 1985;8: S13–S19.
- Bentzen SM, Tucker SL. Quantifying the position and steepness of radiation dose-response curves. Int J Radiat Biol 1997;71:531–42.
- Deasy JO, Blanco AI, Clark VH. CERR: A computational environment for radiotherapy research. Med Phys 2003; 30:979–85.
- Ritter M. Rationale, conduct, and outcome using hypofractionated radiotherapy in prostate cancer. Semin Radiat Oncol 2008;18:249–56.
- Ronde HS, Hoffmann L. Validation of Varian's AAA algorithm with focus on lung treatments. Acta Oncol 2009; 48:209–15.
- Korreman SS, Juhler-Nottrup T, Fredberg PG, Navrsted PA, Enmark M, Nystrom H, . The role of image guidance in respiratory gated radiotherapy. Acta Oncol 2008;47:1390–6.
