To the Editor,
The standard curative care of inoperable non-small cell lung cancer (NSCLC) patient is definitive radiotherapy (RT) [Citation1]. Lung and oesophageal toxicity has been regarded as the main toxicity in definitive radiotherapy of NSCLC. Little concern has been offered cardiac toxicity, despite there is increasingly focus on cardiac toxicity and cardiac death due to radiotherapy in patients with other cancer diseases such as early stage breast cancer and patients with lymphoma. Several studies in these diseases have concluded that there is an increased risk of cardiac death following thoracic radiotherapy [Citation2]. The reason why cardiac toxicity has not been considered to be a problem in unresectable NSCLC is probably due to the poor prognosis for these patients [Citation3], but with increasing life expectancy for this group of patients, it may turn out to be an issue of concern in the future. In this study we report the heart toxicities in NSCLC patients treated with definitive RT at our centre.
Material and methods
This is a retrospective study. From September 1, 1995 through February 28, 2007, 328 inoperable patients with stage I–III or recurrent NSCLC received RT with curative intention at our centre, Odense University Hospital, Denmark. All patients had cytological or histological proven NSCLC. Patients were treated at different dose levels: 60 Gy/30–33 F, 66 Gy/33 F and 80 Gy/35–40 F. Induction chemotherapy with a platin-doublet and concomitant chemotherapy were used in patients in good performance and advanced stage since 1999. We excluded 78 patients from study population, 58 patients due to cardiac disease prior to radiotherapy, another eight patients did not fulfil radiotherapy, and nine patients had concomitant chemotherapy. In three cases, patient file or radiotherapy plan were missing. This left 250 patients for further analyses.
We used three-dimensional conformal radiotherapy. Elective nodal irradiation was not performed. Retrospectively, the heart was delineated (by two clinicians) in following three volumes: left ventricle, both ventricles and whole heart excluding pericardium. Mean doses to these volumes were calculated.
Data were obtained from patient files. No patients were lost to follow-up. The patients were followed every third month for two years and every sixth month for another three years with clinical examination, chest x-ray and measurement of lung function.
Cardiac toxicity was defined as having a cardiac event occurring after start of radiotherapy. Patients alive with no cardiac event were categorised as having no cardiac toxicity. Cardiac event included myocardial infarction, congestive heart disease, pulmonary embolism, supraventricular tachycardia, ventricular arrhythmia, sudden death (not otherwise specified), pericardial effusion, and non-specific cardiac disease. Only the first cardiac event was recorded.
Results
Basic study population characteristics are summarised in Table I. Survival was calculated from date of RT start. Data were analysed February 28, 2010, when 226 patients had died, the majority (85%) from NSCLC. Eight patients (3%) died of treatment related toxicity (pneumonitis and one patient of haematemese). The median survival was 17.3 month and overall survival for one, two and five years was 66%, 37% and 15%, respectively. Median potentially follow-up was 95 months (range 36–173 months).
One hundred and ninety patients (84%) died without evidence of cardiac event. In eight cases we could not exclude a cardiac event just before death. We have not included these as cardiac events. After RT 38 patients (15%) developed cardiac disease. The majority was supraventricular tachycardia (14 patients) and myocardial infarctions (eight patients).
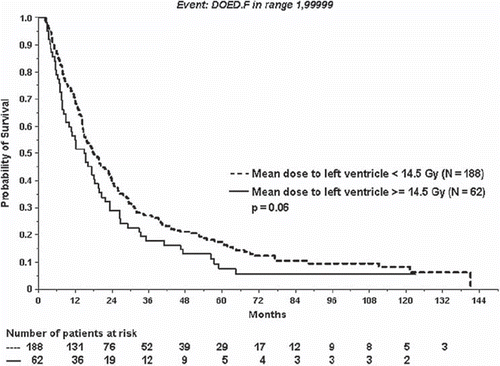
We defined high mean-doses to the heart as mean-dose level that exceeded the doses that 75% of the patients received (MHDup, up stating the upper quartile of the patients). This MHDup was 14.5 Gy to left ventricle (MHD range 0.1–57.8 Gy), 17.2 Gy to ventricles combined (MHD range 0.1–53.7 Gy) and 24.7 Gy to the whole the heart (MHD range 0.3–50.7 Gy), respectively. There was no correlation between having mean-heartdose above or below MHDup to either the whole heart, left- or both ventricles and having a cardiac event. Neither was any association between having mean-heartdose above or below MHDup to any of the three heart volumes and survival. Although there was a trend at worse survival in the group of patients with doses to the left ventricle above MHDup (p=0.06) ().
Discussion
In this retrospective analysis of a consecutive series of NSCLC patients treated with definitive radiotherapy, we did not find a correlation between high mean-dose to three different volumes of the heart (left ventricle, both ventricles or whole heart) and cardiac toxicity defined as having an cardiac event after radiotherapy start. This is not as shown in studies with other diseases treated with radiotherapy. Darby et al. recently published a review concerning radiation related heart disease [Citation2]. They reported a significantly worse survival beyond ten years for breast cancer patients receiving radiotherapy. Some studies reported mortality from heart disease increased by 27%. In Hodgkin lymphoma patients an increased risk value of three to five for cardiac morbidity in general compared to general population [Citation4] and relative risk of death from myocardial infarction compared with general population in range 2 to 4 [Citation4,Citation5]. There may be several possible reasons why we did not experience a significant toxicity despite the high doses we delivered to the heart compared with patients receiving RT for breast cancer and lymphoma. Only relative few NSCLC patients live long enough to experience cardiac disease either due to lung cancer itself or comorbidity as a competitive risk factor. In our study the five year survival was 15% leaving very few patients at risk for developing cardiac disease. Without long-term survivors cardiac toxicity does not seem to be a problem, and this suggests that we should aim to increase tumour control by administrating larger doses of radiotherapy to the tumour and/or by adding concurrent chemotherapy. However, the latter may increase the risk of cardiac toxicity by itself, and the results given in present study, may not be extrapolated to this situation. Another reason might be that if NSCLC patients develop dyspnoea, chest pain, etc. it is interpreted as being due to a relapse of lung cancer and not cardiac disease.
There are several studies indicating that large doses to the heart are associated with higher risk of cardiac toxicity [Citation6]. This is why the upper quartile was chosen to be definition of high mean dose in this study. In addition, it is not known which part of the heart which is the most radiosensitive part, nor which structures at risk should be chosen as a reference point for tolerance doses in clinical practice [Citation2]. In the present study, the endpoint was cardiac event. When analysing larger cohorts the primary end point is often cardiac death. This was not appropriate here because the sample size was too small.
Conclusion
This study did not find a relation between high mean-dose to different volumes of the heart and cardiac toxicity defined as cardiac event. This means that one should not compromise on dose to the tumour in order to reduce dose to the heart, when treating NSCLC patients with definitive radiotherapy.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Clinical practice guidelines for the treatment of unresectable non-small-cell lung cancer. Adopted on May 16, 1997 by the American Society of Clinical Oncology. J Clin Oncol 1997;15:2996–3018.
- Darby SC, Cutter DJ, Boerma M, Constine LS, Fajardo LF, Kodama K, . Radiation-related heart disease: Current knowledge and future prospects. Int J Radiat Oncol Biol Phys 2010;76:656–65.
- Auperin A, Le PC, Rolland E, Curran WJ, Furuse K, Fournel P, . Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181–90.
- Aleman BM, van den Belt-Dusebout AW, De Bruin ML, van ‘t Veer MB, Baaijens MH, de Boer JP, . Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood 2007;109:1878–86.
- Swerdlow AJ, Higgins CD, Smith P, Cunningham D, Hancock BW, Horwich A, . Myocardial infarction mortality risk after treatment for Hodgkin disease: A collaborative British cohort study. J Natl Cancer Inst 2007;99:206–14.
- Gagliardi G, Constine LS, Moiseenko V, Correa C, Pierce LJ, Allen AM, . Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys 2010;76(3 Suppl):S77–85.