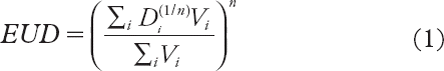Abstract
Background and purpose. The rectum is a major dose-limiting organ at risk (OR) in radiotherapy (RT) of prostate cancer. Methods to predict adverse effects in the rectum are therefore important but their precision often limited, not the least by the internal motion of this organ. In this study late rectal morbidity is investigated in relation to the internal motion of the rectum by applying the ‘Planning organ at Risk Volume’ (PRV) concept. Materials and methods: Late rectal morbidity was analysed in 242 prostate cancer patients treated to 70 Gy with conformal RT to either the prostate, the prostate and seminal vesicles or the whole pelvis (initial 50 Gy only). Late rectal morbidity was classified by the late gastro-intestinal (GI) RTOG toxicity scoring system. Cumulative dose-volume histograms (DVHs) were derived for the rectum OR and six rectum PRVs i.e. the OR expanded with six different margins (narrow/intermediate/wide in anterior direction or in both anterior and posterior direction). The difference in rectum dose-volume parameters between patients with Grade 0–1 vs. Grade 2 or higher morbidity was investigated by logistic regression and permutation tests. Results: Late Grade 2 or higher morbidity was observed in 25 of 242 (10%) patients. The logistic regression analysis and the permutation tests reached significance (p ≤ 0.05) for only one dose level of the rectum OR (40 Gy). For the PRVs, several dose levels were found to be significant (p-value range: 0.01–0.046), most pronounced for the PRV with narrow margins of 6 mm anterior and 5 mm posterior with five intermediate (38–42 Gy) and ten high (62–71 Gy) dose levels. Conclusions: The statistical methods applied displayed consistently a small though significant difference in DVH parameters between patients with vs. without Grade 2 or higher late rectal morbidity for intermediate and high dose levels. The difference became most evident when using a PRV with narrow margins.
The rectum is a major dose-limiting organ at risk (OR) in radiotherapy (RT) of prostate cancer, due to risk of developing late rectal complications [Citation1,Citation2]. Three-dimensional conformal radiotherapy (3DCRT) as well as the subsequent intensity-modulated RT (IMRT) techniques allow for precise radiation delivery to the prostate and limit the irradiated rectum volume [Citation3]. Nevertheless, to take full advantage of these technologies, detailed knowledge of the dose response of the rectum is required [Citation4]. In 2001, Jackson and colleagues studied features of the dose volume histogram (DVH) for the rectum OR in patients with prostate cancer and found significant relations between late rectal morbidity and several dose levels [Citation5]. A number of later studies have investigated the relationship between rectum OR dose/volume parameters and late rectal morbidity. In general, most of these found associations between rectum OR dose-volume parameters and late rectal morbidity for doses ≥ 60 Gy [Citation6,Citation7]. Still, considerable uncertainties about the dose/volume response of rectal morbidity remain, e.g. the influence of patient and treatment-related factors, including the internal motion of this organ [Citation2].
Previous studies have shown that the rectum undergoes considerable internal motion during a course of RT, in magnitude similar to the axial extent of the rectum [Citation8,Citation9]. Organ motion causes both random and systematic errors which will blur and shift, respectively, the dose distribution relative to the target [Citation10]. In order to predict rectal complications after prostate RT, knowledge of how the internal rectal motion influences the dose/volume parameters is central to obtain accurate information of the dose/volume constraints [Citation8]. A simple strategy to account for internal organ motion and set-up uncertainties and capture the dose distribution in the volume space in which the OR is likely to move within is to expand (i.e. add margins to) the OR [Citation11]. This approach is commonly denoted the planning organ at risk volume (PRV) concept and was first introduced in the International Commission on Radiation Units and Measurements (ICRU) Report 62 [Citation11]. A previous study has explored whether use of rectum PRVs improved the association between DVH parameters and acute gastro-intestinal (GI) toxicity in a series of 132 prostate cancer patients [Citation9]. For the DVHs of the PRVs, two to three times as many dose levels were found significantly related to toxicity compared to rectum only [Citation9,Citation12].
In this study, the persistent late rectal morbidity is investigated in relation to the internal motion of the rectum in a larger patient series, applying the population-based measure of motion represented by the PRV concept.
Material and methods
Patient material, dose prescription and treatment technique
Between 2000 and 2001, 247 prostate cancer patients were treated at Haukeland University Hospital (Bergen, Norway) with curative RT to 70 Gy with 10–15 MV photon beams in daily 2 Gy fractions five days a week. During the first five weeks the patients received 50 Gy to a large target volume followed by two weeks of 20 Gy to a reduced volume, the boost volume (further details on target volumes follow in the next section). The treatment planning was performed in Helax TMS (Helax TMS v 6.1A, Helax AB, Uppsala, Sweden). All patients were treated by multi-leaf collimator (MLC)-based 3DCRT with a four-field box technique (anterior, posterior and two lateral fields) except for one patient treated with a six-field technique (anterior, posterior and four lateral oblique fields).
Of these 247 patients, 86% had more advanced tumours [Citation13] and received hormone therapy commencing three to four months before and prolonging two months after start of the 3DCRT to reduce the prostate volume and hence the dose delivered to the ORs, i.e. the rectum and the bladder. The primary tumour was staged according to the 1997 tumour, node and metastasis (TNM) classification for prostate cancer [Citation13] and histopathology was classified according to the Gleason pattern score [Citation14].
The prescribed dose was defined as the mean dose to the internal target volume with a dose variation within the planning target volume meeting the ICRU criteria (−5% and +7%) [Citation15]. Patient specific data, further details of the treatment procedure and the late effects have been described previously [Citation16], but the relevant information for this study is briefly summarized below.
Patient groups and organ outlining
The patients were separated according to their risk factors (TNM stage, PSA level and Gleason score) and divided into three groups [Citation16]: 153 patients in Group Prostate (P), 49 patients in Group prostate and seminal vesicles (PSV) and 45 patients in Group modified pelvic fields (MPF). The patients in Group P and Group PSV received initially 50 Gy to the prostate respectively prostate and the prostate and seminal vesicles, followed by 20 Gy to the prostate solely. Patients in Group MPF received RT to 50 Gy to a larger volume followed by a reduced volume of the prostate and seminal vesicles to 20 Gy. Two margins were added to the CTV to obtain the PTVs: 15 mm to the initial target volume and 10 mm to the boost volume except in posterior direction towards rectum where 10 mm respectively 5 mm were used. The lateral margin for the MPF patients was 1 cm beyond the anatomic pelvis and limited to 10 mm from the prostate and seminal vesicles.
On the planning computer tomography (CT) scan, the responsible oncologist outlined the prostate, the seminal vesicles and the ORs. The rectum was defined by the volume within the outer wall contour including the contents using the first CT slice below the recto-sigmoid flexure as superior limit and the first CT slice above the anal verge as inferior limit.
For the purpose of this study, six rectum PRVs were defined by volume expansion from the rectum OR using six different sets of margins (narrow/intermediate/wide in anterior direction or in both anterior and posterior direction; see ). These margins were based on a previous study on rectum motion in bladder cancer patients [Citation8] with the narrow and large margins enclosing approximately 50% and 75% respectively of the observed rectum variation. As dose gradients primarily were observed along the anterior and posterior direction, margins were applied in these directions only [Citation9].
Table I. The different combinations of rectum margins [mm] applied: PRV 1–3 expanded in anterior direction only and PRV 4–6 in both anterior and posterior direction.
Late morbidity and follow-up
All patients were followed prospectively every six months the first year and then annually. Late complications were identified as adverse effects developed more than 90 days after the RT or as those starting earlier and persisting longer than 90 days after completed RT. The RTOG scoring system [Citation17] was used to grade the late lower gastrointestinal (GI) toxicity from three months up to five years after RT. The follow-up was standardized in terms of using a fixed questionnaire with the same physician conducting at least 90% of the follow-up sessions.
Our late rectal morbidity end-point was defined as the maximum recorded late lower GI Grade, even if a certain adverse effect later subsided. Patients were separated into two groups: Late GI Grade 0-1 and late GI Grade 2 or higher toxicity. In an additional analysis, we also included patients with prolonged late GI Grade 1 on two subsequent follow-up occasions into the maximum late GI Grade 2 or higher toxicity group.
DVH calculation
Relative DVHs for the rectum OR and PRVs were calculated for the summed plans to 70 Gy in steps of 1 Gy (from 0 to the maximal dose) for 242 patients (exclusion due to early death in two patients; data transfer problems in three patients). The DVH-based parameters, i.e. the dose/volume data was extracted and processed into cumulative DVHs in MATLAB (MATLAB v 7.6.0 (R2008a), The Mathworks, Inc, MA, US).
The generalised equivalent uniform dose (gEUD) analysis
The generalised equivalent uniform dose (gEUD) concept condenses information about the whole DVH into one single dose parameter, using a parameter n to describe the volume dependence of the dose-response relationship [Citation18]. The gEUD is assumed to be related to the risk of experiencing normal tissue complications in a certain organ and has shown to be useful also in clinical setting [Citation19]. The gEUD was calculated for all patients by means of the cumulative DVHs for the rectum only and the rectum PRVs applying the following relation [Citation20]:
where ΣiVi is the number of voxels of the current rectum structure and DiVi signifies the dose associated with the i-th voxel. By definition, the gEUD represents the mean dose when n = 1, whereas when n approaches 0 (but non-negative) the gEUD approaches the maximum dose. There is general consensus that the n parameter for rectum is relatively low (< 0.25–0.15) indicating that high-dose regions is the predominant factor in determining the risk of rectal toxicity [Citation6]. The n parameter for the rectal wall was proposed to be 0.12 by Burman et al. [Citation21] some 20 years ago. Several recent studies have assigned divergent values to the n parameter [Citation22,Citation23]. In this study, gEUD calculations were performed using a range of values for the n parameter (0.08, 0.12 and 0.23).
Statistical analysis
Two statistical methods were used to investigate possible differences between the two morbidity populations (see section Late morbidity and follow-up for details): Logistic regression and permutation tests. The permutation tests estimate p-values from Monte Carlo simulations and were applied in the present study with 200 simulations in order to validate the results from the logistic regression. Prediction by means of logistic regression will typically misclassify some of the patients in each morbidity population [Citation24]. The morbidity level was coded binary with 1 denoting patients with ≥ Grade 2 morbidity and 0 patients with Grade 0–1 morbidity. The statistical analysis was conducted in a statistical software (STATA/IC 11.0, StataCorpLP, TX, US).
Results
The maximum late lower GI RTOG Grade 2 or higher toxicity was observed in 25 (10%) of the 242 studied patients, with one case of Grade 4 toxicity. The symptoms consisted mainly of increased frequency of bowel movements, incontinence and mucous discharge. Overall, there were no differences in the irradiated average relative rectum volume for none of the dose levels between the two morbidity populations. However, for high dose levels (56–73 Gy) the patients with ≥ Grade 2 toxicity had a 2% larger irradiated average relative rectum volume and 3% larger for intermediate dose levels (33–44 Gy). The permutation tests reached significance (p ≤ 0.05) for only one dose level (40 Gy) for the rectum OR. Nevertheless, for the PRVs a range of dose levels were found to be significant with the logistic regression and validated by the permutation tests (p-value range 0.01–0.046). This was most pronounced with margins added in both the anterior and the posterior direction () with ten significant high (62–71 Gy) and five intermediate (38–42 Gy) dose levels in total. The number of significant dose levels was most distinct with margins of 6 mm anterior and 5 mm posterior (PRV 4) added. The p-values were considerably lower for all PRVs than the rectum OR in the 55–70 Gy dose range while approximately equal to the p-values for doses in the 40–55 Gy range as well as for doses below 40 Gy. The average area under the DVH curve (AUC) for the PRVs extended in both the anterior and posterior direction for the significant dose intervals was generally larger for patients with Grade 2 or higher GI toxicity than for Grade 0–1 () and on average 187 vs. 170 for the high dose levels and 92 vs. 82 for the intermediate.
Figure 1. The p-values from the logistic regression (permutation tests confirmed these p-values) between the maximum recorded rectum late morbidity and the relative volumes receiving a certain dose for rectum only (thin broken line) and with the six different margins (solid black lines) added. Dotted horizontal line indicates significance (p ≤ 0.05).
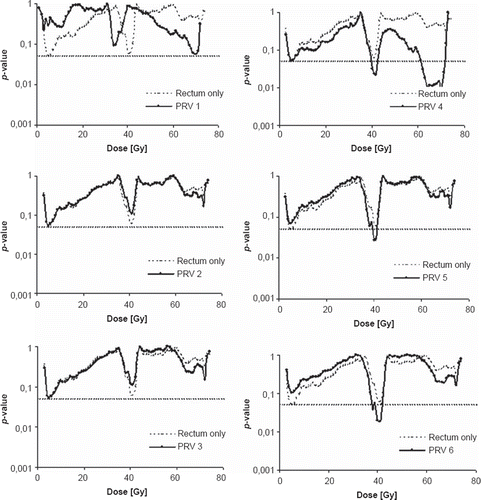
Table II. The calculated average area under the DVH curve (AUC) for the PRVs with intervals of significant dose levels for the high- and the intermediate dose levels. For the maximum RTOG morbidity end-point the AUC is calculated for PRV 4 (high dose levels) and PRV 4, 5 and 6 (intermediate dose levels). The AUC from the maximum RTOG morbidity end-point together with the prolonged GI Grade 1 is instead determined for PRV 1, 3 and 6 (high dose levels) and for the rectum only and all PRVs (intermediate dose levels).
The gEUDs were found to have a higher value for the patients with ≥ Grade 2 toxicity, but the differences did not reach statistical significance. The increase was most evident for large values of the n parameter ().
Table III. The generalized equivalent uniform dose (gEUD) with corresponding SD (in brackets) as well as the p-value for the difference in gEUDs between patients with or without the late maximum GI RTOG Grade 2 or higher rectal morbidity for the rectum only and rectum with margins with different values of the n parameter (0.08, 0.12 and 0.23).
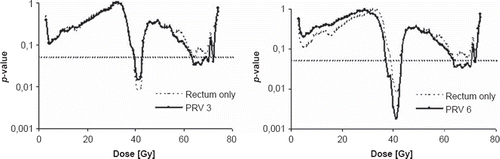
When modifying the maximum late GI Grade 2 or higher toxicity group to include also patients with GI Grade 1 on two subsequent follow-up occasions, the morbidity population consisted instead of 33 (14%) patients. This morbidity population had a 1% larger irradiated average relative rectum volume for all dose levels compared to patients in group Grade 0–1, except for dose levels between 29–73 Gy where it was 3% larger. Statistical significance (p-value range 0.01–0.05) based on the logistic regression and confirmed by the permutation tests was obtained for both the rectum OR and for the PRVs extended in anterior/posterior direction and in anterior direction only. In this case, the number of significant dose levels was most expressed with wide margins of 16 mm anterior added (PRV 3) as well as with wide margins of 16 mm anterior and 11 mm posterior (PRV six) with totally 6 high (64–70 Gy) and five intermediate (38–42 Gy) significant dose levels (). The AUC for the rectum OR and all the PRVs for the significant dose intervals was in analogy with the former findings; larger for patients with Grade 2 or higher GI toxicity (with GI Grade 1 on two subsequent follow-up occasions included) vs. Grade 0–1, though somewhat more pronounced for the intermediate dose levels ().
Figure 2. The p-values from the logistic regression (permutation tests confirmed these p-values) between the maximum late GI Grade 2 or higher toxicity group including also patients with prolonged GI Grade 1 and the relative volumes receiving a certain dose for rectum only (thin broken line) and with wide margins (solid black lines) added in anterior direction only (PRV 3) or in both anterior and posterior direction (PRV 6) added. Dotted horizontal line indicates significance (p ≤ 0.05).
Discussion
In this study we have investigated differences in DVH parameters between patients with Grade 0–1 vs. patients with ≥ RTOG late lower GI Grade 2 toxicity. Overall, the statistical analysis derived from logistic regression, and also verified by the permutation tests, has shown a small though significant difference for intermediate and high individual dose levels. Regarding the main purpose of the study, i.e. evaluating the impact of rectum PRVs, the differences in DVH parameters were found far more evident when margins were applied. PRVs expanded in anterior direction only demonstrated significant dose levels as the morbidity population was expanded whilst margins in both anterior and posterior direction yielded a range of significant dose levels for both investigated morbidity populations. Concerning the size of the margins, the number of significant dose levels peaked with narrow anterior and posterior margins (PRV 4).
Expanding the maximum late GI Grade 2 or higher toxicity group to include also patients with prolonged late GI Grade 1 on two subsequent follow-up occasions provided a stronger association between DVH parameters and morbidity and hence increased the number of significant dose levels: Significant dose levels were found for rectum OR and all PRVs. With this definition of morbidity, the pattern was most pronounced with wide anterior (PRV 3) and anterior and posterior (PRV 6) margins. In general, studying late rectal toxicity with such a longitudinal aspect has shown to contribute with both supplementary and complimentary information compared to using the maximum rectal toxicity end-point only to derive dose-volume constraints [Citation25].
Another common strategy to compare DVH parameters is to analyze the specific values of the relative volumes in the DVHs. Introducing this for 1, 2, 5, 10–100% of the rectal volume to the rectum OR and the PRV with the largest number of significant dose levels (PRV 4) the statistical methods applied did however not reach statistical significance (data not shown). Hence, specific values of the relative volumes had no apparent predictive power differentiating between patients with ≥ vs. without late GI Grade 2 toxicity.
Although there is a general understanding of significant associations between rectum OR dose-volume parameters and late rectal morbidity for doses ≥ 60 Gy there is still no consensus for doses ≤ 45 Gy [Citation6]. Nevertheless, amongst the exceptions the paper by Jackson et al. [Citation5] implies significant dose levels for the rectum OR in the range 40–50 Gy. This is interpreted as when high-dose regions are surrounded by extensive volumes receiving intermediate doses, the ability of these surrounding tissues to aid in the repair of an injury may be depressed (the ‘dose bath effect’) [Citation5]. Likewise these findings and another report from our group supporting significant intermediate dose levels though correlating acute morbidity with rectum OR and expanded with equivalent margins for the rectum PRVs [Citation9], the discoveries in the present study strengthen such an assumption for the rectum OR and in particular for the rectum PRVs. On the other hand, the volumes exposed to a certain dose level are likely to be highly correlated with one another across a wide range of doses, in particular for patients treated with similar techniques at the same institution [Citation6]. Volumes exposed to intermediate doses may therefore appear significant due to their correlation with the more biologically important high dose volumes.
A known limitation of the GI toxicity definition of the RTOG scoring system is the lack of discrimination between small bowel and rectum toxicity [Citation17]. Since the 45 patients in our MPF had a considerable irradiation of also the bowel, we also looked for associations between bowel DVHs (both OR and isotropically expanded PRVs) and our maximum GI endpoint. We did, however, not find any significant relations (data not shown), probably due to the low number of patients. In a future study we therefore aim to explore these relations by expanding the present data material with other clinical series [Citation26,Citation27].
The Monte Carlo based permutation tests were applied to validate the prediction based on the logistic regression. Although we acknowledge the potential bias of repeated testing [Citation28], the p-values used in this analysis are considered as a relative measure of the importance of different dose levels. This enables a comparison with the p-value interpretation of traditional morbidity prediction studies [e.g. Citation5]. Several other statistical methods could have been applied, such as receiver operating characteristics (ROC) analysis, but the issue of repeated testing would still remain.
Using the gEUD concept to describe the dose-response relation will depend on the relevance of the value designated to the n parameter. The values chosen for the n parameter in this study are anchored to the reference work by Burman et al. [Citation21] as well as to more recent studies [Citation22,Citation23], both aiming for a reliable description of the volume dependence in the rectum.
The margins selected for this study have been derived from a repeat CT and electronic portal imaging study on bladder cancer patients [Citation8] measuring the variation of the rectum across the whole height of the rectum but by adding margins in 2D. This was motivated by the fairly fixed location of the rectum in superior-inferior direction and the lack of dose gradients in pelvic RT set-ups [Citation8]. Using margins around ORs is however a simplified method to account for complex internal motion of volume changes, and set-up uncertainties. A more sophisticated approach would apply a model for rectum shape variations [e.g. Citation29]; this will be the topic for future investigations.
The PRV concept has been provided as means of predicting adverse effects also for other ORs after prostate RT. Sanguineti et al. [Citation30] expanded the bowel as the intestinal cavity and found one independent dose level (15 Gy) predicting acute peak Grade 2 diarrhea. In addition to the benefit of using PRVs for improving the predictive power for DVH parameters as shown in the present study, its use to account for organ motion and set-up errors [Citation11] has broad support and application in situations where the dose levels cause unacceptable complications, in particular for serial organs such as, e.g. for the spinal cord and the brain stem in head and neck IMRT planning [Citation10]. Generally, the PRVs are suggested to be defined such that their DVHs will not underestimate the contribution of the high-dose component of the OR in 90% of the cases in order to fulfil the DVH constraints for the ORs and make the treatment plans more robust [Citation10].
In this study and in morbidity prediction studies in general, the dose distribution has been determined from the treatment planning CT which only gives a snapshot of the patient anatomy [Citation31–33]. The dose distributions delivered to the rectal wall across the fractionated course of radiotherapy can vary considerably from the planned dose distributions due to motion and positioning uncertainties of the rectum [Citation29]. This implies that previous approaches have applied a dose distribution that does not mirror the whole picture of the real dose delivered to the rectum [Citation6]. Future improvements in modelling of late rectal morbidity is likely to result from DVHs that more accurately reflect the actual dose distribution to the rectum [Citation34] along with improved understanding in the underlying biology of rectal morbidity [Citation6,Citation32,Citation35,Citation36]. There are yet many steps towards the aim of achieving a more accurate actual dose distribution [Citation29,Citation32]. In future studies we aim to accumulate the dose to the rectum based on available series of repeat CT/Cone Beam CT scans acquired during the course of therapy. With these data it will be possible to take the rectum deformation into account, hopefully resulting in improved relationship between morbidity and the dose actually delivered to the rectum.
In conclusion, this study has displayed significant differences in associating the cumulative rectum dose with morbidity for patients with and without late GI Grade 2 effects, with more distinct differences when using margins around the rectum OR. The findings of this study support the use of rectum PRVs as tools in RT reporting and evaluation.
Acknowledgements
This work has been supported by research grants from the Danish Cancer Society, FSS (The Danish Council for Independent Research), CIRRO – The Lundbeck Foundation Center for Interventional Research in Radiation Oncology – as well as The Danish Council for Strategic Research. Ellen Wasbø and Harald Valen, Haukeland University Hospital (Bergen, Norway), are acknowledged for assistance with data transfer and anonymization.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Schultheiss TE, Lee WR, Hunt MA, Hanlon AL, Peter RS, Hanks GE. Late GI and GU complications in the treatment of prostate cancer. Int J Radiat Oncol Biol Phys 1997;37:3–11.
- Sripadam R, Stratford J, Henry AM, Jackson A, Moore CJ, Price P. Rectal motion can reduce CTV coverage and increase rectal dose during prostate radiotherapy: A daily cone-beam CT study. Radiother Oncol 2009;90:312–7.
- Perez CA, Michalski JM, Purdy JA, Wasserman TH, Williams K, Lockett MA. Three-dimensional conformal therapy or standard irradiation in localized carcinoma of prostate: Preliminary results of a nonrandomized comparison. Int J Radiat Oncol Biol Phys 2000;47:629–37.
- Gulliford SL, Foo K, Morgan RC, Aird EG, Bidmead AM, Critchley H, . Dose-volume constraints to reduce rectal side effects from prostate radiotherapy: Evidence from MRC RT01 trial ISRCTN 47772397. Int J Radiat Oncol Biol Phys 2010;76:747–54.
- Jackson A, Skwarchuk MW, Zelefsky MJ, Cowen DM, Venkatraman ES, Levegrun S, . Late rectal bleeding after conformal radiotherapy of prostate cancer (II): Volume effects and dose-volume histograms. Int J Radiat Oncol Biol Phys 2001;49:685–98.
- Michalski JM, Gay H, Jackson A, Tucker SL, Deasy JO. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys 2010;76(Suppl 3):123–9.
- Fiorino C, Valdagni R, Rancati T, Sanguineti G. Dose-volume effects for normal tissues in external radiotherapy: Pelvis. Radiother Oncol 2009;93:153–67.
- Muren LP, Ekerold R, Kvinnsland Y, Karlsdottir A, Dahl O. On the use of margins for geometrical uncertainties around the rectum in radiotherapy planning. Radiother Oncol 2004;70:11–9.
- Muren LP, Karlsdottir A, Kvinnsland Y, Wentzel-Larsen T, Dahl O. Testing the new ICRU 62 ‘Planning Organ at Risk Volume’ concept for the rectum. Radiother Oncol 2005;75: 293–302.
- McKenzie A, van Herk M, Mijnheer B. Margins for geometric uncertainty around organs at risk in radiotherapy. Radiother Oncol 2002;62:299–307.
- ICRU Report 62. Prescribing, recording and reporting photon beam radiotherapy (supplement to ICRU Report 50). International Commission on Radiation Units and Measurements, Bethesda, MD; 1999.
- Karlsdottir A, Johanesson DC, Muren LP, Wentzel-Larsen T, Dahl O. Acute morbidity related to treatment volume during 3D-conformal radiation therapy for prostate cancer. Radiother Oncol 2004;71:43–53.
- Hermanek P, Hutter RVP, Sobin LH, Spiessi B, Wagner G. TNM atlas. Illustrated guide to the TNM/pTNM classification of malignant tumours. Berlin and Heidelberg: Springer-Verlag; 1997.
- Mellinger GT, Gleason D, Bailar J. The histology and prognosis of prostatic cancer. J Urol 1967;97:331–7.
- ICRU Report 50. Prescribing, recording and reporting photon beam therapy. International Commission on Radiation Units and Measurements. Bethesda, MD; 1993.
- Karlsdóttir A, Muren LP, Wenzel-Larsen T, Dahl O. Late gastrointestinal morbidity after three-dimensional conformal radiation therapy for prostate cancer fades with time in contrast to genitourinary morbidity. Int J Radiat Oncol Biol Phys 2008;70:1478–86.
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;5:1341–6.
- Peeters ST, Lebesque JV, Heemsbergen WD, van Putten WL, Slot A, Dielwart MF, . Localized volume effects for late rectal and anal toxicity after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2006;64:1151–61.
- Wang JZ, Mayr NA, Yuh WT. Behind EUD. Acta Oncol 2008; 47:971–2.
- Mohan R, Mageras GS, Baldwin B, Brewster LJ, Kutcher GJ, Leibel S, . Clinically relevant optimization of 3-D conformal treatments. Med Phys 1992;19:933–44.
- Burman C, Kutcher GJ, Emami B, Goitein M. Fitting of normal tissue tolerance to an analytic function. Int J Radiat Oncol Biol Phys 1991;21:123–35.
- Tucker SL, Dong L, Bosch WR, Michalski J, Winter K, Lee AK, . Fit of a normal-tissue complication probability (NTCP) model to Grade ≥ 2 late rectal toxicity data from patients treated on protocol RTOG 94-06 [Abstract]. Int J Radiat Oncol Biol Phys 2007;69(Suppl 3):8–9.
- Rancati T, Fiorino C, Gagliardi G, Cattaneo GM, Sanguineti G, Borca VC, . Fitting late rectal bleeding data using different NTCP models: Results from an Italian multi-centric study (AIROPROS0101). Radiother Oncol 2004;73:21–32.
- Skwarchuk MW, Jackson A, Zelefsky MJ, Venkatraman ES, Cowen DM, Levegrün S, . Late rectal toxicity after conformal radiotherapy of prostate cancer I: Multivariate analysis and dose-response. Int J Radiat Oncol Biol Phys 2000; 47:103–13.
- Gulliford SL, Partridge M, Sydes MR, Andreyev J, Dearnaley DP. A comparison of dose-volume constraints derived using peak and longitudinal definitions of late rectal toxicity. Radiother Oncol 2010;94:241–7.
- Fokdal L, Honoré H, Høyer M, von der Maase H. Dose-volume histograms associated to long-term colorectal functions in patients receiving pelvic radiotherapy. Radiother Oncol 2005;74:203–10.
- Muren LP, Wasbo E, Helle SI, Hysing LB, Karlsdottir A, Odland OH, . Intensity-modulated radiotherapy of pelvic lymph nodes in locally advanced prostate cancer: Planning procedures and early experiences. Int J Radiat Oncol Biol Phys 2008;71:1034–41.
- Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst 1994;86:829–35.
- Yan D, Jaffray DA, Wong JW. A model to accumulate fractionated dose in a deforming organ. Int J Radiat Oncol Biol Phys 1999;44:665–75.
- Sanguineti G, Somani M, Eugene E, Little M, Chen G, Berilgen J, . Dosimetric predictors of diarrhea during radiotherapy for prostate cancer [Abstract]. Int J Radiat Oncol Biol Phys 2007;69(Suppl 3):346–7.
- Buettner F, Gulliford SL, Webb S, Sydes MR, Dearnaley DP, Partridge M. Assessing correlations between the spatial distribution of the dose to the rectal wall and late rectal toxicity after prostate radiotherapy: An analysis of data from the MRC RT01 trial (ISRCTN 47772397). Phys Med Biol 2009;54:6535–48.
- Grau C, Muren LP, Høyer M, Lindegaard J, Overgaard J. Image-guided adaptive radiotherapy-integration of biology and technology to improve clinical outcome. Acta Oncol 2008;47:1182–5.
- Søndergaard J, Høyer M, Petersen JB, Wright P, Grau C, Muren LP. The normal tissue sparing obtained with simultaneous treatment of pelvic lymph nodes and bladder using intensity-modulated radiotherapy. Acta Oncol 2009;48: 238–44.
- Webb S. The contribution, history, impact and future of physics in medicine. Acta Oncol 2009;48:169–77.
- Jeraj R. Future of physics in medicine and biology. Acta Oncol 2009;48:178–84.
- Van Herk M. Will IGRT live up to its promise? Acta Oncol 2008;47:1186–7