Abstract
Background. Expression of the peptide hormones ghrelin and obestatin has previously been demonstrated in human mammary glands. However, the clinical implications of the expression of these peptides in breast cancer are unclear. The aim of this study was to investigate the potential clinical value of ghrelin and obestatin as breast cancer biomarkers. Methods. A tissue microarray containing breast cancer specimens from 144 patients was immunostained with antibodies directed towards ghrelin and obestatin. Using varying cut-offs, the expression of the two peptides was evaluated and correlated to previously known prognostic factors in breast cancer and to the outcome. Cox regression analysis was used to assess whether these markers may predict survival of breast cancer patients. Results. Moderate to strong immunoreactivity for ghrelin and obestatin was observed in 71.5% and 77.1% of the cases, respectively. Ghrelin and obestatin expression was significantly but weakly correlated to low histological grade, estrogen receptor positivity, small tumor size and low proliferation. Only ghrelin expression was significantly correlated to better recurrence-free and breast cancer-specific survival (HR = 0.3–0.4, p = 0.02–0.05) in both uni- and multivariate analyses. The optimal cut-off was any ghrelin expression versus none. Reproducibility between the two readers was very good for both stainings with kappa values of 0.94–1.00. Conclusions. Patients with tumors expressing ghrelin had 2.5–3 times lower risk for recurrence or breast cancer death than those lacking ghrelin expression. Ghrelin expression is easily assessable with high reproducibility using immunohistochemistry. Further investigations are needed to establish the clinical significance of ghrelin as a biomarker in breast cancer.
Breast cancer is one of the most common malignancies in women worldwide and the leading cause of female cancer-related mortality [Citation1]. Prognostic and predictive markers are used to identify breast cancer patients which may benefit from adjuvant systemic treatment. Recently, the updated version of St Gallen International Expert Consensus on the primary treatment of early breast cancer (2011) was published, recommending classification of breast cancer into subtypes using clinicopathological determination of estrogen and progesterone receptors (ER and PgR), HER2 and Ki-67 [Citation2,Citation3]. Since genetic profiling is not established for clinical routine, the use of immunohistochemical markers for subtyping should still be considered state of the art for assessing risk of relapse and estimating probable effect of systemic therapy [Citation4].
Despite the use of above prognostic/predictive markers, we are still not able to accurately identify patients that will benefit from adjuvant systemic treatment and those that do not need it. A continued search for additional markers to further improve treatment stratification of breast cancer patients is therefore of importance.
Ghrelin is a peptide hormone which is involved in the pathway regulating growth hormone (GH) secretion. It is the natural ligand of the GH-secretagogue receptor (GHSR) type 1a. Ghrelin is a 28 amino acid peptide that is generated by processing of a 117 amino acid peptide, preproghrelin, and is stored in secretory vesicles of endocrine cells. Ghrelin can be further processed by addition of an octanoyl group to a serine residue, and this acylation was initially thought to be important for the biological activity of the peptide. Ghrelin is multifunctional, being involved in a range of processes including stimulation of appetite and regulation of gut motility [Citation5–9].
In 2005, another ghrelin-gene derived peptide was discovered and termed obestatin. It was initially reported to have the opposite effects to those of ghrelin. However, these actions have been disputed [Citation10,Citation11].
It has previously been shown that several regulatory peptides, including obestatin and ghrelin, are expressed in human breast tissue [Citation12]. Ghrelin and its receptor have also been shown to be present in cell lines and breast cancer tissues. It has been reported that growth factors may influence normal and neoplastic breast tissue, since specific mRNAs for growth hormone releasing hormone (GHRH) have been demonstrated in these tissues, as well as specific binding sites for GH secretagogues (GHS). Ghrelin has been proposed as the natural ligand for these binding sites, and there is emerging evidence that ghrelin could play a role in breast cancer since it may regulate proliferation of breast cancer cell lines [Citation13–18]. However, the presence and function of ghrelin and obestatin in mammary tissue still warrants further investigation.
Given these observations, we wanted to test the hypothesis that ghrelin/obestatin is correlated to survival in breast cancer patients and may be used as prognostic markers. The aim of this study was to investigate the possible immunohistochemical expression of ghrelin and obestatin in breast cancer tissue, and to test if any of these proteins could be useful as markers of clinical outcome.
Material and methods
Patient and tumor characteristics
In this study, tissue samples from 144 patients with invasive breast cancer, diagnosed between 2001 and 2002 at the Department of Pathology, Skåne University Hospital, Malmö, Sweden, were included [Citation19]. The cohort is non-consecutive and cases were selected to contain a fairly equal proportion of grade 1–3 tumors. The median age of patients at the time of diagnosis was 64 years (range: 34–97 years). All tumors were reclassified regarding histological subtype and Nottingham Histological Grade (NHG) before TMA-construction. Tumor diameter ranged from 6–145 mm (median: 20 mm; mean: 26 mm). Patient characteristics including age, tumor size, grade, expression of hormone receptors, Ki67-index, lymph node status and HER2 are summarized in . The assessment of ER, PgR, HER2 and Ki67 has been previously described [Citation20].
Table I. Patients’ characteristics.
Median duration of follow-up in patients was 6.55 years (range: 0.33–7.55 years). Mean overall survival (OS) was 5.78 years and recurrence-free survival (RFS) was 5.45 years. The 5-year OS and RFS were 71% and 69%, respectively.
Tissue microarray construction
Formalin-fixed and paraffin-embedded specimens were collected. Two 1-mm tissue cores were taken using a semi-automated arraying device (TMArrayer, Pathology Devices, MD, USA), mounted in recipient blocks, cut in 4 μm sections and transferred to glass slides. The slides were stored at 4°C.
Antibodies
The primary antibodies used for immunohistochemical staining were anti-obestatin for which the production and characterization has been described previously [Citation21] and anti-ghrelin (Phoenix Pharmaceuticals, Belmont, CA, USA). Both antibodies were diluted 1:2000.
Immunohistochemistry
Immunohistochemical staining was performed using the Dako EnVision Plus-HRP Detection Kit (Dako, Glostrup, Denmark) according to the manufacturer's instructions. For antigen retrieval the sections were subjected to pre-treatment (microwave heating for 10 min at 750 W followed by 15 min at 380 W using Tris-HCl buffered saline, pH 8.0). The sections were incubated with the primary antibodies in PBS with 1% BSA over night at 4°C. Bound antibodies were visualized by incubation with liquid 3, 3′-Diaminobenzidine substrate chromogen for 5 min.
The analysis of the ghrelin and obestatin immunostainings was performed by two independent observers (MG and ETJ). Individual samples were examined and graded as non-immunoreactive (non-IR, A), weak (B), moderate (C) and strong (D). In case of conflicting results, a third evaluation was performed and consensus was reached. Each of the two samples from every tumor core on the array was examined and scored separately. If one sample from a tumor core was lost, the remaining one was used for scoring.
Photographs were taken using a Zeiss Observer Z1 microscope and the Axiovision software (Carl Zeiss, Göttingen, Germany).
Controls
The specificity of the antibodies has been evaluated and presented previously [Citation21]. Normal human gastric mucosa was used as positive control.
Data analysis
To investigate the optimal cut-off values we systematically analyzed hazard ratios (HR) for different cut-offs. The optimal cut-off was defined as the value that best separated a poor prognostic group from a good prognostic group (i.e. the highest/lowest HR with a low p-value).
Spearman's rank correlation test was used for correlations between variables. Kaplan-Meier plots were used for survival analysis, and the log-rank test was used to compare curves separated according to ghrelin or obestatin expression. Recurrence free survival (RFS) and breast cancer specific survival (BCSS) were used as events. RFS was defined as time from diagnosis until recurrence and breast cancer-specific survival (BCSS) was defined as time from diagnosis until breast cancer-related death.
Univariate Cox analyses were performed to test how each of the parameters correlated to breast cancer recurrence and survival. Multivariate Cox proportional hazards regression model including established prognostic parameters (age at diagnose, tumor size, nodal status, NHG, HER2) was used to estimate HR.
To evaluate the reproducibility of the scoring of the immunohistochemical results, comparison of the agreement of the two observers’ results was performed. Both investigators manually scored the material using a light microscope. The degree of concordance between the two investigators was quantified as the chance-corrected measure of agreement, known as kappa [Citation22]. The commonly applied definition for the interpretation of different kappa values that was used here is as follows: <0.20 poor; 0.21–0.40 fair; 0.41–0.60 moderate; 0.61–0.80 good; 0.81–1.00 very good.
REMARK criteria
A description of the fulfillment of REMARK [Citation23] criteria for biomarker studies is provided in Supplementary Table I to be found online at http://informahealthcare.com/abs/doi/10.3109/0284186X.2011.631576.
Ethics
Ethical approval was obtained from the Ethics Committee at Lund University (ref no 447-07), whereby informed consent was deemed not to be required other than by the opt-out method.
The research protocol was approved by the local ethics committees at Uppsala University Hospital and Lund University.
Results
Immunoreactivity in tumor breast tissue samples
For both markers, immunoreactivity was detected exclusively in the cytoplasm of the tumor cells whereas the stroma was non-IR. When present, both peptides were generally expressed in the majority of tumor cells and, therefore, only the staining intensity was accounted for in the statistical analyses. Various patterns of immunostaining intensity were observed. Obestatin generally showed a stronger immunostaining pattern than ghrelin. A total of 11 cases showed difference at the immunoreactive vs. the non-IR level between the antibodies. Ghrelin was IR in two cases where obestatin was non-IR, and obestatin was IR in nine cases where ghrelin was non-IR.
One hundred and thirty-seven cases were available for immunohistochemical evaluation of ghrelin as well as for obestatin. The remaining cases were either lost during technical preparation or did not contain a sufficient number of tumor cells (> 200 cells). The ghrelin array had seven completely detached cases, and 22 cases with only one core, and the obestatin array had seven detached cases and 11 cases with one core.
One hundred and thirty-four of 137 (97.8%) evaluable cases immunostained for ghrelin showed the same degree of immunostaining intensity between the two cores samples which was the same for 130 of 137 (94.9%) cases of the obestatin samples. Altogether, in three (2.1%) cases the immunostaining-score (from 0–3) varied between the two ghrelin samples whereas seven (4.9%) obestatin cases showed variation. Of these, only one (0.7%) ghrelin case and two (1.4%) obestatin cases showed a major variance resulting in a difference between IR and non-IR within the same case. Most often the differences were seen between grade 1 and 2. summarizes the results from the immunohistochemical scoring. Representative photos from the ghrelin immunostainings are shown in .
Figure 1. Ghrelin expression in invasive breast cancer tissue was analyzed by immunohistochemistry. Representative images of ghrelin with A (non-immunoreactive), B (weak), C (moderate) and D (strong) immunostaining. Scale bar = 100 μm.
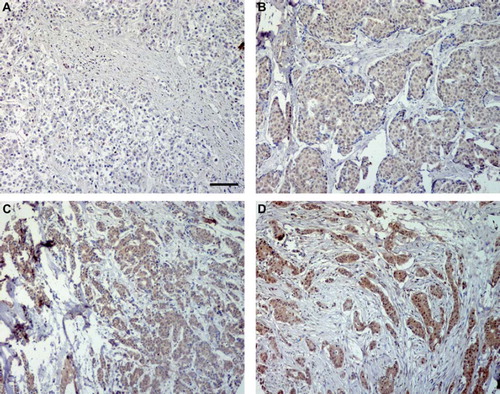
Table II. Results from the immunohistochemical scorings.
Reproducibility
The two investigators examined the ghrelin and obestatin immunostainings independently and blinded to clinical data. The kappa value for ghrelin was 0.94 and for obestatin 1.00.
Correlations to relevant clinicopathologic parameters
As shown in , both ghrelin and obestatin showed similar correlation patterns with a positive correlation to ER status, and negative correlations to tumor size, tumor grade and Ki67. Neither of the two markers showed any significant association to PgR status, HER2 status, age, menopausal status or node status. A positive significant correlation was also found between ghrelin and obestatin.
Table III. Ghrelin and obestatin expression in breast cancer in relation to clinicopathologic variables.
Association between the peptides and prognosis
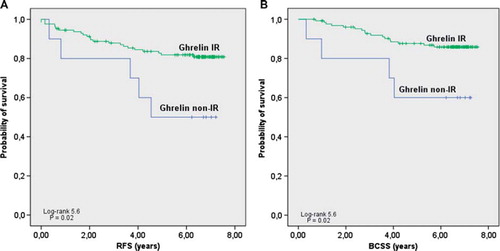
In univariate analysis, ghrelin expression showed a significant association to RFS (HR = 0.3, p = 0.02) as well as BCSS (HR = 0.3, p = 0.03) (). These associations remained significant in multivariate analysis adjusted for age, tumor size, nodal status and NHG ().
Table IV. Univariate analysis of prognostic parameters.
Table V. Multivariate analysis of prognostic markers.
Kaplan–Meier analysis confirmed that patients with tumors expressing ghrelin had a significantly longer RFS (, p = 0.02) and BCSS (, p = 0.02) compared to those with no ghrelin expression.
Figure 2. Survival among breast cancer patients by ghrelin expression. Ghrelin immunoreactive, scores 1–3; ghrelin non-immunoreactive, score 0. A, RFS according to ghrelin. B, BCSS according to ghrelin.
For obestatin, no correlation to BCSS or RFS was detected by univariate (), multivariate or Kaplan–Meier analysis (data not shown).
Control experiments
In all experiments the positive control showed immunoreactive cells in the deeper third part of the gastric mucosa as expected.
Discussion
In this study, the expression of ghrelin and obestatin was evaluated using immunohistochemistry in a well-characterized cohort of invasive breast cancer cases, to evaluate their biological and prognostic relevance.
In a previous study, we identified both ghrelin and obestatin in the epithelial cells of human breast, together with other neuroendocrine markers, such as chromogranin B [Citation12]. The presence of these peptides could suggest a possible endocrine function of these cells. However, both ghrelin and obestatin have also been demonstrated in human milk, where their function could be connected to affecting appetite and the regulation of energy balance of the new-born child, thus their function in this concept would rather be exocrine [Citation24–26]. Both ghrelin mRNA and protein have previously been detected in human breast tissue [Citation18,Citation27]. However, studies of the distribution of ghrelin and, especially, obestatin in normal and neoplastic mammary tissue are still very limited and more studies are warranted.
There are several studies that demonstrate the presence of ghrelin and its receptor in human tumors, but it still has not been concluded whether ghrelin promotes or inhibits carcinogenesis. There are reports where the effect of ghrelin on breast cancer cell lines has been investigated, however with contradictory results. An anti-proliferative effect has been documented by some [Citation13], whereas others have described a potential tumor-promoting role of ghrelin [Citation18]. Nevertheless, the use of cell lines and administrated dose of ghrelin in these studies may not represent physiological conditions. Further studies made in vivo are needed.
In this study, ghrelin and obestatin correlated positively to ER expression, and negatively to proliferation rate, tumor size and NHG status, demonstrating a relation between ghrelin and obestatin immunoreactivity in breast tumors and known factors associated with prognosis.
Ghrelin expression was associated with a prolonged survival, which is in line with the negative correlation to tumor grade, tumor size and proliferation rate. Furthermore, ghrelin retained its value as an independent prognostic marker after adjustment for established prognostic factors. The negative correlation to Ki67 and NHG also indicate that ghrelin may be a marker for less aggressive tumors and better prognosis. Ghrelin has a similar HR as established prognostic factors, which gives the hormone a possible role as a new prognostic marker for invasive breast cancer. Accordingly, patients with tumors non-IR for ghrelin have a 2.5–3 times higher risk for breast cancer recurrence and breast cancer-specific death.
The optimal cut-off, defined as the value that best separated a poor prognostic group from a good prognostic group, was IR (1+2+3) vs. non-IR (0) tumors. The evaluation separating only IR from non-IR tumors, without any counting of cells, was easy and quickly performed with a high reproducibility which is an advantage for possible future use in the clinical setting.
Ghrelin and obestatin were also demonstrated to be significantly correlated to each other. However, in this study obestatin did not provide any prognostic information, despite its close relationship to ghrelin. The reason for this is unclear; however, due to relatively few events in our studies it cannot be ruled out that also obestatin is a prognostic factor, even though we could not show this. One explanation for the different protein distribution of the peptides in the same tumor could be the complexity of the ghrelin gene. Recently, a revision of the structure of the human ghrelin gene has demonstrated the presence of novel exons, alternative splice variants and natural antisense transcripts. In addition, novel transcripts encoding C-ghrelin and a transcript encoding only for obestatin have been demonstrated. This suggests that ghrelin gene-derived peptides may also be produced independently of preproghrelin since these transcripts may exclude exon 1 of preproghrelin, which encodes for the conventional signal peptide of preproghrelin, and the first five amino acids of ghrelin [Citation28].
Obesity is associated with a poorer prognosis in breast cancer [Citation29]. Since ghrelin is an appetite-stimulating peptide, further studies regarding the possible relationship among ghrelin, obesity and breast cancer would be of interest.
To estimate the reproducibility of ghrelin and obestatin results, the agreement of two observers’ results was evaluated. The reproducibility of the ghrelin and obestatin assessments was very good (kappa values 0.94 and 1.00). We believe that the reproducibility of the results strengthen the robustness of the evaluation. The combination of easily scored material and good reproducibility makes this method easy to adapt for routine use.
In conclusion, we have identified expression of ghrelin and obestatin in invasive breast cancer. Significant correlations were found between ghrelin immunoreactivity and breast cancer relapse and survival. Ghrelin is a possible new interesting candidate in the field of prognostic markers. Further studies with a larger study material should be performed to confirm or refute the role of ghrelin as a new prognostic marker for breast cancer.
http://informahealthcare.com/abs/doi/10.3109/0284186X.2011.631576
Download PDF (39.6 KB)Acknowledgements
We thank Åsa Forsberg for excellent technical assistance. This work was supported by the Swedish Cancer Society and the Lions Foundation for Cancer Research at the Uppsala University Hospital. There is no conflict of interest to be declared.
References
- Hortobagyi GN, de la Garza Salazar J, Pritchard K, Amadori D, Haidinger R, Hudis CA, . The global breast cancer burden: Variations in epidemiology and survival. Clin Breast Cancer 2005;6:391–401.
- Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes – dealing with the diversity of breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011;22:1736–47.
- Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, . Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 2009;101:736–50.
- Gnant M, Harbeck N, Thomssen C. St. Gallen 2011: Summary of the Consensus Discussion. Breast Care (Basel) 2011;6:136–41.
- Arvat E, Di Vito L, Broglio F, Papotti M, Muccioli G, Dieguez C, . Preliminary evidence that Ghrelin, the natural GH secretagogue (GHS)-receptor ligand, strongly stimulates GH secretion in humans. J Endocrinol Invest 2000;23:493–5.
- Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, . Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 2001;120:337–45.
- Inui A. Ghrelin: An orexigenic and somatotrophic signal from the stomach. Nat Rev Neurosci 2001;2:551–60.
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999;402:656–60.
- Tack J, Depoortere I, Bisschops R, Delporte C, Coulie B, Meulemans A, . Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut 2006;55:327–33.
- Tang SQ, Jiang QY, Zhang YL, Zhu XT, Shu G, Gao P, . Obestatin: Its physicochemical characteristics and physiological functions. Peptides 2008;29:639–45.
- Zhang JV, Ren PG, Avsian-Kretchmer O, Luo CW, Rauch R, Klein C, . Obestatin, a peptide encded by the ghrelin gene, opposes ghrelin's effects on food intake. Science 2005;310:996–9.
- Gronberg M, Amini RM, Stridsberg M, Janson ET, Saras J. Neuroendocrine markers are expressed in human mammary glands. Regul Pept 2010;160:68–74.
- Cassoni P, Papotti M, Ghe C, Catapano F, Sapino A, Graziani A, . Identification, characterization, and biological activity of specific receptors for natural (ghrelin) and synthetic growth hormone secretagogues and analogs in human breast carcinomas and cell lines. J Clin Endocrinol Metab 2001;86:1738–45.
- Laban C, Bustin SA, Jenkins PJ. The GH-IGF-I axis and breast cancer. Trends Endocrinol Metab 2003;14:28–34.
- Chen A, Laskar-Levy O, Koch Y. Selective expression of neuropeptides in the rat mammary gland: Somatostatin gene is expressed during lactation. Endocrinology 1999;140:5915–21.
- Kahan Z, Arencibia JM, Csernus VJ, Groot K, Kineman RD, Robinson WR, . Expression of growth hormone-releasing hormone (GHRH) messenger ribonucleic acid and the presence of biologically active GHRH in human breast, endometrial, and ovarian cancers. J Clin Endocrinol Metab 1999;84:582–9.
- Schally AV, Varga JL. Antagonistic analogs of growth hormone-releasing hormone: New potential antitumor agents. Trends Endocrinol Metab 1999;10:383–91.
- Jeffery PL, Murray RE, Yeh AH, McNamara JF, Duncan RP, Francis GD, . Expression and function of the ghrelin axis, including a novel preproghrelin isoform, in human breast cancer tissues and cell lines. Endocr Relat Cancer 2005;12:839–50.
- Elkabets M, Gifford AM, Scheel C, Nilsson B, Reinhardt F, Bray MA, . Human tumors instigate granulin-expressing hematopoietic cells that promote malignancy by activating stromal fibroblasts in mice. J Clin Invest 2011;121:784–99.
- Borgquist S, Holm C, Stendahl M, Anagnostaki L, Landberg G, Jirstrom K. Oestrogen receptors alpha and beta show different associations to clinicopathological parameters and their co-expression might predict a better response to endocrine treatment in breast cancer. J Clin Pathol 2008;61: 197–203.
- Gronberg M, Tsolakis AV, Magnusson L, Janson ET, Saras J. Distribution of obestatin and ghrelin in human tissues: Immunoreactive cells in the gastrointestinal tract, pancreas, and mammary glands. J Histochem Cytochem 2008; 56:793–801.
- Altman D. Practical statistics for medical research. London, UK: Chapman & Hall; 1991.
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 2006;100:229–35.
- Aydin S, Aydin S, Ozkan Y, Kumru S. Ghrelin is present in human colostrum, transitional and mature milk. Peptides 2006;27:878–82.
- Aydin S, Ozkan Y, Erman F, Gurates B, Kilic N, Colak R, . Presence of obestatin in breast milk: Relationship among obestatin, ghrelin, and leptin in lactating women. Nutrition Epub 2008 May 20.
- Kierson JA, Dimatteo DM, Locke RG, Mackley AB, Spear ML. Ghrelin and cholecystokinin in term and preterm human breast milk. Acta Paediatr 2006;95:991–5.
- Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, . The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab 2002;87:2988.
- Seim I, Collet C, Herington AC, Chopin LK. Revised genomic structure of the human ghrelin gene and identification of novel exons, alternative splice variants and natural antisense transcripts. BMC Genomics 2007;8:298.
- Sinicrope FA, Dannenberg AJ. Obesity and breast cancer prognosis: Weight of the evidence. J Clin Oncol 2011; 29:4–7.
