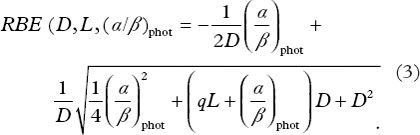Abstract
Background. The biological effects of particles are often expressed in relation to that of photons through the concept of relative biological effectiveness, RBE. In proton radiotherapy, a constant RBE of 1.1 is usually assumed. However, there is experimental evidence that RBE depends on various factors. The aim of this study is to develop a model to predict the RBE based on linear energy transfer (LET), dose, and the tissue specific parameter α/β of the linear-quadratic model for the reference radiation. Moreover, the model should capture the basic features of the RBE using a minimum of assumptions, each supported by experimental data. Material and methods. The α and β parameters for protons were studied with respect to their dependence on LET. An RBE model was proposed where the dependence of LET is affected by the (α/β)phot ratio of photons. Published cell survival data with a range of well-defined LETs and cell types were selected for model evaluation rendering a total of 10 cell lines and 24 RBE values. Results and Conclusion. A statistically significant relation was found between α for protons and LET. Moreover, the strength of that relation varied significantly with (α/β)phot. In contrast, no significant relation between β and LET was found. On the whole, the resulting RBE model provided a significantly improved fit (p-value < 0.01) to the experimental data compared to the standard constant RBE. By accounting for the α/β ratio of photons, clearer trends between RBE and LET of protons were found, and our results suggest that late responding tissues are more sensitive to LET changes than early responding tissues and most tumors. An advantage with the proposed RBE model in optimization and evaluation of treatment plans is that it only requires dose, LET, and (α/β)phot as input parameters. Hence, no proton specific biological parameters are needed.
The number of proton therapy centers is increasing and to date there are more than 30 facilities worldwide that have treated more than 65 000 patients in total [Citation1]. The primary advantage of proton therapy over conventional radiotherapy with photons and electrons is that the dose can be more focused to the tumor. Proton beams are considered especially well-suited for irradiation of, e.g. tumors located close to critical, radiosensitive structures in cases where the steep dose fall-off at the distal end of the proton range is advantageous, and for pediatric tumors due to the reduced integral dose. While the physical properties of proton beams are relatively well known, there are still questions regarding their biological effects. To take advantage of the experience gained from conventional radiotherapy with x-rays, the biological effects of particles are often expressed in relation to that of photons. The conversion from a (physical) particle dose to a (biological) photon equivalent dose is made using the concept of relative biological effectiveness (RBE). The physical proton dose multiplied with the RBE gives the biologically equivalent photon dose, or the so called effective dose. Equivalently, the prescription dose of protons can be determined by dividing the intended photon dose by the RBE. Therefore, it is essential that a correct RBE value is used since an incorrect RBE value may result in another effective dose than intended with a discrepancy larger than the dose accuracy of ± 3% usually requested [Citation2]. Also, an incorrect RBE value may propagate into an incorrectly chosen proton prescription dose.
There is experimental evidence that the RBE varies with the linear energy transfer (LET), the dose, the cell or tissue type, and the chosen endpoint [Citation3]. However, currently in proton therapy, a generic RBE of 1.1 is usually applied [Citation4] regardless of the physical properties of the proton beams and the biological system. This means that a given proton dose is assumed to be equivalent to a 10% higher photon dose for all tissues, tumors, doses and energies. A constant RBE is recommended in the report by the International Commission on Radiation Units and Measurements (ICRU) [Citation5], but in the same report it is warned for the increased RBE at the distal end of the proton range. A constant RBE for protons is a simplification and values both higher and lower than 1.1 have been observed. The use of a constant RBE has largely been due to the relatively small differences in RBE together with relatively large uncertainties in the experimentally obtained RBE values and considerable variations across experiments which make it difficult to discover systematic trends between different beam setups and biological systems [Citation5]. In this study, we will investigate if more clear trends in the variation of RBE are revealed by accounting for cell type.
One way of characterizing the cell type or tissue type in terms of sensitivity to radiation is by the parameters α and β of the well-known linear- quadratic (LQ) model [Citation6]. A low α/β ratio after exposure to photons has been found to be associated with late-responding tissues whereas a high α/β ratio is linked to early-responding tissues and most tumors. Therefore, the α/β ratio has an impact on dose fractionation effects and has been established and tabulated for many tissues and tumors. One question addressed in this study is if the cells’ sensitivity to photon radiation, quantified by the α/β ratio, can be used to estimate their sensitivity to proton radiation.
There are several cell survival studies where the parameters of the LQ model for protons are obtained, and several research groups have studied the dependence of these parameters on LET. A linear dependence of α as a function of LET up to LET values around RBE maximum have been suggested by, e.g. Hawkins [Citation7], Wilkens and Oelfke [Citation8], and Tilly et al. [Citation9]. The LET dependence of β is less clear with some studies pointing to an increasing β with increasing LET [Citation10,Citation11] whereas others report a decreasing trend [Citation12,Citation13]. Often a constant β is assumed for protons [Citation8,Citation9,Citation14].
A cell dependence of the parameter α has been suggested in various forms. Tilly et al. [Citation9] used two discrete slopes of the linear dependence on LET depending on which α/β ratio of the reference radiation the cell was grouped into (α/β ≈2 or ≈10). Dale and Jones [Citation14] introduced a parameter RBEmax, defined as the ratio of α for high LET over α for low LET, which depends on dose and the α/β ratio of the low-LET radiation (but no LET dependence is included). Hawkins [Citation7] and Frese et al. [Citation15] use an α/β ratio-dependence within the framework of the microdosimetric-kinetic model and the repair-misrepair-fixation model, respectively. Other models have a free parameter that needs to be determined for each cell type [Citation8].
The aim of this study is to develop a model to predict the RBE for protons based on dose, LET, and the tissue specific parameter α/β of photons. Moreover, the model should capture the basic features of the RBE, using a minimum of assumptions that are supported by experimental data. The α and β parameters will be studied with respect to their dependence on LET. We hypothesize: 1) a linear relation between α and LET; with 2) a slope that depends on cell type where the cell type is represented by the α/β ratio of photons; and finally, 3) we assume a parameter β independent of LET. Statistical analyses of experimental data will be performed to verify the validity of these assumptions. In order to study how the parameters α and β vary with LET and cell type, published in vitro clonogenic cell survival data where the LET and cell type are well-defined were chosen.
Material and methods
The RBE model
A first expression of the RBE can be derived from the LQ model, which describes the survival fraction as a function of dose and the radiosensitivity parameters α and β, together with the definition of RBE (see Appendix: available online at http://www.informahealthcare.com/doi/abs/10.3109/0284186X.2012.705892). This expression includes three ratios of interest: (α/β)phot, α/αphot, and β/βphot. The subscript phot will denote the reference photon radiation (e.g. 60Co γ-rays or x-rays) in this report whereas all other quantities pertain to proton radiation unless otherwise stated. The first quantity (α/β)phot is simply the ratio of the LQ parameters from photon exposure which is often used to characterize the radiosensitivity of a cell type or tissue. For the other two ratios, analytical expressions are proposed to describe how they vary with LET, and then evaluated using statistical methods (see Parameter estimation and model evaluation).
The ratio α/αphot has shown to increase with increasing LET up to around 30 keV/µm for protons, after which it decreases [Citation10]. Exactly how α/αphot increases with LET is not fully determined. There are few publications that report α for monoenergetic proton beams especially for LETs < 5 keV/µm. Due to the limited number of experimental data sets, a simple linear dependence to the LET, L, is assumed here where α for protons approaches αphot for photons, when LET decreases:
Equation 1 is not valid for LETs higher than 30 keV/µm since it does not account for the decrease at higher LETs. However, that high values are of little practical relevance with the energies used in clinical proton therapy due to straggling effects and, therefore, not considered in this work. Different studies report different α/αphot values for similar LET values. The difference is possibly due to differences between cell lines. We assume that the slope k is affected by the cell type and propose an inverse relationship between the slope and the tissue response related parameter (α/β)phot so that k decreases as (α/β)phot increases (see Equation A6 in Appendix). This means that the cell survival of cell lines with high (α/β)phot ratio depends less on LET because of the smaller slope k compared to cell lines with low (α/β)phot. The resulting expression for α/αphot becomes
where q is a free parameters of the expression.
The dependence of the ratio β/βphot on LET is more unclear since different research groups come to different conclusions. On this basis, the correlation between β and LET seems weaker than that for α and we will assume a constant β equal to βphot, i.e. β/βphot = 1.
The resulting expression for the RBE, obtained by inserting the expression for α/αphot (Equation 2) and β/βphot = 1 into the original RBE expression (Equation A4 in Appendix), depends only on the proton dose, LET, and α/β ratio of photons:
The constant q is the same regardless of the physical characteristics of the proton beam and the biological system. This expression is for single doses or dose per fraction.
Parameter estimation and model evaluation
For the purpose of our study, published clonogenic cell survival data with well-defined LET and reported α, αphot, β, and βphot values, were selected for the model evaluation. Experimental data of 10 different cell lines were included where the cells had been irradiated with near monoenergetic proton beams with LET values ranging from 6 to 30 keV/µm, i.e. up to LET values in the region of RBE maximum, giving in total 24 experimental data points [Citation10–13,Citation16,Citation17]. All the cell lines with their experimental α and β values used in this study are tabulated in .
Table I. The cell lines used in this study with the published α and β values given in the order of increasing α/β of the reference radiation.
The study by Perris et al. [Citation18] met our criteria of selection but was not included in our study. They studied the survival of Chinese hamster V-79 cells following irradiation with monoenergetic protons and 60Co γ-rays as a reference radiation. However, they report an (α/β)phot ratio of 25 which is far from the values around 5 that usually are reported for V-79 cells. A possible explanation could be that the cells were irradiated in different growth conditions. Due to the large discrepancy to other V79 cell lines, we omitted these experimental data.
To determine if the general assumptions made in the RBE model are adequate and supported by the experimental cell survival data, a series of statistical analyses were carried out. The significance level was set consistently to 5%. First, linear regression was used to test the assumption that there exists a positive relationship between α/αphot and LET. The null hypothesis was that the slope equals zero. Additionally, a 95% confidence interval (CI) for the intercept of this linear relation was constructed to determine if the assumption that the intercept equals one is adequate.
Second, the influence of cell type to the dependence between α/αphot and LET was studied. The assumption that the slope k from the linear relation between α/αphot and LET changes with (α/β)phot ratio was tested with Vuong's test for non-nested models. This is a method for model selection based on the likelihood ratio test statistic. The models to be compared here are the expression derived in this study where the slope is assumed to vary with the (α/β)phot ratio, Equation 2, and the expression where the slope is constant, Equation 1. The null hypothesis is that these models are equally close to the “true” model (a theoretical, unknown model that provides a perfect fit do data) against the alternative hypothesis that one model is closer than the other. Voung's test gives a test statistic that follows a standard normal distribution, so that a test statistic > 1.96 favors the (α/β)phot-dependent expression in Equation 2 over the (α/β)phot-independent expression in Equation 1 at the 5% significance level. As a complement to this analysis, to test for which cell types the slope is significantly larger than zero, the experimental data were grouped into four groups depending on their (α/β)phot ratio (2.7–3.1, 7.7–7.7, 15–18 and ≥ 70). The slope was studied in each group.
Finally, the assumption that there is no relation between β/βphot and LET was tested by constructing a 95% CI for the slope of the regression line.
For the derived model, the expression of α/αphot, Equation 2, was fitted to the experimentally obtained α/αphot ratios with least square optimization for all the cell lines and LETs used in this study to get a global value of the parameter q, i.e. a constant value regardless of the cell types and LET values used.
The resulting RBE model, Equation 3, was then evaluated by comparing the analytically obtained RBE values with experimental RBE values, and the model was further characterized by studying the effects of varying its input parameters.
Results
The results from the statistical analyses of the assumptions made in the RBE model show a positive relation between α/αphot and LET; for the whole data set, the slope k was greater than zero with statistical significance with a one-tailed p-value < 0.05. When grouping the experimental data based on the cell type, here measured by the (α/β)phot ratio, the slope was statistically greater than zero at the 5% significance level for the two groups with low (α/β)phot ratios (2.7–7.7). On the other hand, for the two groups with high (α/β)phot ratios (≥ 15) where the slope is smaller, the null hypothesis could not be rejected. The 95% CI for the intercept in the linear relation between α/αphot and LET was 0.79 ± 1.1, which includes the assumption of an intercept equal to one.
In the analysis on whether the relation between α/αphot and LET is influenced by the cell type, i.e. the assumption of a varying slope k depending on the (α/β)phot ratio, the Vuong's test resulted in test statistic of 2.7. Hence, the expression including a slope k that vary with (α/β)phot, Equation 2, gives a statistically significantly better fit to the experimental data compared to the expression with a constant slope k, Equation 1, at the 5% significance level.
For the β/βphot ratio no statistically significant relation with LET was seen and the 95% CI for the slope was -0.01 ± 0.05, which includes zero.
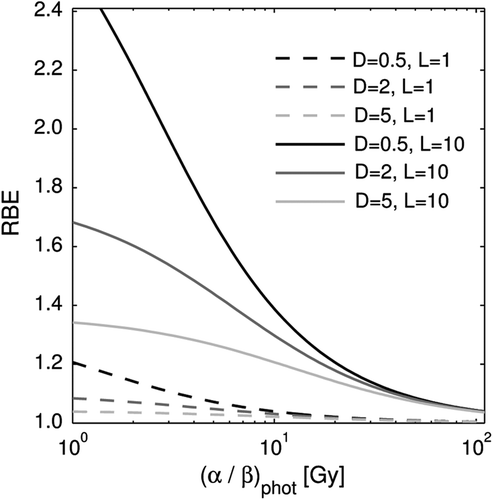
The value of parameter q of Equation 2 that best fitted the experimental α/αphot data was found to be 0.434 [95% CI (0.366, 0.513)] (Gy µm/keV). This q value is used in all the figures in this study. The α/αphot dependence on LET and (α/β)phot is visualized in . The resulting slopes of the curves shown in the figure are presented in . Generally, α/αphot increased with increasing LET, but this relation between α/αphot and LET was influenced by the (α/β)phot ratio. The sharpest increase was seen at low (α/β)phot ratios (panel A in ), but for very high (α/β)phot ratios α/αphot displayed no trend with LET (panel D in ). shows that with increasing (α/β)phot ratio the slope k decreases, and in k as a function of the (α/β)phot ratio, obtained with Equation A6 in Appendix, is shown.
Figure 1. Experimentally obtained α/αphot values as a function of LET. Panel A with (α/β)phot 2.7−3.1: V79-379A cells [12], V79-753B cells [10], and DLD1 cells [17]. Panel B with (α/β)phot 7.7−7.7: SQ20B [13], and C1-1 cells [16]. Panel C with (α/β)phot 15Ȣ18: C3H10T1/2 [11], and SCC25 [13]. Panel D with (α/β)phot ≥ 70: HCT116 [17], M/10 cells and HF19 cells [13]. The error bars show the 95% CI. The dashed lines are obtained with Equation 2 where the highest and lowest (α/β)phot in each panel is used.
![Figure 1. Experimentally obtained α/αphot values as a function of LET. Panel A with (α/β)phot 2.7−3.1: V79-379A cells [12], V79-753B cells [10], and DLD1 cells [17]. Panel B with (α/β)phot 7.7−7.7: SQ20B [13], and C1-1 cells [16]. Panel C with (α/β)phot 15Ȣ18: C3H10T1/2 [11], and SCC25 [13]. Panel D with (α/β)phot ≥ 70: HCT116 [17], M/10 cells and HF19 cells [13]. The error bars show the 95% CI. The dashed lines are obtained with Equation 2 where the highest and lowest (α/β)phot in each panel is used.](/cms/asset/68bf5944-cc64-4f31-9e0c-99401d778286/ionc_a_705892_f0001_b.gif)
Figure 2. k is the slope from the linear relation between α/αphot and LET (see Equation 1) and is here plotted as a function of the α/β ratio of the reference radiation using Equation A6 in Appendix with the parameter q = 0.434. The shaded areas under the curve show the (α/β)phot ranges used in the different panels of Figure 1.
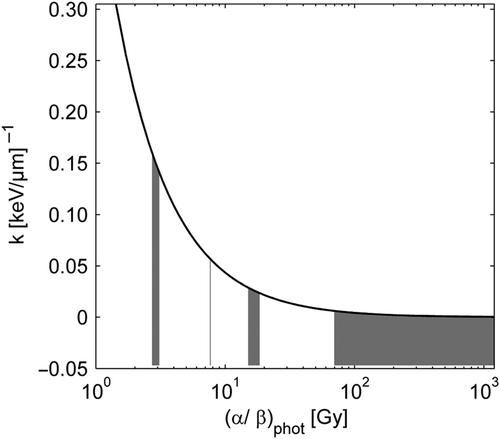
Table II. The resulting slope k of Equation A6 used in .
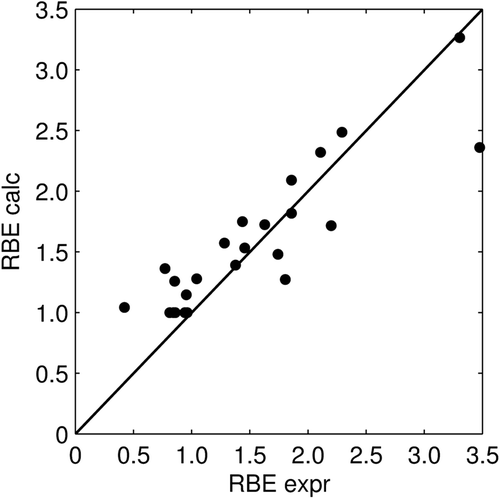
The comparison between the analytically obtained RBE values from the proposed model presented in Equation 3 and the experimental RBE values is shown in . The resulting coefficient of determination r2 for RBE values of all cell lines and LETs was 0.76. The RBE model gave a statistically significantly better fit to data, compared to the standard model, which assumes a constant RBE. The resulting p-value was < 0.01. In , the RBE for the cell lines with the lowest and highest (α/β)phot ratio, 2.7–3.1 and ≥ 70, respectively, are shown displaying a higher RBE for the cells with low (α/β)phot ratio than for the cells with high (α/β)phot ratio. For cells with low (α/β)phot ratio, the RBE increased with increasing LET whereas no such trend was seen for the cells with high α/βphot ratio. In fact, the experimental RBE values for the cell lines with very high (α/β)phot ratio were smaller than unity. In , the analytically obtained RBE values for all cell lines and LETs are compared with the experimental RBE values. Pearson's correlation coefficient was 0.89. Both in and , the RBE at the survival level obtained for the reference radiation at 2 Gy was used.
Figure 3. RBE as a function of LET at 2 Gy photon dose for cell lines with (α/β)phot ratio < 4 (circles) and (α/β)phot ratio ≥ 70 (squares). The experimental data is shown with filled symbols and the calculated data from Equation 3 has open symbols.
Figure 4. Analytically obtained RBE values (see Equation 3) vs. experimental RBE values for all cell lines used in this study. Pearson's correlation coefficient is 0.89. The RBE is obtained at 2 Gy photon dose.
shows how the input parameters (α/β)phot, dose, and LET of Equation 3 affect the RBE. The RBE generally increased with decreasing (α/β)phot, with decreasing dose, and with increasing LET, but at high (α/β)phot the change in RBE between different doses and LETs was small. Similarly, at low LETs and high doses, the RBE change due to variations in (α/β)phot was small.
Discussion
We have developed an RBE model that is able to estimate the RBE for protons. The proposed model is simple in the way that it is based on the well-known linear-quadratic model and the only information needed is the dose, LET, and the α/β ratio of the reference radiation. The constant q is the same regardless of the physical characteristics of the proton beam and the biological system. Hence, no proton specific biological parameters are needed. With this model, investigations on how the RBE is affected by changes in dose, LET, and cell type can easily be made.
With the aim to develop a model using few assumptions that are supported by experimental data, statistical analyses were made to test our expressions. A statistically significant positive relation between the experimental α/αphot values and LET was found, which supports our first assumption that α/αphot increases with increasing LET. The 95% CI for the intercept of that relation included our assumed value of one, even though the spread was large. From the experimental data included in this study, it is difficult to determine exactly how α/αphot varies with LET for low LET values. We have assumed a simple linear relation between α/αphot and LET also for low LETs, but it is possible that α/αphot approaches 1, or another value on the intercept, non-linearly with decreasing LET.
Upon a closer examination, it was the cell lines with low (α/β)phot that contributed to the positive relation between α/αphot and LET. The cell lines with (α/β)phot ≥15 showed no statistically significant relation between α/αphot and LET, i.e. the slope k was not significantly larger than zero. We have developed an expression where the sensitivity to LET changes is linked to the α/β ratio of photons, Equation 2, so that α/αphot increases with increasing LET with a slope that decreases with increasing (α/β)phot. Statistical analyses supported these assumptions.
When it comes to the β/βphot ratio, no support for an LET dependence was found, and hence the assumption of a constant β equal to β for photons is reasonable. In conclusion, the assumptions made in the development of the RBE model were all supported by the experimental data used in this study.
The resulting RBE model fitted the experimental RBE values well (r2 = 0.76), and significantly better compared to the standard constant RBE (p-value < 0.01). The proposed model predicts an increasing RBE with increasing LET in a cell dependent way: the α/β ratio of photons determines the strength of the positive relation between RBE and LET of protons. In other words, different cell lines differ in their sensitivity to LET changes which is determined by their α/β ratio of photons. So, clearer trends in the variation of RBE with LET are revealed by stratifying the data: for low (α/β)phot cell lines the highest RBE values are found and a distinct increase in RBE with LET is observed, whereas for high (α/β)phot, the RBE is low and no LET dependence is found (see ). In fact, experimental RBE values lower than one are seen indicating that photon radiation might be more effective in cell killing than protons in this group of cells. Our results have the implication that late responding tissues (low (α/β)phot ≈3) are more sensitive to LET changes than early responding tissues and most tumors (high (α/β)phot ≈10). Moreover, in a treatment planning situation, caution in selecting an (α/β)phot ratio for a tissue is most important in the low range of (α/β)phot values since a small difference in this value gives a large change in the slope k (see ) and, ultimately, a large change in the RBE.
The tendency of a higher RBE for cells with low (α/β)phot is in agreement with the conclusions made by Gerweck and Kozin [Citation4]. They summarized the results of published experimental data where the measurements of RBE were obtained for different spread-out Bragg peaks (SOBP). SOBP are used to obtain a homogeneous dose distribution throughout the tumor by modulating the proton energy. This means that the SOBP is composed of multiple superimposed Bragg peaks of different energies and, hence, there will be a spectrum of LETs in the SOBP. In this study, cell survival data obtained from unmodulated proton beams was chosen instead to have a well-defined LET in order to better identify relations between RBE, LET, and (α/β)phot.
Our evaluation of the RBE model indicates that, not only the sensitivity of the RBE to LET variations, but also its sensitivity to dose variations depends on the (α/β)phot. The model shows an increasing RBE with decreasing dose as seen in where the effect is most pronounced at low (α/β)phot and high LETs. For high (α/β)phot only a small or no dose effect is seen. Paganetti et al. [Citation3] have reviewed published RBE values of in vitro and in vivo systems in a SOBP and concluded that a statistically significant increase in RBE is found for low doses per fraction in both systems, but less pronounced in vivo. Of all the in vitro experiments they studied, only three did not show this trend. Interestingly, two of these cell lines, human colon carcinoma LS174T [Citation19] and human squamous cell carcinoma SCC25 [Citation20], had a high (α/β)phot (the ratio was infinite and 47.5, respectively) and would therefore not be expected to show this trend according to the RBE model presented here. In the third study, where Chinese hamster ovary cells with an (α/β)phot of 1.83 were used [Citation21], an increasing RBE with decreasing dose was observed in the end of a 3-cm SOBP and in the middle of a 0.5-cm SOBP, but the opposite relation was found in the beginning of a 3-cm SOBP and in the initial plateau of the beam. Perhaps this could be explained by a low LET in the initial plateau and the beginning of a SOBP so that small RBE changes could not be distinguished.
All taken together, the highest RBE values are expected for cell lines and tissues with low (α/β)phot, receiving high LET and low dose per fraction. This combination can be obtained, e.g. when the end of the proton range is located in late-responding normal tissue. This is not an unusual situation since tumors located in the immediate vicinity of organs at risk are often selected for proton radiotherapy, and high-weighted spots or the distal end of a SOBP are frequently situated at the distal edge of the tumor in order to obtain a homogeneous dose distribution. Also in the laterally peripheral parts of a beam this combination of high LET, low dose per fraction, and low (α/β)phot can be obtained. An increased RBE in normal tissue may, if not taken into account, result in a too high prescription dose selected and a higher biological effect than expected. For example, a late reacting tissue with (α/β)phot = 3, situated immediately downstream of the target volume, receiving 1 Gy per fraction and 6 keV/µm would obtain an RBE of 1.48 according to the RBE model.
Tumors often receive relatively high LET components in proton radiotherapy since Bragg peaks are placed inside the target volume. However, the biological effect of the increased LET depends on the (α/β)phot as seen in this study. For some slowly growing tumors that have low (α/β)phot, such as prostate cancer, it might be a significant gain with this increased LET. However, most tumors have relatively high (α/β)phot values and, therefore, the increased LET might not result in a favorable increased RBE. RBE values even lower than 1.1 may be obtained when using a simultaneous integrated boost technique if the clinical target volume (CTV) surrounding the boosted gross tumor volume (GTV) receives dose mainly from the plateau region of the proton beam. A CTV with (α/β)phot = 10 receiving 2 Gy per fraction and 2 keV/µm gives a predicted RBE value of 1.06. With the RBE model presented in this study the effects of different LET values, α/β values of photons, and doses can simultaneously be taken into account.
In this study, in vitro data have been used to evaluate the RBE model. Ideally, there should exist relevant in vivo data for human tissue where well-defined LETs were used, but these data are at present scarce. RBE variations are expected to be smaller for in vivo systems [Citation3]. Until more data are available, in vitro data can be used to estimate the potential relative effects rather than absolute values.
After the completion of the study at hand, Carabe et al. [Citation22] published an extension of the radiobiological model originally presented by Dale and Jones [Citation14]. In their article they propose an expression similar to ours for the α/αphot ratio (called RBEmax in their study). Their approach for parameter determination differs from the one used in this study in that they fit their model only to experimental data from V79 cells. From this data they extrapolate to other cell lines. The strength of our approach is that we fit to experimental data of a range of different (α/β)phot ratios and LET values simultaneously, and that we use statistical model selection methods to empirically evaluate the form of our parametric function.
In conclusion, the sensitivity of cells to x-rays, characterized with the α/β ratio, is useful in estimating their sensitivity to proton radiation. By accounting for α/β ratio of photons, clearer trends between RBE and LET was revealed. The RBE was found to increase with increasing LET for cell lines with low (α/β)phot ratio, whereas limited variability in RBE was observed at high ratios. The proposed RBE model accounts for an RBE that depends on dose, LET and α/β ratio of the reference radiation.
http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.705892
Download PDF (914 KB)Acknowledgements
The authors thank Dr. Nina Tilly for an initial literature review of the experimental studies, and Dr. Johanna Kempe for the pre-study. We also thank Assoc. Prof. Iuliana Toma-Daşu for her constructive and insightful comments, and her careful reading of the manuscript. The research was supported by the Swedish Research Council (VR).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- PTCOG: Particle Therapy Co-Operative Group. [updated 2012 Mar 12; cited 2012 May 30]. Available from: http://ptcog.web.psi.ch.
- Dale RG, Jones B, Cárabe-Fernández A. Why more needs to be known about RBE effects in modern radiotherapy. Appl Radiat Isot 2009;67:387–92.
- Paganetti H, Niemierko A, Ancukiewicz M, Gerweck LE, Goitein M, Loeffler JS, . Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys 2002;53:407–21.
- Gerweck LE, Kozin SV. Relative biological effectiveness of proton beams in clinical therapy. Radiother Oncol 1999; 50:135–42.
- Report 78. Prescribing, Recording, and Reporting Proton-Beam Therapy. J ICRU 2007;7:21–28.
- Sinclair WK. The shape of radiation survival curves of mammalian cells cultured in vitro. In: Biophysical aspects of radiation quality. Technical Reports series no. 58. Vienna: IAEA; 1966.
- Hawkins RB. A microdosimetric-kinetic theory of the dependence of the RBE for cell death on LET. Med Phys 1998;7(Pt 1):1157–70.
- Wilkens JJ, Oelfke U. A phenomenological model for the relative biological effectiveness in therapeutic proton beams. Phys Med Biol 2004;49:2811–25.
- Tilly N, Johansson J, Isacsson U, Medin J, Blomquist E, Grusell E, . The influence of RBE variations in a clinical proton treatment plan for a hypopharynx cancer. Phys Med Biol 2005;50:2765–77.
- Belli M, Cera F, Cherubini R, Dalla Vecchia M, Haque AM, Ianzini F, . RBE-LET relationships for cell inactivation and mutation induced by low energy protons in V79 cells: Further results at the LNL facility. Int J Radiat Biol 1998;74:501–9.
- Bettega D, Calzolari P, Marchesini R, Noris Chiorda GL, Piazzolla A, Tallone L, . Inactivation of C3H10T1/2 cells by low energy protons and deuterons. Int J Radiat Biol 1998;73:303–9.
- Folkard M, Prise KM, Vojnovic B, Newman HC, Roper MJ, Michael BD. Inactivation of V79 cells by low-energy protons, deuterons and helium-3 ions. Int J Radiat Biol 1996;69: 729–38.
- Belli M, Bettega D, Calzolari P, Cera F, Cherubini R, Dalla Vecchia M, . Inactivation of human normal and tumour cells irradiated with low energy protons. Int J Radiat Biol 2000;76:831–9.
- Dale RG, Jones B. The assessment of RBE effects using the concept of biologically effective dose. Int J Radiat Oncol Biol Phys 1999;43:639–45.
- Frese MC, Yu VK, Stewart RD, Carlson DJ. A mechanism-based approach to predict the relative biological effectiveness of protons and carbon ions in radiation therapy. Int J Radiat Oncol Biol Phys 2012;83:442–50.
- Sgura A, Antoccia A, Cherubini R, Dalla Vecchia M, Tiveron P, Degrassi F, . Micronuclei, CREST-positive micronuclei and cell inactivation induced in Chinese hamster cells by radiation with different quality. Int J Radiat Biol 2000;76:367–74.
- Baggio L, Cavinato M, Cherubini R, Conzato M, Cucinotta F, Favaretto S, . Relative biological effectiveness of light ions in human tumoural cell lines: Role of protein p53. Radiat Prot Dosimetry 2002;99:211–4.
- Perris A, Pialoglou P, Katsanos AA, Sideris EG. Biological effectiveness of low energy protons. I. Survival of Chinese hamster cells. Int J Radiat Biol Relat Stud Phys Chem Med 1986;50:1093–101.
- Blomquist E, Russell KR, Stenerlöw B, Montelius A, Grusell E, Carlsson J. Relative biological effectiveness of intermediate energy protons. Comparisons with 60Co gamma-radiation using two cell lines. Radiother Oncol 1993;28:44–51.
- Bettega D, Calzolari P, Chauvel P, Courdi A, Herault J, Iborra N, . Radiobiological studies on the 65 MeV therapeutic proton beam at Nice using human tumour cells. Int J Radiat Biol 2000;76:1297–303.
- Gueulette J, Grégoire V, Octave-Prignot M, Wambersie A. Measurements of radiobiological effectiveness in the 85 MeV proton beam produced at the cyclotron CYCLONE of Louvain-la-Neuve, Belgium. Radiat Res 1996;145:70–4.
- Carabe A, Moteabbed M, Depauw N, Schuemann J, Paganetti H. Range uncertainty in proton therapy due to variable biological effectiveness. Phys Med Biol 2012;57: 1159–72.