Abstract
Purpose. Investigation of clonogenic cell survival and cell proliferation following single dose and fractionated delivery of high dose rate flattening filter free (FFF) irradiation compared to conventional dose rates. Material and methods. The human astrocytoma D384, glioma T98 and lung carcinoma SW1573 cell lines were irradiated using either a single dose (0–12 Gy) or a fractionated protocol of 5 daily fractions of 2 Gy (D384) or 3 Gy (SW1573). Cells were irradiated inside a phantom using fixed gantry beams of a linear accelerator. A sliding window technique created homogeneous dose distributions over the surface of the cell cultures. Irradiations using standard beams (6 MV, 600 MU/min.) and high dose rate FFF beams (10 MV, 2400 MU/min.) were compared. Cell survival was determined by clonogenic assay. In the fractionated irradiation set-up, the number of clonogenic cells was estimated by including tumor cell proliferation during the overall treatment time in the analysis. Results. All cell lines showed equal cell survival following irradiation using either the FFF beams or conventional flattened (FF) beams. This was observed after single dose exposure (0–12 Gy) as well as after fractionated irradiation (p = 0.08 for D384 and 0.20 for SW1373 cell lines). Conclusion. FFF irradiation with a dose rate of 2400 MU/min and four times higher dose per pulse compared to irradiation with FF beams did not change cell survival for three human cancer cell lines up to a fraction dose of 12 Gy compared to irradiation using FF beams.
Radiotherapy traditionally uses flattened (FF) beams, which facilitate the creation of homogeneous dose distributions to target volumes using only open and wedged beams. FF beams are created using flattening filters, which attenuate the photons on the central axis more than at the outside, since the initial photon fluence distribution after the target in the head of the accelerator is conically shaped. Using flattening filters, the fluence distribution becomes more homogeneous resulting in an as homogeneous dose as possible to be delivered at a depth of 10 cm in a water tank. The disadvantage of these FF beams is a reduction of dose rate (typically to 600 MU/min) and a higher dose outside the field caused by photons scattered in the flattening filter.
Intensity modulated radiation therapy (IMRT) has become a standard irradiation technique. By modulating the radiation beam using a static gantry or as a volumetric modulated arc therapy [Citation1], conformal dose distributions to the target volume can be obtained while minimizing the dose to adjacent critical normal tissues. As IMRT beams have to be modulated anyway, the shape of the original beam profile is less important and either flat or conically shaped beam profile can be used.
Flattening filter free (FFF) beams can deliver higher dose rates, potentially shortening the delivery times. This benefit is especially evident in stereotactic body radiotherapy SBRT where high fraction doses are delivered with long beam-on times [Citation2,Citation3]. Resulting dose rates can be as high as 2400 MU/min, leading to a maximum dose rate of 24 Gy/min in an object at a surface source distance of 100 cm, at the center of a 10 cm × 10 cm 10 MV beam of a Varian TrueBeam™ linear accelerator [Citation4]. In addition to the higher average dose rate, the dose per pulse is also increased by a factor of four [Citation5], leading to instantaneous dose rates of > 104 Gy/min.
The resulting very high instantaneous dose rates of an FFF beam have re-introduced discussions about the radiobiological consequences of high dose rates for both normal tissues and tumors, in spite of a previously published review [Citation6] that discussed that there is no difference between high and conventional dose rates. Lohse et al. [Citation7] found that use of FFF beams with a higher dose per pulse in vitro leads to a reduced clonogenic cell survival for doses > 5 Gy compared to treatment with conventional dose rates, although Sørensen et al. found no difference [Citation8]. Therefore, in the present study, the effect of high dose rates created by FFF beams was investigated on three human cancer cell lines in vitro and compared to irradiation with standard dose rate FF beams. Cell survival was determined following single dose irradiation and in a fractionated irradiation with five daily fractions. The fractionation approach, rather than single dose irradiation, better mimics the clinical situation where treatment is delivered in multiple fractions. This strategy allows for the effect of cell proliferation as well as to enlarge possible differences in cell survival between the two irradiation methods.
Material and methods
Cell culture
SW1573 human lung cancer cells, T98 human malignant glioma and D384 human astrocytoma cells [Citation9] were cultured in L-15 medium (Leibovitz). The medium was supplemented with 10% fetal calf serum, 2 mM L-glutamine and 100 iU/mL penicillin/streptomycin. Cells were cultured as monolayers in 25 cm2 culture flasks at 37°C. The flasks were filled with 10 cm3 L-15 medium, resulting in a 4 mm water layer above the cells.
Irradiation and treatment
Cells were irradiated in the exponential growth phase. Both for the single dose and fractionated irradiations – with 5 fractions on subsequent days, three cell culture flasks were positioned between two layers, each 4 cm thick, polystyrene slabs of 30 cm × 30 cm. A computed tomography (CT) scan of the flasks between the slabs was made for accurate treatment planning. Three cell culture flasks were irradiated simultaneously from posterior with 6 MV FF beams or 10 MV FFF beams on a TrueBeam™ linear accelerator (Varian Medical Systems, Palo Alto, USA). Irradiation was conducted at room temperature and all flasks were outside the incubator for the same time.
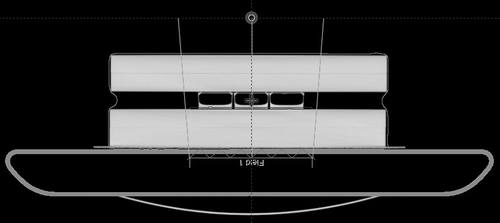
FF beams were delivered at their maximum dose rate of 600 MU/min, FFF beams at 2400 MU/min, both with 360 pulses per second and pulse width of approximately 4.5 μs. The FFF beam was ‘homogenized’ using an inversely optimized sliding window MLC in Eclipse™ (Varian Medical Systems). On the CT scan, a surrogate 2 mm thick target volume was contoured for the optimization (). The highest dose rate of the beam at the target was only delivered on the central axis of the beam, and the dose rate at the borders of the field (14 cm × 15.8 cm in the isocenter) was limited to ∼75% of the maximum dose rate. Therefore, the surface source distance (SSD) was chosen at 86 cm to ensure that the three flasks received a dose rate of the beam at the target between 21 and 29 Gy/min (mean of 24 Gy/min and average instantaneous dose rate during the pulse of 1.5∙104 Gy/min).
The plans for the FF beams also used an inversely optimized sliding window, in order to provide a similar treatment technique as for FFF beams. The three flasks were positioned at a SSD of 95 cm, delivering a dose rate of the beam at the target between 5.6 and 5.9 Gy/min (mean of 5.86 Gy/min and average instantaneous dose rate during the pulse of 3.6∙103 Gy/min).
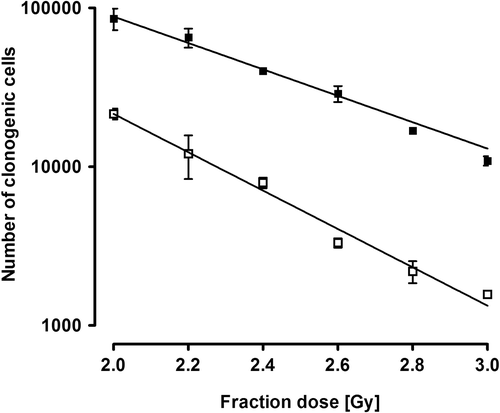
The single fraction experiment was performed for all three cell lines. Sets of three flasks were irradiated to doses of 2, 4, 6, 8, 10 and 12 Gy, using either FF or FFF beams. Due to a limitation in the maximum allowed number of monitor units per plan, for the FF irradiations, combinations of plans of 2 and 4 Gy were used, and for the FFF plans combinations of plans of 2, 4 and 8 Gy were used. The fractionated experiment was performed for the SW1573 and the D384 cell lines. On five subsequent days, two times a set of three flasks was irradiated with fraction doses of 3 Gy for the SW1573 and 2 Gy for the D384 cell lines. Thus six flasks were used per cell line and per beam. The fraction sizes were selected after a dose finding experiment with 0.2 Gy dose steps, aimed to estimate an isoeffective cell survival fraction size for the two cell lines (). In that experiment, two cell culture flasks were irradiated per experiment on a 60Co-unit with 5 daily fractions sized between 2 and 3 Gy.
Figure 2. Number of clonogenic cells (plating efficiency × total number of cells after 5 daily fractions) for SW1573 (closed squares) and D384 (open squares) cells for different fraction doses. Error bars represent the standard error of the mean (n = 2).
The dosimetric accuracy was measured for the sliding window fields using double Gafchromic EBT films just below the flasks, thus 2 mm lower than the actual cells, for the 2 and 4 Gy FF plans and the 2, 4 and 8 Gy FFF plans.
Clonogenic assay and cell growth analysis
For determination of clonogenic cell survival, after the treatment, cells were washed with phosphate buffered saline (PBS), trypsinized (with trypsine + EDTA) and then resuspended. Cells were counted on the coulter counter (Coulter® ZTM series). In the fractionation experiments, cell proliferation was scored by counting the number of cells after the last radiation fraction, as previously described [Citation10]. A predetermined number of cells ranging from 500 to 10 000, dependent on the irradiation dose, were plated in 25 cm3 culture flasks and allowed to grow into colonies. After 12 days, cells were washed with PBS, fixed in ethanol and stained with a 10% Giemsa solution (Merck, Darmstadt, Germany). Colonies of 50 cells or more were counted using a stereomicroscope. Surviving fractions were calculated by dividing the number of colonies by the number of plated cells and then correcting by the plating efficiency of non- irradiated control cells. Following fractionated irradiation, the ‘number of clonogenic cells’ was obtained by multiplying cell survival (plating efficiency) with the total number of cells after 5 daily fractions, i.e. at the end of treatment. Cell survival was estimated combining six-fold data and fitting the average survival levels by least squares regression using the linear quadratic model. P-values for significance were calculated by applying an unpaired t-test, using Prism 4 (GraphPad software, Inc.©).
Results
As sliding window techniques were used to irradiate the cells, the total delivery time was higher than for open beams. Typically, delivery of a fraction of 4 Gy lasted 58 seconds for the FF beam and 28 seconds for the FFF beam.
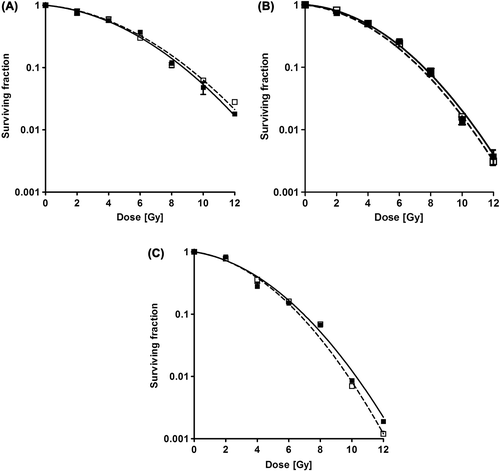
shows cell survival curves following single fraction irradiation with the two methods. No significant difference in cell survival is observed following irradiation with either the FFF or the FF beams for all three cell lines. Error bars represent the standard error of the mean for the three flasks of each irradiation.
Figure 3. Normalized cell survival curves for SW 1573 (A), D384 (B) and T98 (C) cells. Error bars represent the standard error of the mean (n = 3). Open squares = FF, Closed squares = FFF.
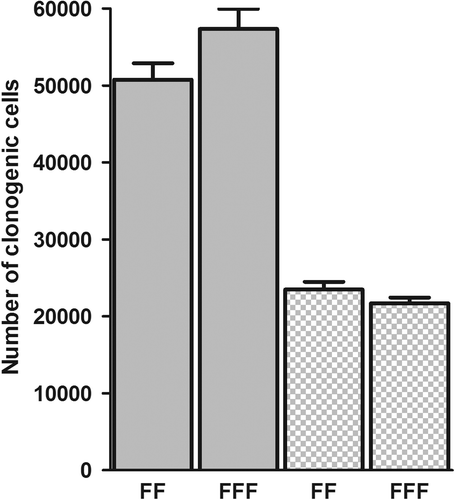
In the fractionation experiment, using dose steps of 0.2 Gy (), isoeffective single doses appeared to be approximately 2 Gy for the D384 and 3 Gy for the SW1573 cell line. The number of clonogenic cells following irradiation with 5 daily fractions with either an FFF or FF beam is presented in . Average cell survival after 5 fractions was 0.056 and 0.026, respectively, for the D384 and SW1573 cell lines. No significant difference was noticed between the two irradiation techniques for both the D384 (p = 0.08) and SW1573 cell-lines (p = 0.20).
Figure 4. The number of clonogenic cells following flattening filter and FFF fractionated irradiation of D384 (5 × 2 Gy, p = 0.08) and SW1573 (5 × 3 Gy, p = 0.20, dotted bars) cells. Error bars represent the standard error of the mean (n = 6).
Gafchromic film measurements for 2 and 4 Gy FF plans resulted in average doses (± standard deviation of dose measurements) of 2.00 ± 0.016 Gy and 4.00 ± 0.037 Gy, respectively. Dose was averaged over the area of the flasks. For the 2, 4 and 8 Gy FFF plans, the measured doses were 1.99 ± 0.015 Gy, 3.95 ± 0.046 Gy and 8.13 ± 0.086 Gy, respectively.
Discussion
The present data show that an approximate four-fold increase in instantaneous dose rate, increasing the average dose rate of the beam at the target from 6 Gy/min using a FF beam to 24 Gy/min using an FFF beam, results in equal biological effects, both for using the endpoints ‘clonogenic cell survival’ and ‘number of clonogenic cells’. This was observed for three human cancer cell lines, the astrocytoma cell line D384, the malignant glioma cell line T98 and the small cell lung cancer cell line SW1573, after single fraction irradiation up to 12 Gy and for the D384 and SW1573 cell lines after fractionated irradiation as well.
The results of the single dose experiments are in contrast with the results from Lohse et al. [Citation7], who reported a lower cell survival for cells irradiated with an FFF beam to a dose of 8 Gy or higher. The T98 malignant glioma cell line used in their study was also used in our experiments. Conversely, our data are consistent with those of Sorensen et al. [Citation8] who also reported no dependence of cell survival on the instantaneous dose rate. Their experiments were performed on two cell lines that were irradiated with single doses up to 10 Gy. However, instead of a real FFF beam, they used a regular FF beam where the distance of the cells to the accelerator was varied, thus enhancing the dose rate up to 29.9 Gy/min, and comparing to dose rates of 5 Gy/min. Lohse et al. compared the effect of dose rates varying between 0.2 and 24 Gy/min and they concluded that for the high instantaneous dose rate only, a higher cell killing was observed, even if this was delivered with a lower average dose rate. In the present study, irradiation using high instantaneous dose rate and high average dose rate of the beam at the target (average 24 Gy/min) was compared with irradiation using lower instantaneous and average dose rate (5.86 Gy/min). Although the effective average dose rates were lower due to the sliding window technique (4.1 Gy/min for FF and 8.6 Gy/min for FFF for a 4 Gy delivery), this should not influence the result as they are both in the range of the conventional clinically applied average dose rates, and the concern was for the higher instantaneous dose rates.
Experimental data on the effects of dose rate – in the very high dose rate range – are scarce. With traditional external beam irradiation techniques, clinical irradiation is typically applied at maximum dose rates in the order of 5 Gy/min which is now increased to over 20 Gy/min for FFF beams. Radiobiological data from the past showed similar clonogenic cell survival for a range of dose rates between 0.6 Gy/min up to ultra high dose rates of ˜6 × 1011 Gy/min, following single dose Co60-gamma irradiation or irradiation with electrons under normal oxygenated conditions [Citation11,Citation12]. Auer et al. [Citation13] also did not observe a significant difference between cell survival for pulsed and continuous proton beam irradiation, where the pulsed beam typically delivers an instantaneous dose rate in the order of 1010 Gy/min, which is much higher than the instantaneous dose rate achieved with FFF beams.
Different from the experimental procedure used by Lohse et al. and Sorensen et al. [Citation7,Citation8], we used a sliding window to deliver a homogeneous dose from the conical FFF dose profile. This way, we were able to irradiate three flasks simultaneously, however, with a ± 20% variation in instantaneous dose rate. The average increase in instantaneous dose is a factor of four and the variation has not led to a higher uncertainty in survival for the cells irradiated with FFF beams. For consistency, a similar sliding window, although with a slightly larger window width, was also created for the irradiation with the FF beam. Another difference was the use of different beam energies (6 MV and 10 MV). This should have no impact on cell survival because the RBE for these photon beams is the same.
The film measurements assured that the measured doses were all within 1.5% of the planned doses. The importance and accuracy of such film measurements have been described earlier [Citation14].
The difference in cell survival, observed by Lohse et al., was mainly seen for fraction doses > 5 Gy with the effect becoming significant for doses between 10 Gy and 20 Gy. We did not perform single fraction irradiations with doses higher than 12 Gy because the survival would become so low that the presence of sterile cells in the experiment could cause a significant contribution to the clonogenic fraction. In the fractionated irradiation set-up with 5 daily fractions (10–15 Gy total dose), cell proliferation during the treatment course was taken into account and final survival approached that of Lohse et al. for their 10 Gy single fraction irradiation [Citation7].
Although fractionated irradiation is common clinical practice, it is hardly used for in vitro experiments. No data had yet been reported on the effects of ultra-high dose rate irradiations with multiple fractions. If FFF would have a higher radiobiological effect for fraction doses > 10 Gy, e.g. through an increased accumulation of non-repairable DNA double strand breaks, this effect would be insignificant in a fractionated irradiation with multiple conventional daily fractions up to a total dose of 15 Gy.
So far, radiobiological experiments were restricted to in vitro studies. Of concern could be a possible higher in vivo radiobiological effect of FFF beams on normal tissues and organs during patient irradiation. FFF beams are nowadays used at maximum dose rate of 2400 MU/min for lung and liver SBRT treated with RapidArc® (Varian Medical Systems) at fraction doses above 11 Gy [Citation15]. For lower fraction doses, the average dose rate will drop. However, this occurs by removal of pulses from the beam and not by lowering the dose per pulse. In spine SBRT, a higher radiobiological effect could have implications as the spinal cord is often treated up to the maximum tolerance dose. However, the cord is kept at much lower dose than the tumor, in the range of doses used in our experiments in which no differential effect between the two techniques was observed.
In conclusion, there has been controversy about the radiobiological effect of the high dose FFF beams. One study reported a higher cell kill effect, the other reported no difference. We investigated the effect for three different cell lines, for both single fraction irradiation up to 12 Gy and for fractionated irradiation up to 15 Gy in 5 fractions, and conclude that up to these doses, there are no radiobiological differences that could limit the clinical use of FFF beams.
Acknowledgements
Chin Loon Ong, Dennis van de Water and Mustafa Zahir are acknowledged for technical assistance.
Declaration of interest: The Department of Radiation Oncology of VUmc has a research collaboration with Varian Medical Systems.
References
- Palma DA, Verbakel WF, Otto K, Senan S. New developments in arc radiation therapy: A review. Cancer Treat Rev 2010;36:393–9.
- Kuijper IT, Dahele M, Senan S, Verbakel WF. Volumetric modulated arc therapy versus conventional intensity modulated radiation therapy for stereotactic spine radiotherapy: A planning study and early clinical data. Radiother Oncol 2010;94:224–8.
- Ong CL, Verbakel WF, Cuijpers JP, Slotman BJ, Lagerwaard FJ, Senan S. Stereotactic radiotherapy for peripheral lung tumors: A comparison of volumetric modulated arc therapy with 3 other delivery techniques. Radiother Oncol 2010;97:437–42.
- Hrbacek J, Lang S, Klöck S. Commissioning of photon beams of a flattening filter-free linear accelerator and the accuracy of beam modeling using an anisotropic analytical algorithm. Int J Radiat Oncol Biol Phys 2011;80: 1228–37.
- Titt U, Vassiliev ON, Pönisch F, Dong L, Liu H, Mohan R. A flattening filter free photon treatment concept evaluation with Monte Carlo. Med Phys 2006;33:1595–602.
- Ling CC, Gerweck LE, Zaider M, Yorke E. Dose-rate effects in external beam radiotherapy redux. Radiother Oncol 2010; 95:261–8.
- Lohse I, Lang S, Hrbacek J, Scheidegger S, Bodis S, Macedo NS, . Effect of high dose per pulse flattening filter-free beams on cancer cell survival. Radiother Oncol 2011;101:226–32.
- Sørensen BS, Vestergaard A, Overgaard J, Praestegaard LH. Dependence of cell survival on instantaneous dose rate of a linear accelerator. Radiother Oncol 2011;101:223–5.
- Balmforth AJ, Ball SG, Freshney RI, Graham DI, McNamee HB, Vaughan PF. D-1 dopaminergic and beta-adrenergic stimulation of adenylate cyclase in a clone derived from the human astrocytoma cell line G-CCM. J Neurochem 1986;47:715–9.
- Van Nifterik KA, van den Berg J, Stalpers LJ, Lafleur MV, Leenstra S, Slotman BJ, . Differential radiosensitizing potential of temozolomide in MGMT promoter methylated glioblastoma multiforme cell lines. Int J Radiat Oncol Biol Phys 2007;69:1246–53.
- Michaels HB, Epp ER, Ling CC, Peterson EC. Oxygen sensitization of CHO cells at ultrahigh dose rates: Prelude to oxygen diffusion studies. Radiat Res 1978;76:510–21.
- Ling CC, Spiro IJ, Mitchell J, Stickler R. The variation of OER with dose rate. Int J Radiat Oncol Biol Phys 1985; 11:1367–73.
- Auer S, Hable V, Greubel C, Drexler GA, Schmid TE, Belka C, . Survival of tumor cells after proton irradiation with ultra-high dose rates. Radiat Oncol 2011;6:139.
- Claridge Mackonis E, Suchowerska N, Naseri P, McKenzie D. Optimisation of exposure conditions for in vitro radiobiology experiments. Australas Phys Eng Sci Med 2012;35:151–7.
- Scorsetti M, Alongi F, Castiglioni S, Clivio A, Fogliata A, Lobefalo F, . Feasibility and early clinical assessment of flattening filter free (FFF) based stereotactic body radiotherapy (SBRT) treatments. Radiat Oncol 2011;6:113.