Abstract
In the current study IMPT plan robustness was evaluated with respect to inter-fractional patient positioning for various beam arrangements and two tumor indications in the cranial region. Material and methods. For 14 patients suffering from tumors in the cranial region [skull base (SB; n = 7) and paranasal sinus (PS; n = 7)] the CTV and OARs were delineated. A safety margin of 3 mm was applied to the CTV. A prescribed dose of 2 GyE was planned via three beam arrangements (α, β, γ). Beam arrangement α consisted of lateral opposed fields for both tumor groups while beam arrangement β was optimized according to respective tumor and OAR locations, using two beams only. Beam arrangement γ applied four beams in the SB group and three beams in the PS group. Dose distributions were recalculated subjected to virtual patient translations along the major anatomical axes. The following dosimetric indices were evaluated and compared to original plans: target coverage (TC), target dose homogeneity (HI), CTV median and average dose (Dmedian, Dmean). For OARs near maximum dose and average dose (D2%, Dmean) were evaluated. Results. Dose distributions were distorted after introducing shifts. In the SB group, TC and HI were significantly different for caudal, cranial and anterior shifts for all beam arrangements. For PS patients, all but right shifts differed significantly from the original plans for all beam arrangements, although clinical relevance was not reached for arrangement γ (ΔTC < 1.5%). In general, beam arrangement γ exhibited the least spread of data regarding target indices and was consequently considered the most robust. Dosimetric parameters regarding the brainstem were mostly influenced by shifts along the anterio-posterior axis. Conclusion. For cranial IMPT, set-up uncertainties may lead to pronounced deterioration of dose distributions. According to our investigations, multi-beam arrangements were dosimetrically more robust and hence preferable over two beam arrangements.
One of the well-known and documented advantages of proton therapy over advanced photon beam therapy techniques is excellent target conformity with improved sparing of neighboring critical structures, especially in the medium to low dose range, and the general reduction of irradiated healthy tissue (e.g. [Citation1,Citation2]). This advantageous physical selectivity of protons has been utilized especially for cranial targets where highly sensitive organs at risk, such as the optic tract or the brainstem, challenge treatment planning especially with photons [Citation3,Citation4]. On the other hand, radiotherapy in the cranial region is less troublesome with respect to organ motion, i.e. it is common practice to assume rigid anatomy. Nevertheless, patient set-up errors can still be present and patients can move inside a non-invasive immobilization device, even if it is a precision mask system [Citation5]. Such set-up errors and positioning uncertainties deteriorate highly conformal isodose distributions with steep dose gradients. The sensitivity of charged particles with respect to tissue density variations along the beam path pronounces these effects.
So far, image guidance in proton beam therapy is mostly based on planar x-ray imaging. Volumetric CT or cone beam CT is acquired seldom prior to each fraction in order to extract 6 degree of freedom information with respect to set-up uncertainties including effects of density variations. The advent of technological solutions for image guidance in advanced photon beam therapy and the more detailed insight into set-up uncertainties influenced treatment plan robustness considerations. Treatment plan robustness has played an important role in proton therapy, although the main concerns were related to range uncertainties and stopping power conversion [Citation6], CT artifacts [Citation7] or temporal redistribution of density heterogeneities within the patient [Citation8,Citation9]. As shown by several groups, the latter is a very pronounced source of error, especially outside the cranial region, which potentially leads to a vast degradation of a previously accepted dose distribution [Citation10].
While the outcome regarding CT calibration uncertainties and artifacts can be directly translated to various tumor sites, the difficulty concerning patient positioning must be addressed individually. Recent literature describes the impact of set-up uncertainties and density heterogeneities on the finite range of protons for prostate cancer [Citation11]. So far no similar systematic multi-patient analysis has been performed for cranial targets.
The purpose of the present work was to evaluate dosimetric treatment plan robustness of IMPT plans for cranial malignancies (tumors of the skull base (SB) and the paranasal sinus (PS)) with respect to translational set-up uncertainties. Different beam configurations mimicking 1) a classical parallel opposed field set-up and 2) a two beam approach based on the minimization of normal tissue damage were compared to 3) a highly sophisticated multi-field strategy with respect to treatment plan robustness.
Material and methods
Patients and volumes
Fourteen patients, who had received conventional photon radiation therapy (RT) at the Department of Radiooncology of the MedUniWien/AKH Vienna, were selected for this retrospective treatment planning study. Their target volumes were localized in regions characterized by elevated heterogeneity. The cohort was split into two even groups consisting of either patients suffering from tumors in the SB or in the PS region. All patients were immobilized with a commercially available high-precision mask system (HeadSTEPTM/BiteSTEPTM, Elekta, Crawley, UK), which was equipped with an additional upper jaw fixation for motion reduction in cranio-caudal direction [Citation12]. All cases underwent planning CT acquisition in treatment position with a multi-slice CT scanner (Somatom Volume Zoom, Siemens, Erlangen, Germany) in a 4 mm slice thickness acquisition mode. Representative contours, originally being prepared for photon irradiation according to in-house delineation protocols, were retained for proton treatment planning. They encompassed clinical target volume (CTV), brainstem (BS) and ipsi- as well as contra-lateral optical nerves (OI and OC). Median CTV size was 48.7 (14.6–115.9) cm3 in the SB group and 172.8 (71.3–259.6) cm3 in the PS group. The optic chiasm was neither contoured nor dosimetrically evaluated because it was always fully included in the target volumes. The planning target volume (PTV) covered the CTV plus additional 3 mm safety margin [Citation13]. In case of overlap with OARs it was manually modified towards these structures.
Treatment planning
All IMPT treatment plans were generated with the treatment planning system (TPS) XiO (V4.41; CMS/Elekta, Crawley, UK). The energy dependent spot sigma was calculated internally by the planning software based on an initial spot sigma of 3 mm in air. The lateral spot spacing of the pencil beams was 5 mm. The energy dependent distance between two spot layers was designed to be in the order of a single Bragg peak (width at 80% of the peak maximum) at the respective depth [Citation14]. The dose calculation grid was set to 3 × 3× 3 mm3 and a relative biological effectiveness (RBE) of 1.1 (relative to 60Co) was applied.
Three different beam arrangements, subsequently denoted as α, β and γ, were studied. Beam arrangement α simulated the scenario where only a fixed horizontal beam-line is available, while β and γ represented full gantry strategies. The respective beam configurations were defined as follows. Beam arrangement α was composed of two lateral parallel-opposed beams for SB as well as PS patients and represented a classical approach in light ion therapy. Arrangement β comprised two individualized fields which were chosen to maximize the benefit for OARs [Citation15]. Above ‘simple’ beam arrangements were chosen because in IMPT acceptable dose distributions can be achieved already with a few beams or even a single beam. Beam arrangement γ was intended to represent a more sophisticated, multi-field arrangement. It was made up of four beams (± 70°, ± 110°) in the SB group [Citation16] and three beams (0°, ± 40°) in the PS group [Citation17].
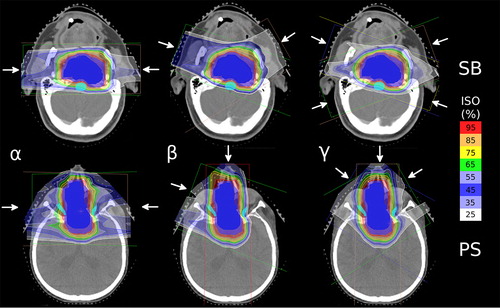
The original proton treatment plans, in the following referred to as P0, were designed to deliver a median dose of 2 GyE to the PTV. P0 plans were considered dosimetrically acceptable if 95% of the PTV was covered by 95% of the prescription dose [Citation18]. shows a transversal isocentric slice of a representative patient of both groups with corresponding original dose distributions (P0).
Figure 1. Representative layer of a SB (upper row) and PS (lower row) case. The left column depicts beam arrangement α (two lateral opposed beam ports). The central column represents one representative setup of beam arrangement β (individual OAR sparing two beam arrangement) while the right column shows beam arrangement γ (four (SB) and three (PS) beam ports, respectively).
Test of treatment plan robustness
In order to test dosimetric robustness of the prepared P0, treatment delivery in presence of systematic translational patient misalignment was simulated. This was achieved by recomputing P0 after introducing shifts of the scanning raster with respect to the planning isocenter. The values of the shifts were derived from an in-house performed, CBCT-based reproducibility analysis of the high-precision mask system mentioned above. From this analysis, the following maximum translational shifts were derived: 3 mm in lateral (left-right) as well as longitudinal (cranio-caudal) and 2.3 mm in vertical (anterio- posterior) direction [Citation12]. Comparable outcome concerning maximum shifts of similar high-precision systems was reported in recent literature [Citation5]. For each of the 14 cases, P0 was recalculated six times according to the above mentioned maximum positive and negative shifts along the major anatomical axes. Shifted plans, labeled as Pshift hereafter, define recomputed plans with shifts applied in the denoted directions. Rotational misalignments of the patients were neglected in the context of this study according to the findings of Sejpal et al. [Citation19] and Meyer et al. [Citation20]. Although they applied larger rotational uncertainties than reported in our institution they observed minimal attributable dose deviations and concluded clinical irrelevance. Hence, 21 plans were generated per patient (P0 and six shifted plans per beam arrangement) and 294 plans in total.
Data analysis
For each recomputed treatment plan (Pshift) the following dosimetric metrics (Mi) were derived for the CTV: Target coverage (TC), homogeneity index (HI), average dose (Dmean) and median dose (Dmedian). TC was defined as the quotient of the target volume receiving a certain dose level (here 95% of the prescribed dose) and the full target volume expressed in percent [Citation21]. HI represents a percental measure of dose homogeneity in the target given as the quotient of dose range and prescribed dose [Citation22].
For P0 plans, above indices were also calculated for the PTV in order to demonstrate initial treatment plan quality. For respective organs at risk (OAR) the average dose Dmean and the dose to 2% of the volume (D2%) being a surrogate for the near maximum dose were calculated. Above metrics were explained in more detail in the literature [Citation18].
For the classification of the impact of set-up errors on the nominal dose distributions boxplots including P0 as well as all Pshift were generated. Additionally, the difference ΔMi
ΔMi = Mi(Pshift) − Mi(P0) (1)
of respective indices Mi in relation to P0 was calculated. ΔMi was used to compare different beam arrangements according to treatment plan robustness. The smaller the values the more robust the respective treatment plans.
For statistical analyses SPSS 17.0 (SPSS Inc., Chicago, USA) was utilized. Paired two-sided Wilcoxon rank sum tests were applied and statistical significance was assumed for a p-value ≤ 0.05.
Results
Initial planning results (P0)
Dosimetric data of the original proton treatment plans P0 are summarized in . The initial planning goal (delivery of 95% prescribed dose to 95% of PTV) was fulfilled for all beam arrangements and both tumor indications except of one case in the PS group whose target volume was fully embedded in extremely low density material (air) . Largest HI values were 16.6 % and 29.3% for SB and PS tumors, respectively. Median HI values decreased from beam arrangement α to γ. Generally, median and mean values and narrow range of all indices indicate high quality of P0 with respect to target volumes. Mi regarding OARs were characterized by high fluctuations due to patient specific individual anatomic situations of the targets.
Table I. Initial treatment planning results (P0) for both tumor indications and all beam arrangements. BS, brainstem; Dmean, average dose; Dmedian, median dose; D2%, near maximum dose (dose to 2% of the respective volume); HI, homogeneity index; OC, contra-lateral optical nerve; OI, ipsi-lateral optical nerve; TC, target coverage.
Treatment plan robustness
Dose distributions were distorted after applying virtual translational set-up errors of the patients and consequently the quality of the recalculated treatment plans decreased. The boxplots in , and show the influence of shift directions and beam set-ups on the above listed indices for both tumor indications. The following paragraphs are dedicated to respective dosimetric parameters Mi. Values in parentheses represent median values of respective Pshift listed in the text to be compared to the values of P0 in , followed by the p-value. Median values were calculated over the patient groups.
Figure 2. Boxplots representing target coverage (TC) as well as dose homogeneity (HI) of both tumor indications influenced by translational shifts of the patients for the three beam arrangements.
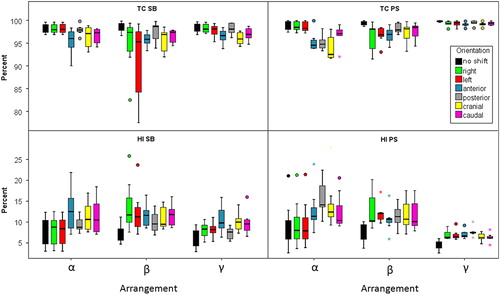
Figure 3. Boxplots representing average (Dmean) and median dose (Dmedian) to the target volume of both tumor indications influenced by translational shifts of the patients for the three beam arrangements.
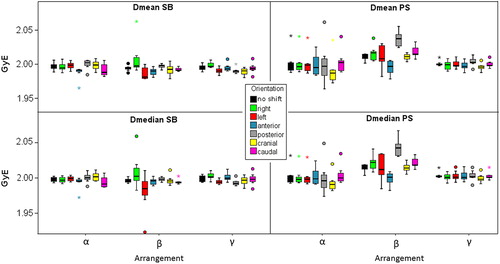
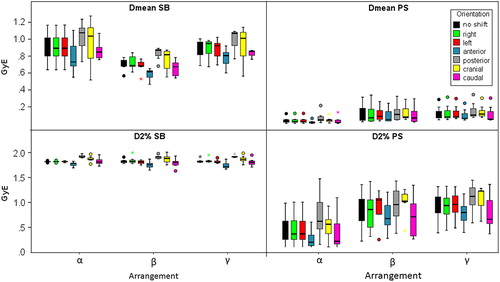
Figure 4. Boxplots representing average (Dmean) and near maximum dose (D2%) to the brainstem of both tumor indications influenced by translational shifts of the patients for the three beam arrangements.
Beam arrangement related treatment plan robustness is addressed thereafter and was evaluated by comparing ΔMi of respective beam configurations α, β and γ within one and the same shift direction. The results are summarized in and for SB and PS patients, respectively. Medians of ΔMi regarding particular shift directions are listed only for those shifts where statistically significant differences were obtained.
Table II. Treatment plan robustness as a function of beam arrangements for the skull base indication. Δ of the various dosimetric indices are calculated according to formula 1. Median values are listed. a, anterior; cd, caudal; cr, cranial; l, left, p, posterior; r, right. Individual Pshift represent respective directions in which statistically significant differences between beam arrangements were obtained.
Table III. Treatment plan robustness as a function of beam arrangements for the paranasal sinus indication. ΔMi are calculated according to formula 1. Median values are listed. a, anterior; cd, caudal; cr, cranial; l, left; p, posterior; r, right. Individual Pshift represent respective directions in which statistically significant differences between beam arrangements were obtained.
Skull base cases
Target coverage.
Shifts in cranial and caudal as well as in anterior direction altered target coverage significantly for all three beam arrangements [Pcranial 97.1% (0.02), Pcaudal 97.3% (0.02), Panterior 96% (0.03) for α; Pcranial 96.9% (0.02), Pcaudal 97.4% (0.02), Panterior 95.8% (0.02) for β; Pcranial 95.9% (0.02), Pcaudal 96.9% (0.02), Panterior 96.6% (0.02) for γ]. Additionally, left lateral shifts caused a statistically significant decrease of the TC for beam arrangement β [Pleft 95.3% (0.03)] and γ [Pleft 98.1% (0.03)].
When comparing different beam set-ups treatment plan robustness concerning target coverage in the left shift direction differed significantly between beam arrangements α and β, being less robust for the latter (median: 0% vs. −4%). Additionally, significant differences were also found between beam arrangement α and γ (0% vs. −0.6%) for Pleft and between β and γ (-2.7% vs. −2%) for Panterior. The latter were not assumed to be clinically relevant. Regarding all other shift directions no significant difference was found for TC when comparing different beam arrangements.
Dose homogeneity.
Concerning HI a similar behavior could be detected, i.e. statistically significant changes for all beam arrangements for Pcranial, Pcaudal and Panterior [Pcranial 10.6% (0.02), Pcaudal 10.5% (0.03), Panterior 12.5% (0.02) for α; Pcranial 9.5% (0.03), Pcaudal 11.8% (0.02), Panrerior 11.6% (0.02) for β; Pcranial 10% (0.02), Pcaudal 9.5% (0.02), Panrerior 9.8% (0.02) for γ]. Furthermore, significant differences were obtained for Pright and Pleft for beam arrangement β and γ.
HI showed big median differences of Δ-values for Pright (0% vs. 5.6%) and Pleft (0.1% vs. 3.5%) between beam arrangement α and β, in favor for the first. Between beam arrangement α and γ significantly different homogeneity was obtained for both left and right shift orientations, being all below 2%.
Average and median dose.
Concerning average and median doses to the CTV beam arrangement γ showed significant differences between P0 and Pright, Pleft and Pposterior. For beam arrangement α and β statistical significances were obtained exclusively for the comparison between P0 and Panterior.
Regarding Dmean as well as Dmedian statistically significant differences between the beam arrangements were, if present, less than 0.6 cGyE and hence considered to be of no clinical relevance.
Organs at risk.
Treatment plan quality regarding the brainstem was mostly affected by shifts along the anterio-posterior axis. Variations of Dmean for the brainstem based on these shift orientations were found to be significant for all of the three beam arrangements [Panterior 0.73 GyE (0.02), Pposterior 1.08 GyE (0.02) for α; Panterior 0.64 GyE (0.02), Pposterior 0.87 GyE (0.02) for β; Panterior 0.81 GyE (0.02), Pposterior 1.01 GyE (0.02) for γ]. The same applied to D2% [Panterior 1.78 GyE (0.03), Pposterior 1.86 GyE (0.02) for α, Panterior 1.75 GyE (0.02), Pposterior 1.91 GyE (0.02) for β, Panterior 1.72 GyE (0.02), Pposterior 1.92 GyE (0.02) for γ]. Due to the close vicinity of the brainstem with respect to the target volumes this can be explained by simple shifts of the high dose regions either further away from or closer to this sensitive structure. Dmean for both optical nerves exhibited a somehow similar behavior, i.e. their dosimetric parameters were most affected by shifts in anterio-posterior direction (all p-values below 0.02). There was a very large spread of D2% for individual patients and hence no systematic statistically significant differences between P0 and any Pshift could be obtained.
For all OAR median ΔMi of respective beam arrangements did not show systematic significant differences. Median differences of the Δ-values were in the order of a few cGyE. For quantitative data refer to .
Paranasal sinus cases
Target coverage.
For patients suffering from tumors in the PS region, statistically significant differences of the TC between the original and the shifted plans were obtained for all three beam arrangements in cranio-caudal [Pcranial 92.6%, Pcaudal 97.1% for α; Pcranial 98.2%, Pcaudal 98.5% for β; Pcranial 99.2%, Pcaudal 99.5% for γ; all p-values = 0.02], anterio-posterior [Panterior 94.6% (0.02), Pposterior 94.8% (0.03) for α; Panterior 97% (0.02), Pposterior 97.9% (0.04) for β; Panterior 99.2% (0.02), Pposterior 99.5% (0.02) for γ] as well as left lateral directions [Pleft 98.3% (0.03) for α; Pleft 96.9% (0.02) for β; Pleft 99.5% (0.02) for γ; all p-values = 0.02]. For the OAR tailored beam arrangement β shifts in right lateral direction were also statistically significantly different from P0 with respect to target coverage [Pright 98.1% (0.03)].
Statistically significant differences regarding beam set-up related TC were obtained for left shift directions between beam arrangement α and β, favoring beam arrangement α. In contrast to this for right and posterior shifts benefits for beam arrangement β were detected. Comparing beam arrangement α and γ for all but the right shifts significant difference were obtained. Beam arrangement γ (maximum ΔTC = −0.6%) showed systematic benefits over the lateral beam arrangement α. This behavior was equal in the comparison between beam arrangement β and γ, showing systematically larger median ΔTC for β.
Dose homogeneity.
Dose homogeneity was mostly influenced by the same shift directions like target coverage (p < 0.02), i.e. all but right, except for beam arrangement α left and caudal directions did not lead to statistically significant differences from P0.
ΔHI differed statistically significantly between beam arrangement α and β for left as well as right shift directions, being very robust for beam arrangement α. Comparing beam arrangement α with beam arrangement γ this holds as well for Pright and Pleft. Regarding posterior (7.6% vs. 3.3%) and cranial (4.5% vs. 2.2%) shifts beam arrangement γ was more robust. Beam arrangement β and γ differed significantly for left and caudal shift orientations, showing benefits for the latter.
Average and median dose.
Considering average and median doses comparing P0 and Pshift, different shift directions led to significant differences for respective beam arrangements: for beam arrangements α and γ the cranial shifts (p = 0.02) while for beam arrangement β anterior and posterior shifts (p = 0.02).
Although significant differences were found between all beam arrangements for average and median doses to the CTV, medians of ΔDmean and ΔDmedian were very small and hence not assumed to be clinically relevant.
Organs at risk.
Regarding Dmean to the brainstem, set-up errors in anterior [0.02 GyE (0.02)] as well as in posterior direction [0.05 GyE (0.02)] caused highest variations when irradiating with lateral opposed beam ports (α) in relation to P0. The same trend was observed for D2% [0.21 GyE, 0.63 GyE; both p-values = 0.02]. Similar to SB cases this was caused by shifts away from or towards the high dose regions. For beam arrangement β the only significant difference was obtained for caudal shifts concerning Dmean [0.07 GyE (0.04)] as well as for D2% [0.72 GyE (0.04)]. In case of treatment with three beams (γ) all but caudal shifts caused significantly different Dmean values (all p < 0.03). Regarding D2% shifts in anterior [0.8 GyE (0.03)], posterior [1.13 GyE (0.02)], cranial [1.23 GyE (0.03)] and caudal [0.67 GyE (0.02)] direction caused statistically significant differences from P0. For beam arrangement α, Dmean of the optic nerves varied significantly for shifts in anterior [contra: 1.27 GyE (0.04), ipsi: 1.91 GyE (0.02)] and posterior [contra: 1.1 GyE (0.02), ipsi:1.87 GyE (0.03)] direction, while for other Mi and beam arrangements no statistically significant difference was obtained for optic nerves.
Rather large differences were obtained for the comparison between lateral (α) and the OAR sparing (β) beam arrangements concerning the near maximum dose to the brainstem, especially for patient set-up errors in posterior (−16.7 cGyE vs. −8.4 cGyE) and anterior (26 cGyE vs. 9.6 cGyE) direction. The differences were in favor for beam arrangement β. The comparison of beam arrangement α and γ resulted in a similar order of magnitude for Pcranial and Pcaudal for the benefit of γ. Average brainstem doses were found to be quite stable, regardless of shift orientation. Refer to for numerical values regarding optical nerves.
Discussion
In this paper a dosimetric treatment plan robustness investigation analyzing potential impacts of inter-fractional translational patient misalignment in relation to the planned scanning raster for IMPT was performed. The entire study aimed to give an insight in what could happen if patients receiving IMPT in the cranial region were systematically displaced. This might be the case if image guidance tools with a systematic error were used or patients would move due to tension or relaxing on a regular basis.
The study was based on three different beam arrangements, which were characterized by a different number of beams as well as differing incident beam angles. They were chosen as follows. Beam arrangement α applied a very simple option, namely two lateral opposed beams and represented a classical approach in light ion therapy. The second consisted also of two beams which were not restricted to fixed incident beam angles but tried to avoid critical structures or at least not to directly penetrate them. Structures of highest interest were the brainstem in the SB group and the contra-lateral optic nerve in the PS group [Citation15]. The reason why the brainstem in the latter group was not treated as primary OAR for beam arrangement β was that it was mostly situated relatively far away from the target volumes. The third beam set-up employed additional beams which have been reported in recent literature [Citation16,Citation17]. The four beams in the SB group were distributed ‘star-like’ around the target while for PS cases the two ports of beam arrangement β were maintained and the ipsi-lateral port was mirrored by the anterio-posterior axis to the contra-lateral part. At this point it should be emphasized again that only the gantry degree of freedom was exploited (coplanar treatment), i.e. the treatment couch was always fixed at 0°. By utilizing only two beam ports (arrangement α and β), the degrees of freedom were restricted, despite achieving adequate initial plan quality (see ). Thus, such beam arrangements are more vulnerable to patient set-up errors. Comparing different beam arrangements it was found that for both tumor indications under investigation, the multi-beam constellations (beam arrangement γ) showed benefits with respect to stability of treatment plans regarding the target indices TC and HI. This is obvious with respect to the smaller scattering range of the data (above all for PS patients) of the mentioned beam arrangement (see and ), although better performance was not reaching statistical significance. By adding additional beams, the fraction of dose to be delivered by individual beams is reduced and misalignment can be better compensated for. If one beam is strongly influenced by a certain shift orientation, the others may not be.
Regarding beam arrangement α it is obvious that shifts along the lateral patient axis had almost no influence on treatment plan quality because no component of the shift is perpendicular to the incident beams. Hence, geographical miss can be ignored and only range effects play a role. ΔMi after shifts in anterio-posterior as well as cranio-caudal directions were more pronounced because they were exclusively perpendicular.
For arrangement β every shift direction has its perpendicular components and hence interplay effects of range and geographical miss of the targets were pronounced along each shift direction for the SB group. For a few SB patients the target coverage dropped below 85%, e.g. shifts in left lateral direction. Consequently, for this tumor indication, beam arrangement β with the aim of sparing OARs failed to fulfill robustness criteria in the SB group for some patients and are often inferior compared to simple lateral beam ports (beam arrangement α). For PS cases beam arrangements α and β were comparable to each other, sometimes even showing benefits in favor for arrangement β. Although differences of treatment plan robustness between the beam arrangements were obtained for both patient groups regarding average and median doses, they were not considered to have clinical impact.
According to the knowledge of the authors there is no investigation available addressing dosimetric treatment plan robustness for the cranial region in a cohort of patients. In a case study, Lomax [Citation10] and Albertini et al. [Citation16] showed a sophisticated method for the analysis of dosimetric treatment plan robustness adopting a concept of providing a full dose difference map of the original and the recalculated dose distribution. Similar to the underlying study they applied shifts along all major axes while rotational set-up errors of the patient were ignored. In addition to that they reported a worst case scenario based on the directions which most influenced the dose distribution. In contrast to this, our comparison was trying to characterize treatment plan robustness by evaluating commonly accepted dose volume histogram (DVH) parameters, e.g. TC, HI, D2% and Dmean. They are more intuitive to interpret and easier to perform for more patients and plans. We do believe that for clinical routine, besides visual inspection of the shifted dose distributions by an experienced radiation oncologist, the use of such ‘simple’ evaluation metrics is inevitable. Additionally, by not simulating a voxel by voxel worst case scenario, the dosimetric impact of individual shift directions on the global treatment plan quality can be studied. We decided to recalculate each initial treatment plan (P0) six times in order to cover misalignment scenarios along all major anatomic axes of the patient. Combined set-up errors, up to an absolute value of 4.2 mm with our initial input parameters, were not considered in this context.
The use of a uniformly inflated margin for proton therapy is a matter of debate because set-up errors of the patient (i.e. shifts of the planning isocenter) generally do not relate to rigid shifts of the dose distributions. Beam specific individualization of the PTV was proposed by Park et al. [Citation23]. To guarantee a simulation of realistic clinical treatment circumstances we pursued the isotropically inflated PTV concept. To study the influence of margins was beyond the scope of this study. Nevertheless, several cases were evaluated by the use of an inflated PTV, i.e. 5 mm. In general, a bigger margin did not affect the outcome of treatment plan robustness as a function of beam arrangement.
Based on the data reported in this article and recent findings from other groups [Citation10,Citation16], translational set-up errors represent a non-negligible source of error in IMPT. Various options in order to include robustness criteria already into the optimization process at the time of treatment planning have been proposed by several groups. In brief, Fredrikson et al. [Citation24] performed optimization aiming at minimizing the objective function in a pre-calculated worst-case scenario utilizing stochastic programing and thus providing a boundary for how much the plan quality can decrease due to errors. Chen et al. [Citation25] suggested multi-criteria optimization. This allows exploring the trade-off between different objectives as well as the trade-off between robustness and nominal plan quality at the same time, navigating through Pareto fronts. Additionally, by the application of such optimization strategies the above mentioned problem about safety margins becomes obsolete because optimization is tailored directly to the CTV. In the long run, such optimization techniques incorporating alignment uncertainties of the patient a priori will be inevitable.
In conclusion, a systematic multi-case investigation evaluating dosimetric treatment plan robustness with respect to inter-fraction translational set-up errors was performed and evaluated as a function of beam arrangement. Already relatively small shifts distorted initially accepted dose distributions severely, depending on the shift directions. Hence it is obligatory to account for them and further research in this direction should be promoted. Comparing two and multi-beam arrangements benefits were obtained for the latter. Nonetheless, individual anatomic patient and treatment circumstances must be carefully considered for judging which beam arrangement to apply.
Acknowledgements
The current investigation was performed in the context of the EC project ULICE (Grant agreement number: 228436). The authors would like to thank the European Commission, the Austrian Federal Ministry of Economy, Family and Youth and the National Foundation for Research, Technology and Development for financial support.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Lomax A, Rutz EPH, Goitein G. The clinical potential of intensity modulated proton therapy. Z Med Phys 2004;14:147–52.
- Palm Å, Johansson K. A review of the impact of photon and proton external beam radiotherapy treatment modalities on the dose distributions in field and out-of-field; implications for the long-term morbidity of cancer survivors. Acta Oncol 2007;46:462–73.
- Blomquist E, Bjelkengren G, Glimelius B. The potential of proton beam radiation for intracranial and ocular tumours. Acta Oncol 2005;44:862–70.
- Weber DC, Schneider R, Goitein G, Koch T, Ares C, Geismar JH, . Spot scanning-based proton therapy for intracranial meningiomas. Int J Radiat Oncol Biol Phys 2012;83:865–71.
- Georg D, Bogner J, Dieckmann K, Pötter R. Is mask-based stereotactic head-and-neck fixation as precise as stereotactic head fixation for precision radiotherapy? Int J Radiat Oncol Biol Phys 2006;66:61–6.
- Schneider U, Pedroni E, Lomax AJ. The calibration of CT Hounsfield units for radiotherapy treatment planning. Phys Med Biol 1996;41:111–24.
- Jäkel O, Reiss P. The influence of metal artefacts on the range of ion beams. Phys Med Biol 2007;52:635–44.
- Lomax AJ. Intensity modulated proton therapy and its sensitivity to treatment uncertainties 1: The potential effects of calculational uncertainties. Phys Med Biol 2008;53:1027–42.
- Lassen-Ramshad Y, Vestergaard A, Muren LP, Hoyer M, Petersen JBB. Plan robustness in proton beam therapy of a childhood brain tumour. Acta Oncol 2011;50:791–6.
- Lomax AJ. Intensity modulated proton therapy and its sensitivity to treatment uncertainties 2: The potential effects of inter-fraction and inter-field motions. Phys Med Biol 2008;53:1043–56.
- Góra J, Stock M, Lütgendorf-Caucig C, Georg D. Is there an advantage in designing adapted, patient specific PTV margins in intensity modulated proton beam therapy for prostate cancer? Int J Radiat Oncol Biol Phys Epub 2012 Jul 17.
- Homayuni AR, Georg D, Dieckmann K, Stock M, Pötter R, Osztavics A. Evaluation of the reproducibility of patient set-up for a double-mask system using additional thermoplastic pellets for precision radiotherapy. Radiother Oncol 2011;99:560.
- Rutz HP, Weber DC, Goitein G, Ares C, Bolsi A, Lomax AJ, . Postoperative spot-scanning proton radiation therapy for chordoma and chondrosarcoma in children and adolescents: Initial experience at Paul Scherrer Institute. Int J Radiat Oncol Biol Phys 2008;71:220–5.
- Hillbrand M, Georg D. Assessing a set of optimal user interface parameters for intensity-modulated proton therapy planning. J Appl Clin Med Phys 2010;11:93–104.
- Fukumitsu N, Okumura T, Mizumoto M, Oshiro M, Hashimoto T, Kanemoto A, . Outcome of T4 (International Union Against Cancer Staging System, 7th edition) or recurrent nasal cavity and paranasal sinus carcinoma treated with proton beam. Int J Radiat Oncol Biol Phys 2012;83: 704–11.
- Albertini F, Hub EB, Lomax AJ. Is it necessary to plan with safety margins for actively scanned proton therapy? Phys Med Biol 2011;56:4399–413.
- Zenda S, Kohno R, Kawashima M, Arahira S, Hishio T, Nishio T, . Proton beam therapy for unresectable malignancies of the nasal cavity and paranasal sinuses. Int J Radiat Oncol Biol Phys 2011;81:1473–8.
- International Comission on Radiation Units and Measurements. ICRU Report No. 78 Prescribing, recording, and reporting proton-beam therapy. Tech Report 2007.
- Sejpal SV, Amos RA, Bluett JB, Levy LB, Kudchadker RL, Johnson J, . Dosimetric changes resulting from patient rotational setup errors in proton therapy prostate plans. Int J Radiat Oncol Biol Phys 2009;75:40–8.
- Meyer J, Bluett J, Amos R, Levy L, Choi S, Nguyen Q, . Spot scanning proton beam therapy for prostate cancer: Treatment planning technique and analysis of consequences of rotational and translational alignment errors. Int J Radiat Oncol Biol Phys 2010;78:428–34.
- Lomax NJ, Scheib SG. Quantifying the degree of conformity in radiosurgery treatment planning. Int J Radiat Oncol Biol Phys 2003;55:1409–19.
- Yoon M, Park SY, Shin D, Lee SB, Pyo HR, Kim DY, . A new homogeneity index based on statistical analysis of the dose-volume histogram. J Appl Clin Med Phys 2007; 8:9–17.
- Park PC, Zhu XR, Lee AK, Sahoo N, Melancon AD, Zhang L, . A beam-specific planning target volume (PTV) design for proton therapy to account for setup and range uncertainties. Int J Radiat Oncol Biol Phys 2011;82:329–36.
- Fredrikson A, Forsgren A, Hordemark B. Minimax optimization for handling range and setup uncertainties in proton therapy. Med Phys 2011;38:1672–84.
- Chen W, Unkelbach J, Trofimov A, Madden T, Kooy H, Bortfeld T, . Including robustness in multi-criteria optimization for intensity-modulated proton therapy. Phys Med Biol 2012;57:591–608.
