Abstract
Purpose. To evaluate early treatment results and toxicity in patients with meningiomas treated with particle therapy. Material and methods. Seventy patients with meningiomas were treated with protons (n = 38) or carbon ion radiotherapy (n = 26). Median age was 49 years. Median age at treatment was 55 years, 24 were male (34%), and 46 were female (66%). Histology was benign meningioma in 26 patients (37%), atypical in 23 patients (33%) and anaplastic in four patients (6%). In 17 patients (24%) with skull base meningiomas diagnosis was based on the typical appearance of a meningioma. For benign meningiomas, total doses of 52.2–57.6 GyE were applied with protons. For high-grade lesions, the boost volume was 18 GyE carbon ions, with a median dose of 50 GyE applied as highly conformal radiation therapy. Nineteen patients were treated as re-irradiation. Treatment planning with MRI and 68-Ga-DOTATOC-PET was evaluated. Results. Very low rates of side effects developed, including headaches, nausea and dizziness. No severe treatment-related toxicity was observed. Local control for benign meningiomas was 100%. Five of 27 patients (19%) developed tumor recurrence during follow-up. Of these, four patients had been treated as re-irradiation for recurrent high-risk meningiomas. Actuarial local control after re-irradiation of high-risk meningiomas was therefore 67% at six and 12 months. In patients treated with primary radiotherapy, only one of 13 patients (8%) developed tumor recurrence 17 months after radiation therapy (photon and carbon ion boost). Conclusion. Continuous prospective follow-up and development of novel study concepts are required to fully exploit the long-term clinical data after particle therapy for meningiomas. To date, it may be concluded that when proton therapy is available, meningioma patients can be offered a treatment at least comparable to high-end photon therapy.
Meningiomas represent a heterogeneous group of tumors, ranging from slow growing, low-grade tumors, to highly aggressive anaplastic tumors. Especially in low-grade meningiomas, preservation of quality of life (QOL) and neurocognitive functioning is equally as important as long-term tumor control; this holds true since diagnosis is often in younger, predominantly female patients, and progression-free survival (PFS) and overall survival (OS) are very long [Citation1].
Radiation therapy (RT) has become a valid treatment alternative over the last decades associated with a convincing risk-benefit profile. The complex anatomy of meningiomas especially in the skull base region have hindered the neurosurgeon from complete resections in substantial numbers of patients, and surgical interventions may be associated with severe morbidity due to the close vicinity of critical organs at risk (OAR). Modern highly-conformal RT, such as fractionated stereotactic radiotherapy (FSRT) or intensity modulated radiotherapy (IMRT) have enabled the radiation oncologist to deliver high local doses even in complex anatomy while sparing OARs. Local control has been reported to be up to 95%, with rates of side effects consistently below 5% [Citation2].
With proton therapy, reduction of integral dose may lead to an improved clinical outcome in patients with meningiomas. Several institutions have reported on smaller series of meningiomas, with convincing outcome [Citation3–7]. Most centers have used passive beam technologies. Only few data is available using scanning beam technology. Compared to protons, carbon ions additionally offer an increased relative biological effectiveness (RBE). Previously we had shown that a combination of photon radiotherapy and a carbon ion boost in patients with high-risk meningiomas leads to excellent clinical results [Citation8].
For treatment planning, addition of 68Ga-DOTATOC has been shown to improve detection rate of meningiomas; additionally, for target volume definition, additionally PET-examinations can help distinguish between meningeal thickening versus extension of meningiomas, especially in regions of the dural tail or of bony invasion; moreover, in meningiomas infiltrating into soft tissue PET-imaging can help differentiate meningioma tissue from normal tissue or scar tissue. Therefore, this examination is performed as our in-house standard for treatment planning in patients with meningiomas.
In the present manuscript we report our initial results in patients with meningiomas with special focus on treatment planning and early toxicity.
Patients and methods
We treated 70 patients with meningiomas of different pathological grading with protons (n = 38) or carbon ion radiotherapy (n = 26) between November 2009 and May 2012. All patients were followed prospectively within a continuous observational program and documented within a dedicated database for particle therapy [Citation9].
Patients’ characteristics
At primary diagnosis, median age was 49 years (range 21–83 years). Median age at treatment was 55 years (range 27–83 years), 24 were male (34%), and 46 were female (66%). The main locations included skull base meningiomas (x = 55; 79%), olfactory meningiomas (x = 1) and optic nerve sheath meningioma (ONSM) extending through the optic canal with intracranial extensions in one patient, supratentorial tumors along the falx (n = 12; 17%). shows site distribution of the tumors. Histology was benign meningioma in 26 patients (37%), atypical in 23 patients (33%) and anaplastic in four patients (6%). In 17 patients (24%) with skull base meningiomas no histopathological evaluation was available and diagnosis was based on the typical appearance of a meningioma in the skull base region; for analysis, they were attributed to the low-grade meningioma group. In these patients, additional 68Ga-DOTATOC-PET was mandatory for treatment.
Table I. Patients’ characteristics of 70 patients treated with proton or carbon ion radiotherapy for benign or high-grade meningiomas.
Forty-one patients were treated with protons (59%), and 29 patients (41%) were treated with carbon ions. Of the latter, 16 were treated with a carbon ion boost in combination with photons for high-grade meningiomas, and in 13 patients carbon ions only were applied as re-irradiation.
Benign meningiomas
This group consists of the histologically confirmed low-grade (WHO Grade I) meningiomas, as well as the patients without pathological evaluation. Of these, all were skull base meningiomas, and one olfactory meningioma as well as one optic nerve sheath meningioma (ONSM). The median age at diagnosis was 51 years (range 26–83 years), the median age at particle therapy was 57 years (range 32–83 years). In 30 patients neurosurgery had been conducted after primary diagnosis: In 17 patients one surgical intervention, and two or three surgeries were conducted in 10 and two patients, respectively. In one patient, five resections had been performed. For patients having undergone neurosurgical resection the resection status was partial except in two patients where only a biopsy was performed for diagnosis confirmation. Radiation therapy was applied for tumor recurrence after neurosurgical resection, or in cases without neurosurgical resection, for progressing lesions and/or progressing clinical symptoms. The median time interval between diagnosis and radiotherapy was 40 months (range 1–238 months), the median time between neurosurgical resection and radiotherapy was 46 months (range 3–235 months).
High-grade meningiomas
Of 27 high-grade lesions, 23 (85%) were atypical (WHO Grade II) and four (15%) anaplastic (WHO Grade IV) meningiomas. Of all, 12 (44%) were located along the falx or in the parafalcial region close to the venous sinus, 14 (56%) were skull base meningiomas, and one olfactory meningioma. Of these, 14 were treated for recurrent tumors after re-irradiation, and in 13 patients particle therapy was performed as primary radiotherapy after resection, or for recurrence after resection. All patients were treated with a partial resection at primary diagnosis, except one patient treated with a biopsy only. Ten patients received one resection, two resections were performed in nine patients, four patients each were treated with three and four resections, and in one patient seven resections were conducted prior to radiation therapy. Indication for resection and/or postoperative radiotherapy had been taken as individual decisions outside the Heidelberg Medical Center, and patients were referred to radiation therapy thereafter. The median time after primary diagnosis (and resection) to radiotherapy for patients treated with primary radiotherapy was 52 months (range 32–75 months).
Particle radiotherapy
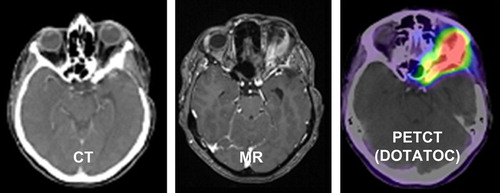
Individually manufactured head masks were made for each patient with Scotch cast™ or aquaplast. CT-imaging in mask fixation was obtained with and without contrast enhancement. Contrast-enhanced MRI as well as 68Ga-DOTATOC-PET was acquired for target volume definition. Target volume definition is conducted using the Siemens Dosimetrist and Oncologist software tools (Siemens, Erlangen, Germany) matching CT, MRI and PET-Data. A typical imaging situation including CT, MRT and 68Ga-DOTATOC-PET is shown in .
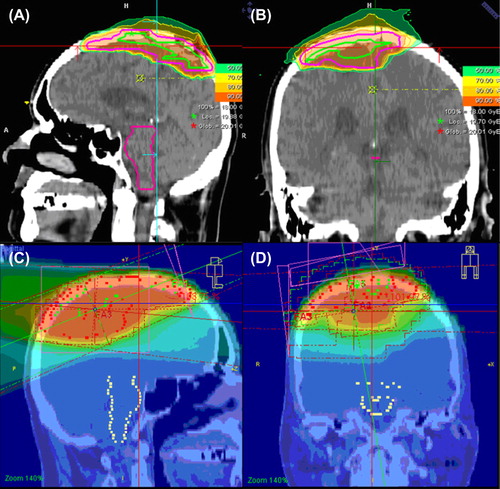
Figure 1. Imaging for target volume definition for proton radiotherapy of a skull base meningioma treated including CT, MRT and 68-Ga-Dotatoc.
The gross tumor volume (GTV) is defined as the macroscopic tumor on T1-weighted MRI and/or 68Ga-DOTATOC-PET-positive regions, and a safety margin of 1–2 mm for benign meningiomas as the clinical target volume (CTV). For high-risk meningiomas treated with primary radiotherapy, the GTV is encompassed by 5 mm for the CTV. Photon radiotherapy is additionally applied up to 50 GyE in 2 GyE single fractions to the GTV plus a safety margin of 20–30 mm (CTVPhotons) to account for the potential microscopic spread.
For benign meningiomas, total doses of 52.2–57. 6 GyE in single doses of 1.8 GyE or 2 GyE were applied with protons. For high-grade lesions, the boost volume is treated with 18 GyE carbon ions, with a median dose of 50 GyE in 2 GyE single fractions applied as highly conformal radiation therapy. A typical treatment plan including three-dimensional (3D)-photon treatment and the plan for the carbon ion boost is depicted in .
Re-irradiation for tumor progression
Nineteen patients were treated as re-irradiation, of which five were low-grade, and 14 high-risk meningiomas; in these cases, target volume definition depended on the clinical presentation of the patient, histology, dose and target volume of the previous radiotherapy as well as anatomical location and neighboring risk structures. In general, the GTV included the macroscopic tumor, and a safety margin of 5 mm was added for the CTV.
Of these, 14 patients were treated with carbon ions with doses from 45–51 GyE in 3 GyE single fractions. Five patients were treated with protons, two after initial small-volume radiosurgery, and three after fractionated photon treatments with total doses of 54 Gy and 50 Gy. The proton dose in recurrent tumors was 54 GyE or 57.6 GyE in single fractions of 1.8 GyE.
Target volume definition and evaluation of PET-MRI-planning
For evaluation of target volume definition based on MRI and 68Ga-DOTATOC-PET, we defined two volumes: Volume A was a GTV_CT/MRI based on information obtained from CT and MRI with and without contrast enhancement. Volume B was GTV_PET defined as 68Ga-DOTATOC-PET-positive regions.
Treatment planning
For treatment planning, the system ‘Syngo PT Planning’ developed by Siemens Oncology Care Systems (OCS, Erlangen, Germany) was implemented. Biological plan optimization is based on the local effect model (LEM) developed by GSI; it is designed for RBE-calculation in different tissue types and for selected endpoints. The optimization of the scan control parameters for the raster scanning technique within the treatment planning system (TPS) is done with respect to the biological effective dose of the particles. A fixed value for the relative biological effectiveness (RBE) of 1.1 is used clinically for proton treatments. For carbon ions, the optimization is based on the LEM model, and we used an α/β value of 2 for the treatment in line with our previous clinical experience. Prior to each fraction patient positioning is checked using orthogonal x-rays, which are correlated with planning CT DRRs focusing mainly on bony landmarks was used for position corrections.
Follow-up and statistics
All patients are followed prospectively and follow-up data are documented homogeneously. For skull base tumors, a detailed questionnaire on signs and symptoms typical for this anatomical region are retrieved at every visit. Patient data is documented in a large database designed to collect data on particle therapy (ULICE-Database), allowing rapid access to the data and providing all essential information for valid clinical evaluation.
The median follow-up time was six months (range 2–22 months). Local control (LC) is calculated from the initial date of radiotherapy to the date of tumor progression, or, in the case of censored data, to the last date of follow-up. All statistical evaluations were performed using the Statistica 6.1 Software (StatSoft, Germany) [Citation9].
Results
Evaluation of target volume definition
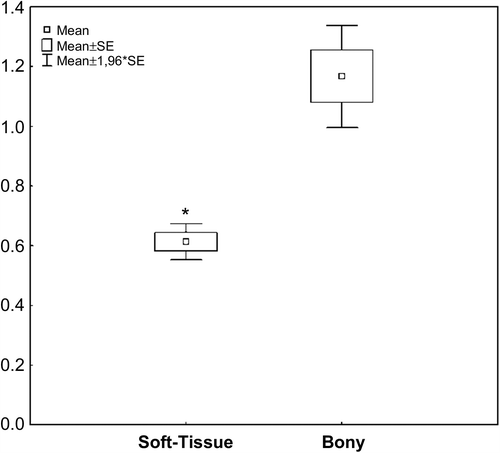
The median volume A was 30.95 ml (range 11.5–166.1 ml), the median volume B was 35.85 ml (range 13.4–112.9 ml). The intersection region (MRI-visible and PET-positive regions) were determined, the median volume was 22.95 ml (range 7.4–104.9 ml). We calculated the sum of MRI- and PET-based target volumes, defined as volume D; median volume D was 41.25 ml (range 17.3–166.8 ml). Both enlargements as well as reductions of GTV-volume were seen after correlation with PET as compared to MRI-alone. Reduction in volume was seen in 40% of the patients. These volumes were, on MRI, large volumes with median GTV-MRI of 127.4 ml (range 106.1–166.1 ml). All of these were volumes extending into soft tissue regions (pterygoid muscles, pharyngeal structures). PET-GTV in these meningiomas had a median volume of 61.3 ml (range 50.5–112.9 ml). In smaller meningiomas of typical skull base location such as within the cavernous sinus or sphenoorbital bony region, differences in Volume A and B were comparably small, and lead to increase in volume rather than reduction. Median volume A in these patients was 20.35 ml (range 11.5–37.8 ml) and median volume B was 18 ml (range 16.5–39.7 ml).
Regions PET-positive visible on MRI were defined as the ratio of Volume B/Volume A. For soft-tissue infiltrating meningiomas, mean ratio was 0.61 ± 0.06. For typical meningiomas along bony structures, the mean ratio was 1.17 ± 0.29 (). This difference in ratio was significantly different at p = 0.02.
Side effects
In all patients particle therapy was well-tolerated and could be completed without interruptions due to treatment-related toxicity. Thirty-three patients of 70 (47%) developed focal alopecia within the treatment portals.
Clinical symptoms prior to radiation therapy included headaches (n = 20; 29%), dizziness (n = 17; 24%), nausea (n = 16: 23%), motor deficits (n = 17; 24%) and sensory deficits (n = 16; 23%). In one patient seizures were observed, which were controlled under antiepileptic medication. Double vision was present in 17 patients (24%), and other visual deficits in 21 patients (30%). Detailed cranial nerve impairment prior to radiation was seen as oculomotor paresis in four patients (6%), trigeminal deficits in five patients (7%), abducens paresis in five patients (7%), a postoperative facial lesion in one patient (1%), and hypoglossal impairment in one patient (1%).
During short-term follow-up, no patient developed new cranial nerve deficits, especially no new visual impairment. Motor and sensory deficits improved in four and one patients, respectively, no patients developed new treatment-related motor or sensory deficits. Nausea and dizziness improved after radiotherapy in seven patients, and in two patients these symptoms worsened during the first six months of follow-up.
No other treatment-related toxicity was observed; in all patients, with special focus on patients treated as re-irradiation, no development treatment-related edema or necrosis to normal tissue was observed during short-term follow-up.
Local tumor control and survival
For low-grade meningiomas, all patients remained locally controlled over a median follow-up time of six months (range 2–22 months). No tumor recurrences developed. All patients remained alive at the time of analysis.
In the group of patients with high-grade meningiomas, five of 27 patients (19%) developed tumor recurrence during follow-up. Of these, four patients had been treated as re-irradiation for recurrent high-risk meningiomas. Actuarial local control after re-irradiation of high-risk meningiomas was therefore 67% at 6 and 12 months.
In patients treated with primary radiotherapy, only one of 13 patients (8%) developed tumor recurrence 17 months after radiation therapy (photon and carbon ion boost).
All patients remained alive at the time of analysis.
Discussion
Actively delivered particle therapy with protons and carbon ions provides a safe and precise method for treatment of patients with various histologies. In our group of 70 meningiomas treated at HIT, horizontal beam lines were used. Early toxicity is very mild and tolerability of treatment is excellent. To date, tumor control is high, however, considering the benign histology in most patients, longer follow-up times for evaluation of full efficacy are required. Evaluation of treatment planning confirmed that 68Ga-DOTATOC-PET adds valuable information for target volume definition, especially in larger volumes extending into soft tissue regions.
Although active beam delivery has been available over the last decades, only few centers are actively treating using a scanned ion beam. However, the superiority or a scanning approach has been shown for several aspects: In passive beam delivery, patient specific hardware is required to achieve acceptable dose distributions, which lead to prolonged setup and treatment times, and can be quite costly. Additionally, distal conformity of the dose distribution is not always optimal; challenges are also attributed with active beam delivery, such as the treatment of moving organs, however, these are not in focus for non-mobile tumors, such as meningiomas. However, as always when bringing new techniques into clinical application, quality measures are necessary, not only on the biological and physics side. Clinically, close monitoring of patients and detection of toxicity as well as tumor control after treatment are of importance.
The physical benefits of the particle beam with low dose deposition in the entry channel and a high local dose peak (Bragg Peak) in a defined depth lead to a reduction of integral dose. Several treatment planning comparisons have shown the superiority of particle beams compared to even highly advanced photon techniques with special regard on reduction of dose to normal tissue. Most recently, we could show that using horizontal fixed beams excellent dose plans can be calculated for tumors of the skull base, and the benefit of a gantry might be minimal in this tumor location [Citation10].
Early clinical results of proton therapy for meningiomas have been reported by several institutions. Austin-Seymour reported on 13 patients with meningiomas within a group of 110 patients with skull base tumors [Citation11]; passively delivered protons were delivered with a median dose of 59.4 Gy, and two patients developed higher grade side effects: one patient showed unilateral necrosis of the pons 11 months after treatment, another developed optic neuropathy also 11 months after proton therapy. However, all patients remained controlled with respect to the tumor at the time of publication. More advanced data from the same institution were reported by Wenkel and colleagues: Recurrence-free survival was 100% and 88% at five and 10 years in 46 patients with meningioma of different histological and anatomical regions [Citation12]. Survival without severe toxicity was 80% at 10 years; this consisted of opthalmological side effects, neurologic as well as otologic complications. In these patients, doses to the risk structures exceeded the defined tolerance doses, mostly due to the location and the histology of the tumor.
In Sweden, 19 patients have been treated with hypofractionated protons [Citation5]. Of these patients, only two developed steroid-dependent edema six months after treatment, which deteriorated over time; no recurrences had been observed after a median follow-up of 36 months. Vernimmen et al. published 27 patients, of which 23 were skull-base lesions [Citation6]. 88% remained locally controlled after a median follow-up of 40 months. Acute side effects were observed in two patients (transient new cranial nerve neuropathy) which resolved completely, two other patients developed long-term side effects (hearing loss, temporal lobe epilepsy), but no patient required interventions for complications. Other institutions also reported results using a passive beam delivery.
For active beam delivery, Weber et al. published outcome in 40 patients with primary (n = 32) and recurrent (n = 8) meningiomas [Citation7]. The median dose was 56 GyE in 1.8–2 GyE single fraction as delivered by spot scanning. After a mean follow-up time of 62 months, five-year actuarial local control was 84.4%; late adverse effects were observed in 41% of the patients, including pituitary dysfunction, optic neuropathy, brain edema, brain necrosis, retinitis, xerophtalmie and ischemic stroke.
For high-grade meningiomas, a dose-response relationship has been shown [Citation13]. Proton and carbon ion radiotherapy have been applied for high-risk meningiomas. Compared to protons, carbon ions are associated with an enhanced relative biological effectiveness, which is between two and five depending on cell line or end point [Citation14]. For several tumors, a clinical benefit of high linear energy transfer (LET)-beams has been shown, such as for chordomas and chondrosarcomas, pancreatic cancer, liver cancer, and malignant salivary gland tumors [Citation15]. For high-risk meningiomas, a small group of patients treated with a carbon ion boost has shown high local control and survival rate and a very low rate of side effects [Citation16]. Therefore, the prospective MARCIE trial is evaluating this concept [Citation8].
In the present work we present the largest group of patients with meningiomas treated with actively delivered particle beams; the safety and early toxicity data confirm that scanning beams can be applied safely in clinical routine, for benign and high-risk meningiomas of different histological subtypes. No high-grade side effects were observed, even in patients treated with re-irradiation for recurrent tumors. However, follow-up times are still limited, and close observation is required to determine further acute and long-term side effects. To precisely define the target volume, PET-imaging based on 68Ga-DOTATOC is performed at our institution, providing a better distinction between some areas of normal tissue and meningioma residuals, especially after surgical resection. We could show that addition of 68Ga-DOTATOC-PET provides valuable information in all patients. For larger meningiomas extending into soft tissue, PET-imaging leads to significant decrease of volumes, as it helps distinguish meningioma infiltration from normal tissue or scar tissue. For smaller lesions and/or typical lesions extending into bony regions such as the cavernous sinus of the sphenoorbital region, PET consistently lead to enlargement of volumes better identifying regions of bony involvement less visible on CT and MRI.
Continuous prospective follow-up and development of novel study concepts are required to fully exploit the long-term clinical data after particle therapy for meningiomas. Such studies are currently recruiting patients, e.g. the MARCIE-Protocol [Citation8]. However, to date, it may be concluded that when proton therapy is available, meningioma patients can be offered a treatment at least comparable to high-end photon therapy. In the long run, the reduction of integral dose by protons may lead to clinical improvement with respect to side effects; however, to date no data to proof this hypothesis are yet available. Carbon ions offer beneficial biological characteristics, and should be further evaluated within clinical trials.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bondy M, Ligon BL. Epidemiology and etiology of intracranial meningiomas: A review. J Neurooncol1996;29:197–205.
- Debus J, Wuendrich M, Pirzkall A, Hoess A, Schlegel W, Zuna I, . High efficacy of fractionated stereotactic radiotherapy of large base-of-skull meningiomas: Long-term results. J Clin Oncol2001;19:3547–53.
- Boskos C, Feuvret L, Noel G, Habrand JL, Pommier P, Alapetite C, . Combined proton and photon conformal radiotherapy for intracranial atypical and malignant meningioma. Int J Radiat Oncol Biol Phys2009;75:399–406.
- Combs SE, Kieser M, Rieken S, Habermehl D, Jakel O, Haberer T, . Randomized phase II study evaluating a carbon ion boost applied after combined radiochemotherapy with temozolomide versus a proton boost after radiochemotherapy with temozolomide in patients with primary glioblastoma: The CLEOPATRA trial. BMC Cancer2010; 10:478.
- Gudjonsson O, Blomquist E, Nyberg G, Pellettieri L, Montelius A, Grusell E, . Stereotactic irradiation of skull base meningiomas with high energy protons. Acta Neurochir (Wien.)1999;141:933–40.
- Vernimmen FJ, Harris JK, Wilson JA, Melvill R, Smit BJ, Slabbert JP. Stereotactic proton beam therapy of skull ase meningiomas. Int J Radiat Oncol Biol Phys2001;49: 99–105.
- Weber DC, Schneider R, Goitein G, Koch T, Ares C, Geismar JH, . Spot scanning-based proton therapy for intracranial meningioma: Long-term results from the Paul Scherrer Institute. Int J Radiat Oncol Biol Phys2012;83: 865–71.
- Combs SE, Edler L, Burkholder I, Rieken S, Habermehl D, Jakel O, . Treatment of patients with atypical meningiomas Simpson grade 4 and 5 with a carbon ion boost in combination with postoperative photon radiotherapy: The MARCIE trial. BMC Cancer2010;10:615.
- Kessel KA, Bougatf N, Bohn C, Habermehl D, Oetzel D, Bendl R, . Connection of European particle therapy centers and generation of a common particle database system within the European ULICE-framework. Radiat Oncol2012; 7:115.
- Kosaki K, Ecker S, Habermehl D, Rieken S, Jakel O, Herfarth K, . Comparison of intensity modulated radiotherapy (IMRT) with intensity modulated particle therapy (IMPT) using fixed beams or an ion gantry for the treatment of patients with skull base meningiomas. Radiat Oncol2012;7:44.
- Austin-Seymour M, Munzenrider J, Linggood R, Goitein M, Verhey L, Urie M, . Fractionated proton radiation therapy of cranial and intracranial tumors. Am J Clin Oncol1990;13:327–30.
- Wenkel E, Thornton AF, Finkelstein D, Adams J, Lyons S, De La MS, . Benign meningioma: Partially resected, biopsied, and recurrent intracranial tumors treated with combined proton and photon radiotherapy. Int J Radiat Oncol Biol Phys2000;48:1363–70.
- Hug EB, Devries A, Thornton AF, Munzenride JE, Pardo FS, Hedley-Whyte ET, . Management of atypical and malignant meningiomas: Role of high-dose, 3D-conformal radiation therapy. J Neurooncol2000;48:151–60.
- Combs SE, Bohl J, Elsasser T, Weber KJ, Schulz-Ertner D, Debus J, . Radiobiological evaluation and correlation with the local effect model (LEM) of carbon ion radiation therapy and temozolomide in glioblastoma cell lines. Int J Radiat Biol2009;85:126–37.
- Schulz-Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol2007;25: 953–64.
- Combs SE, Hartmann C, Nikoghosyan A, Jakel O, Karger CP, Haberer T, . Carbon ion radiation therapy for high-risk meningiomas. Radiother Oncol2010;95: 54–9.