Abstract
Background. Concurrent chemoradiotherapy (CRT) is the standard of care in patients with limited-stage small cell lung cancer (SCLC). Treatment with conventional x-ray therapy (XRT) is associated with high toxicity rates, particularly acute grade 3+ esophagitis and pneumonitis. We present outcomes for the first known series of limited-stage SCLC patients treated with proton therapy and a dosimetric comparison of lung and esophageal doses with intensity-modulated radiation therapy (IMRT). Material and methods. Six patients were treated: five concurrently and one sequentially. Five patients received 60–66 CGE in 30–34 fractions once daily and one patient received 45 CGE in 30 fractions twice daily. All six patients received prophylactic cranial irradiation. Common Terminology Criteria for Adverse Events, v3.0, was used to grade toxicity. IMRT plans were also generated and compared with proton plans. Results. The median follow-up was 12.0 months. The one-year overall and progression-free survival rates were 83% and 66%, respectively. There were no cases of acute grade 3+ esophagitis or acute grade 2+ pneumonitis, and no other acute grade 3+ non-hematological toxicities were seen. One patient with a history of pulmonary fibrosis and atrial fibrillation developed worsening symptoms four months after treatment requiring oxygen. Three patients died: two of progressive disease and one after a fall; the latter patient was disease-free at 36 months after treatment. Another patient recurred and is alive, while two patients remain disease-free at 12 months of follow-up. Proton therapy proved superior to IMRT across all esophageal and lung dose volume points. Conclusion. In this small series of SCLC patients treated with proton therapy with radical intent, treatment was well tolerated with no cases of acute grade 3+ esophagitis or acute grade 2+ pneumonitis. Dosimetric comparison showed better sparing of lung and esophagus with proton therapy. Proton therapy merits further investigation as a method of reducing the toxicity of CRT.
Small cell lung cancer (SCLC) accounts for about 15% of all lung cancers, 35% of which are limited-stage disease (LD-SCLC) [Citation1]. Adding early thoracic radiotherapy to chemotherapy (CTRT) in LD-SCLC has demonstrated improved outcomes with a median survival of 18–24 months [Citation2–4], while extensive-stage disease (ESD) has a poor prognosis with a median survival of 8–10 months [Citation5]. Concurrent chemoradiotherapy (CRT) is the current standard of care [Citation6–9] in LD-SCLC; however, irradiating large volumes with conventional radiotherapy planning techniques coupled with the use of concurrent chemotherapy leads to high toxicity rates. Acute grade 3+ radiation esophagitis and pneumonitis in these patients have been reported at approximately 30% and 5–10%, respectively [Citation6–9].
Dosimetric studies have demonstrated that patients with non-small cell lung cancer (NSCLC) receive less radiation dose to the esophagus and lung with proton therapy compared to x-ray therapy (XRT) [Citation10–13]. These studies have shown that these improvements exist with proton therapy over three-dimensional conformal radiotherapy (3DCRT) and/or intensity-modulated radiotherapy (IMRT) whether the target is the gross disease or if elective nodal irradiation (ENI) is included. Furthermore, early reports of proton therapy in stage III NSCLC have shown low rates of acute toxicity [Citation14–16] compared to IMRT. Proton therapy may therefore prove to be beneficial in reducing toxicity seen with radiation in SCLC, particularly to the lung and esophagus. We present the first known case series, to our knowledge, of SCLC patients treated with thoracic proton therapy, and we compare lung and esophageal doses with IMRT and proton radiotherapy treatment plans.
Material and methods
Under institutional review board approval, seven patients with LD-SCLC who had been treated with proton therapy between 2009 and 2012 were retrospectively identified from institutional records after having taken part in a study on proton therapy in lung cancer under which data was prospectively collected. One patient did not give informed consent and was excluded. All but one patient who refused underwent 18 fluoroudeoxyglucose (FDG)-positron emission tomography (PET) scanning as well as bronchoscopy, mediastinoscopy, and computed tomography (CT)-guided biopsy confirming small cell histology. Patients were staged as LD-SCLC using the Veterans classification [Citation1] as well as by the 7th edition of the American Joint Commission on Cancer TNM staging system for lung cancer in patients with SCLC [Citation17]. The TNM stage at presentation and tumor location for the six patients treated are shown in . All six patients were treated with radical intent; five patients received concurrent CRT and one was treated sequentially because of a questionable liver lesion at diagnosis that remained stable during chemotherapy.
Table I. Baseline characteristics and staging of the six patients undergoing treatment.
Chemotherapy
Cisplatin and etoposide were used for induction and concurrent chemotherapy. Dose reductions, if required, were instituted at the discretion of the treating medical oncologist.
Radiotherapy
Five patients received 60–66 CGE in 30–34 fractions once daily. One patient received 45 CGE in 30 fractions twice daily. ENI was delivered to doses ranging from 40–60 Gy, except in one patient with prior contralateral chest wall irradition for breast cancer five years prior who received involved-site radiation only. All patients received prophylactic cranial irradiation (PCI) following completion of thoracic CRT.
Treatment planning
Patients were immobilized in the supine position on a wing board and using posthand grips. A vacuum bag stabilized the legs and pelvic area while the arms and head were stabilized on the wing board using a smaller Vac Loc (Civco, Kalona IA, USA). Patient positioning ensured that a gap was present between the cranial and pelvic vacuum bags so that the bags did not obstruct the path of the proton beam. A 4D CT scan was performed with and without contrast (Phillips Brilliance, Phillips Medical Systems, Madison WI, USA). Patients were instructed to breathe normally during simulation scanning and no method of restricting respiratory motion was employed. The 10 respiratory-phase scans and their reconstructed averages were transferred to a MiMvista Work Station (MiMvista Corp, Cleveland, OH, USA). The gross tumor volume (GTV) was contoured using the end-of-inspiration non-contrast CT set with the assistance of the fused contrasted CT set. Deformable registration was used to propagate the GTV contour to all breathing phases and the combined contours were generated to form the internal gross tumor volume (IGTV). This was expanded by 0.6 cm to create a clinical target volume (CTV) which was edited to account for areas of overlap with bone or uninvolved chest wall. A further uniform expansion of 0.5 cm was applied to the CTV to create the planning target volume (PTV) based on previous institutional work in NSCLC [Citation18]. The nearest uninvolved nodal echelons to the CTV were contoured and ENI was delivered to doses between 46 and 60 CGE.
The average 4D CT non-contrast set was used for plan optimization and dose calculation. Target volumes and normal structure volumes were imported into an Eclipse proton treatment planning system (Varian Medical Systems, Palo Alto, CA, USA) and a 3D conformal proton therapy plan was designed using two to four beams. Beam orientations were chosen to minimize dose distribution uncertainties to both the target volumes and critical normal structures, so that no beams were planned to stop immediately proximal to the spinal cord.
The Hounsfield units in the IGTV over 50 (for muscle and solid soft tissue) in the treatment planning system were required to be overridden: the Hounsfield numbers for pixels in the GTV represents an average of normal lung tissue and tumor tissue; therefore, for range-modification calculations and compensator designs, this was done to ensure adequate beam range regardless of tumor position within the IGTV. Dose to critical structures, however, was calculated without using this override and distal margins were applied to the CTV to account for range uncertainty. The margins comprised two components: a CT Hounsfield units-to-proton- stopping-power conversion table uncertainty and an additional component accounting for other range uncertainties, including equipment delivery variations [Citation19]. The estimated range uncertainty due to the accuracy of the CT numbers-to-proton-stopping-power conversion was calculated to be 2.5% of the maximum CTV water-equivalent depth. A standard factor of 0.15 cm included equipment delivery variations derived from in-house quality assurance procedures that documented average and maximum deviations (0.02 cm and 0.15 cm, respectively) from the prescribed range in water phantoms. The proximal margins were the same as the distal margins or larger when the depth of the target was noted to increase with organ motion. For lung treatment fields with approximately 15-cm beam ranges, the distal and proximal margins would be a 0.6-cm water-equivalent distance and this would correspond to a significantly higher physical distance when scaled by the lower stopping power of the lung. The block aperture margins to compensate for penumbra effects were expanded from the PTV and varied by depth from 0.7 cm to 1.2 cm. Margins and blocks were occasionally modified for clinical reasons. Finally, a smearing factor was included in the compensator design and the final range prescription to compensate for variations in beam range related to both intra- and inter-fraction motion of structures in the beam path preceding the target, and for inaccuracies in modeling the range of impact of sharp gradients in the compensator. The smearing adjustment to the range prescription was generally 0.8 cm but increased for fields with a relatively long range.
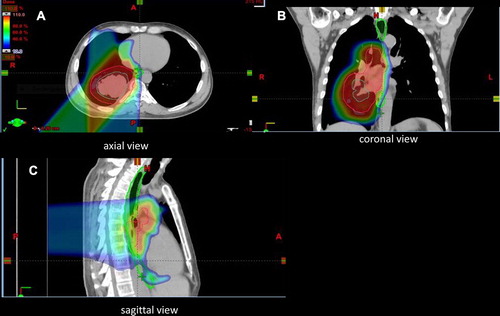
All treatment plans aimed to meet stringent guidelines for target coverage (CTV D99% = 100%) as well as protection of organs at risk including lung V20 < 37% (goal < 30%) and mean lung dose < 20 CGE (goal < 18 Gy); esophagus mean dose < 35 CGE; and spinal cord V50 ≤ 0.1 cm3. All fields were treated each day and verification 4D CT scans were performed weekly or biweekly during treatment to confirm that the proton plan was not significantly affected by minor day-to-day setup errors or treatment-related changes to the tumor or normal tissue. Replanning was performed if target coverage was compromised or if the dose to a critical normal structure was exceeding institutional guidelines. A representative treatment plan of thoracic radiation is seen in .
Dosimetric comparison
The contoured volumes used to create the initial proton plans for all six patients at the time of treatment were used to create IMRT plans for comparison. IMRT plans were generated using a Pinnacle treatment planning system (Philips Electronics, Amsterdam, The Netherlands) with an average of seven fields. The plans were reviewed by the same radiation oncologist to ensure optimal target coverage with minimal dose to organs at risk. Dose-volume histograms were then generated and the relevant percentage lung and esophageal dose levels were recorded. For the purposes of dose comparison, all plans were normalized to 70 Gy. The median percentage for each dose level (v5, 10, 20, 30, 40, 50 and 60 for the lung and v5, 10, 20, 30, 40, 50, and 60 for the esophagus) were calculated as was the mean lung dose and mean esophageal dose.
Toxicity
Patients were routinely evaluated by a physician at the start of treatment, weekly during treatment, one month after treatment, and then every three months for possible treatment-related toxicity and disease response. Toxicities were graded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 3.0, and coded until the date of disease progression or last follow-up. Patient median follow-up time, recurrence-free survival, and overall survival were calculated from the date of diagnosis. In view of the small numbers, simple descriptive statistics were used.
Results
The median age of the six patients treated was 70 years (range 52–76 years) and the median follow-up was 12.0 months (range 7.6–40.6 months). Progression-free survival at 12 months was 66%. Overall survival at one year of follow-up was 83%. The median elapsed treatment time was 42 days and ranged from 22 days (for a patient treated twice daily) to 51 days.
Three patients are currently alive and two are disease-free (). Of the three patients who died, one died of complications following a mechanical fall 36 months after treatment. Magnetic resonance imaging (MRI) brain and CT chest scans showed no evidence of cancer at the time of death. Two patients died of progressive metastatic disease without local progression. Both of these patients had questionable metastatic lesions at initial diagnosis, which were ultimately the site of failure in these patients. This included a patient that developed bone metastases one month after completing concurrent chemotherapy and proton therapy (and died four months later) and another patient who developed liver metastases six months after completing sequential chemotherapy followed by PT (and died two weeks later). One patient is alive, but was found to have a local recurrence and distant bone and adrenal gland metastases 14 months after initial treatment with 45 Gy twice daily. The other two patients are alive and remain disease-free at 12 months of follow-up.
Acute toxicity
Acute treatment toxicity (defined as occurring less than 90 days from the start of proton therapy) is shown in . There were four cases of grade 2 esophagitis (treated with viscous lidocaine) and no cases of grade 2+pneumonitis. One patient developed grade 2 vomiting with grade 2 esophagitis and weight loss in the final week of chemoradiation and required < 24-hour IV hydration and antiemetics due to the vomiting. There were no recorded cases of grade 3+ pneumonitis or esophagitis or any other grade 3+non-hematological toxicities.
Table II. Baseline and acute toxicity maximum grading by Common Terminology Criteria for Adverse Events for six patients undergoing radical radiation treatment (4 concurrent; 2 sequential).
Late toxicity
Late toxicity data was defined as occurring later than 90 days from the start of treatment and was available in five of six treated patients. One patient (patient 5 in ) with preexisting pulmonary fibrosis and atrial fibrillation developed increasing shortness of breath four months after treatment, requiring oxygen and steroids. This patient subsequently developed grade 2 pulmonary fibrosis and fatigue as well as steroid-induced myopathy. This patient's mean lung dose was only 13.9 CGE and V20 was only 24.4%. Another patient developed grade 2 dyspnea (difficulty walking a city block) and grade 2 esophageal stenosis requiring dilatation. This patient had a history of esophageal stenosis prior to his treatment and had also undergone esophageal dilatations. All other late toxicities were grade 1 or less.
Dosimetric comparison
Proton therapy proved superior across all lung and esophageal dose levels. The median lung V5–V60 and esophageal V5–V60 are shown in . The median of the mean lung dose for all six patients was 18.6 Gy (range, 11.6–23.5 Gy) for IMRT versus 12.3 Gy (range, 7.0–18.5 Gy) for proton therapy. The median lung V5 with IMRT was 57% (range, 33–86%) compared to lung V5 of 35% (range, 19–62%) for proton therapy. The median of the percentage differences for V5 between IMRT and proton therapy for all six patients was 17%. The magnitude of the median of the percentage difference between IMRT and proton therapy was greater for lower-dose volumes. While this trend continued across increasing dose levels from V10 TO V60, the magnitude of the median difference decreased with increasing dose level (10% for V10 versus 2% for V60).
Table III. Dosimetric comparison of IMRT to Protons for 6 patients.
With regards to esophageal toxicity, proton therapy was again superior with a greater percentage of difference in median for protons versus IMRT seen at lower dose levels (10% for V5 versus 1% for V60). Nevertheless, the absolute percentage difference was smaller than that seen with the lung. The median of the mean esophageal dose was 19.7 Gy (range, 6–35.5 Gy) for IMRT versus 18.1 Gy (range, 0.8–36 Gy) for proton therapy.
Discussion
We report the first known series of LD-SCLC patients treated with chemotherapy and proton beam therapy. The reported outcomes show good tumor control to date and an acceptable side effect profile with no grade 3 + non-hematological toxicity in patients treated with radical intent.
Of the patients who failed therapy, two recurred in sites suspicious for metastatic disease on pretreatment imaging and, therefore, it is likely that the treatment failures were a result of preexisting metastatic disease at diagnosis rather than a failure to control the primary disease through CRT. Unfortunately, these sites were either too small to sample or presented in a location that was too difficult to biopsy. As two patients are alive and disease-free and another was disease-free at the time of death from a fall, to date, only one patient (treated twice daily) failed locally following CRT.
When treating the primary tumor, proton therapy allows for a reduced radiation dose to critical structures compared with 3D conformal radiotherapy or IMRT. In LD-SCLC, traditionally large treatment fields were employed; as a result, irradiated lung volumes with conventional planning techniques may be high. In the landmark Turissi trial [Citation8], the target volume for thoracic radiotherapy included the gross tumor and the bilateral mediastinal and ipsilateral hilar lymph nodes with the inferior border extending 5 cm below the carina or to a level including the ipsilateral hilar structures, whichever was lower. With the advent of modern radiotherapy and imaging techniques, treatment volumes now tend to be smaller and some authors advocate omitting ENI altogether [Citation20–24]. Despite this, rates of grade 3+ esophagitis and pneumonitis continue to be as high as 30% and 5%–10%, respectively, [Citation6–9] with photon-based radiation therapy.
Many patients presenting with LD-SCLC exhibit poor performance status and, as a result, are unable to tolerate CRT and its associated toxicities. In reducing toxicity, proton therapy might possibly allow for more patients to be treated with CRT in the future than might otherwise have been possible using x-ray-based therapy.
In a review of particle-based therapy in NSCLC, Liao [Citation25] concluded that retrospective and single-arm studies suggested that proton therapy had a better toxicity profile in NSCLC compared to IMRT. As shown in our dosimetric comparison between proton therapy and IMRT, proton therapy is especially beneficial in reducing low-dose exposure of normal lung compared to IMRT. This reduction in a low-dose tissue bath may explain the lack of acute grade 3+ esophagitis and pneumonitis seen in our study.
In a study investigating risk factors for radiation pneumonitis in locally advanced NSCLC, Giroux Leprier et al. [Citation26] found that acute radiation pneumonitis was significantly associated with non-tumor lung volumes irradiated to 13, 20, and 25 Gy as well as mean lung dose. By reducing low-dose radiation exposure of normal lung, proton therapy may prove superior to IMRT in reducing rates of acute pneumonitis. This hypothesis may also be applicable to radiation esophagitis. In a study into the predictors of high-grade esophagitis involving NSCLC patients treated with 3D-conventional radiotherapy, IMRT, and proton therapy, patients with proton therapy had lower rates of acute grade 3+ esophagitis (28% for IMRT vs. 6% for protons) [Citation27], which the authors suggested could be attributable to the low-dose bath to the esophagus with IMRT.
In our current retrospectively analyzed series, the acute toxicity rates in radically treated patients compare well both with previous larger CRT studies in LD-SCLC [Citation6–9] and with studies in NSCLC using proton therapy and concurrent chemotherapy in which only 5% of patients developed grade 3 esophagitis and 2% developed grade 3+ pneumonitis [Citation14–16]. Although our patient numbers are limiting, the absence of acute grade 3+ toxicity (specifically grade 3+ esophagitis and grade 2+ pneumonitis) suggest that proton therapy may merit further investigation in these patients as a method of reducing radiation therapy-related toxicity.
National comprehensive cancer network (NCCN) guidelines [Citation28] state that the optimal dose and schedule of radiotherapy for LD-SCLC has not been established. For twice-daily radiotherapy, the NCCN guidelines recommend a dose of 45 Gy in three weeks while the recommended schedule for once-daily radiotherapy is a total dose of 60–70 Gy in 2 Gy per fraction. Concurrent treatment is recommended over sequential. Patients in our series received a variety of doses and scheduling based on physician preference; development of a prospective study protocol with standardized radiation doses would be helpful in addressing the optimal dose and fractional schedule through future research.
Previously reported results in NSCLC from our institution have demonstrated improved dosimetry with proton therapy compared to IMRT and conformal plans when including elective nodes to 44 Gy and when excluding ENI in stage III lung cancer [Citation13,Citation15,Citation29–31]. These dosimetric findings might be more relevant in the setting of SCLC, where some people still believe there is a benefit to ENI. Incorporating ENI can increase irradiated lung and esophageal volumes using conventional x-ray therapy and increase the risk of developing acute grade 3+ pneumonitis and esophagitis. Indeed, the dosimetric advantage of proton therapy in reducing the volume of irradiated lung and esophagus may explain the lack of acute severe pneumonitis and esophagitis seen in our case series despite the inclusion of ENI.
Long-term follow-up in a larger study population to evaluate the effect of reduced volumes of irradiated lung using proton therapy in LD-SCLC is needed, especially to examine rates of both acute and late radiation pneumonitis and esophagitis. It was not possible to gather complete hematological toxicity data in our patients although there were no reported incidences of severe acute or late hematological toxicity.
Conclusions
We report the first known series of SCLC patients treated with proton therapy. There were no cases of grade 2+ acute pneumonitis or grade 3+ esophagitis in patients treated with radical intent and there did not seem to be any detriment with regards to outcome. Proton therapy also demonstrated better sparing of lung and esophagus in a dosimetric comparison with IMRT. Although this is a small series, the results would suggest that proton therapy merits further exploration in SCLC to reduce treatment-related toxicity, particularly in patients undergoing concurrent treatment.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Stahel R, Ginsberg R, Havemann K, Hirsch FR, Ihde DC, Jassem J. Staging and prognostic factors in small cell lung cancer: A consensus report. Lung Cancer 1989;5:119–26.
- De Ruysscher D, Pijls-Johannesma M, Vansteenkiste J, Kester A, Rutten I, Lambin P. Systematic review and meta-analysis of randomised, controlled trials of the timing of chest radiotherapy in patients with limited-stage, small-cell lung cancer. Ann Oncol 2006;17:543–52.
- Fried DB, Morris DE, Poole C, Rosenman JG, Halle JS, Detterbeck FC, . Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage small-cell lung cancer. J Clin Oncol 2004;22:4837–45.
- Huncharek M, McGarry R. A meta-analysis of the timing of chest irradiation in the combined modality treatment of limited-stage small cell lung cancer. Oncologist 2004;9: 665–72.
- Baka S, Califano R, Ferraldeschi R, Aschroft L, Thatcher N, Taylor P, . Phase III randomised trial of doxorubicin-based chemotherapy compared with platinum-based chemotherapy in small-cell lung cancer. Br J Cancer 2008; 99:442–7.
- Jeremic B, Shibamoto Y, Acimovic L, Milisavljevic S. Initial versus delayed accelerated hyperfractionated radiation therapy and concurrent chemotherapy in limited small-cell lung cancer: A randomized study. J Clin Oncol 1997;15: 893–900.
- Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S, . Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: Results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol 2002;20:3054–60.
- Turrisi AT, 3rd, Kim K, Blum R, Sause WT, Livingston RB, Komaki R, . Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. New Engl J Med 1999;340:265–71.
- Bonner JA, Sloan JA, Shanahan TG, Brooks BJ, Marks RS, Krook JE, . Phase III comparison of twice-daily split-course irradiation versus once-daily irradiation for patients with limited stage small-cell lung carcinoma. J Clin Oncol 1999;17:2681–91.
- Grutters JP, Kessels AG, Pijls-Johannesma M, De Ruysscher D, Joore MA, Lambin P. Comparison of the effectiveness of radiotherapy with photons, protons and carbon-ions for non-small cell lung cancer: A meta-analysis. Radiother Oncol 2010;95:32–40.
- Nakayama H, Sugahara S, Tokita M, Satoh H, Tsuboi K, Ishikawa S, . Proton beam therapy for patients with medically inoperable stage I non-small-cell lung cancer at the university of tsukuba. Int J Radiat Oncol Biol Phys 2010;78:467–71.
- Chang JY, Zhang X, Wang X, Kang Y, Riley B, Bilton S, . Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in Stage I or Stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006;65:1087–96.
- Nichols RCJ, Huh SH, Henderson RH, Zuofeng L, Flampouri S, D’Agostino HJ, . Selective nodal irradiation of regionally advanced non-small-cell lung cancer with proton therapy and IMRT: A dosimetric comparison. Thorac Cancer 2012;3:169–74.
- Oshiro Y, Mizumoto M, Okumura T, Hashimoto T, Fukumitsu N, Ohkawa A, . Results of proton beam therapy without concurrent chemotherapy for patients with unresectable stage III non-small cell lung cancer. J Thorac Oncol 2012;7:370–5.
- Hoppe BS, Flampouri S, Henderson RH, Pham D, Bajwa AA, D’Agostino H, . Proton therapy with concurrent chemotherapy for non-small-cell lung cancer: Technique and early results. Clin Lung Cancer 2012;13: 352–8.
- Chang JY, Komaki R, Lu C, Wen HY, Allen PK, Tsao A, . Phase 2 study of high-dose proton therapy with concurrent chemotherapy for unresectable stage III nonsmall cell lung cancer. Cancer 2011;117:4707–13.
- TNM staging for lung cancer. AJCC Cancer Staging Manual, 7th ed. Chicago, IL: Springer; 2010.
- Yeung AR, Li J, Shi W, Newlin H, Morris CG, Samant S, . Optimal image-guidance scenario with cone- beam computed tomography in conventionally fractionated radiotherapy for lung tumors. Am J Clin Oncol 2010;33: 276–80.
- Kang Y, Zhang X, Chang JY, Wang H, Wei X, Liao Z, . 4D Proton treatment planning strategy for mobile lung tumors. Int J Radiat Oncol Biol Phys 2007;67:906–14.
- De Ruysscher D, Bremer RH, Koppe F, Wanders S, van Haren E, Hochstenbag M, . Omission of elective node irradiation on basis of CT-scans in patients with limited disease small cell lung cancer: A phase II trial. Radiother Oncol 2006;80:307–12.
- Baas P, Belderbos JS, Senan S, Kwa HB, van Bochove A, van Tinteren H, . Concurrent chemotherapy (carboplatin, paclitaxel, etoposide) and involved-field radiotherapy in limited stage small cell lung cancer: A Dutch multicenter phase II study. Br J Cancer 2006;94:625–30.
- van Loon J, De Ruysscher D, Wanders R, Boersma L, Simons J, Oellers M, . Selective nodal irradiation on basis of (18)FDG-PET scans in limited-disease small-cell lung cancer: a prospective study. Int J Radiat Oncol Biol Phys 2010;77: 329–36.
- Shirvani SM, Komaki R, Heymach JV, Fossella FV, Chang JY. Positron emission tomography/computed tomography-guided intensity-modulated radiotherapy for limited- stage small-cell lung cancer. Int J Radiat Oncol Biol Phys 2012;82:e91–7.
- Colaco R, Sheikh H, Lorigan P, Blackhall F, Hulse P, Califano R, . Omitting elective nodal irradiation during thoracic irradiation in limited-stage small cell lung cancer – evidence from a phase II trial. Lung Cancer 2012;76:72–7.
- Liao Z, Lin SH, Cox JD. Status of particle therapy for lung cancer. Acta Oncol 2011;50:745–56.
- Giroux Leprieur E, Fernandez D, Chatellier G, Klotz S, Giraud P, Durdux C. Non-small cell lung cancer: Risk factors of radiation pneumonitis. Cancer Radiother 2012;16: 257–62.
- Gomez DR, Tucker SL, Martel MK, Mohan R, Balter PA, Lopez Guerra JL, . Predictors of high-grade esophagitis after definitive three-dimensional conformal therapy, intensity-modulated therapy, or proton beam therapy for non-small cell lung cancer. Int J Rad Oncol Biol Phys 2012;84: 1010–6.
- Kalemkerian GP, Akerley W, Bogner P, Borghaei H, Chow LQ, Downey RJ, . Small cell lung cancer. JNCCN 2013;11:78–98.
- Nichols RC, Huh SN, Henderson RH, Mendenhall NP, Flampouri S, Li Z, . Proton radiation therapy offers reduced normal lung and bone marrow exposure for patients receiving dose-escalated radiation therapy for unresectable stage III non-small-cell lung cancer: a dosimetric study. Clin Lung Cancer 2011;12:252–7.
- Nichols RC, Huh SH, Hoppe BS, Henderson RH, Li Z, Flampouri S, . Protons safely allow coverage of high-risk nodes for patients with regionally advanced non-small-cell lung cancer. Technol Cancer Res Treat 2011;10:317–22.
- Nichols RC, Henderson RH, Huh S, Flampouri S, Zuofeng L, Bajwa AA, . Proton therapy for lung cancer. Thorac Cancer 2012;3:109–16.