Abstract
Background. Patients receiving chemotherapy for cancer are at increased risk for venous thromboembolism (VTE). We performed a meta-analysis of all randomized controlled trials (RCTs) which evaluated low molecular weight heparin (LMWH) as primary prophylaxis in ambulatory patients with solid malignancies.
Methods. A comprehensive search was conducted until October 2013. Primary outcome was symptomatic VTE. Secondary outcomes were pulmonary embolism (PE), any VTE, deep vein thrombosis (DVT), mortality and adverse events.
Results. Eleven trials met the inclusion criteria, and evaluated a total of 6942 patients. Primary prophylaxis with LMWH reduced symptomatic VTE (RR 0.46, 95% CI 0.32–0.67) and the rate of PE (RR 0.49, 95% CI 0.29–0.84). In the subgroup analysis of VTE in patients with lung and pancreatic cancers LMWH further reduced VTE [RR 0.42 (95% CI 0.25–0.71); RR 0.31 (95% CI 0.18–0.55), respectively]. Meta-analysis of six trials which reported survival outcomes revealed no statistically significant benefit for LMWH in one-year mortality rates (RR 0.93, 95% CI 0.83–1.04). There was no significant increase in major bleeding events (RR 1.28, 95% CI 0.84–1.95).
Conclusions. LMWH reduces the incidence of symptomatic VTE and PE in patients receiving chemotherapy for cancer, with no apparent increase in major bleeding. The benefit is most apparent in pancreatic cancer and also lung cancer. VTE prophylaxis should be considered for these specific populations.
Venous thromboembolism (VTE) is one of the principal causes of morbidity and mortality in cancer patients. It occurs in 4–20% of cancer patients, and it is one of the leading causes of deaths [Citation1,Citation2]. The risk of venous thromboembolic events, including pulmonary embolism (PE) and deep vein thrombosis (DVT) in cancer patients varies according to disease-specific factors, such as site, stage, and type of malignancy [Citation3,Citation4]. The incidence of VTE is higher in pancreas, stomach and lung cancers [Citation5]. VTE risk is further increased by anti-cancer therapies, with significant increases in VTE in cancer patients treated with chemotherapy and hormonal-therapy [Citation6–8]. Several studies have implied that low molecular heparin (LMWH) may confer anti-neoplastic properties, such as inhibiting pathways of angiogenesis, cellular adhesion, and tumor invasion and by inducing apoptosis and hence may affect survival not only by reducing VTE [Citation9–12].
Several studies have evaluated the role of LMWH as primary thromboprophylaxis in cancer patients, yielding inconsistent results. The general recommendation established by four organizations [American College of Chest Physicians (ACCP), American Society of Clinical Oncology (ASCO), National Comprehensive Cancer Network (NCCN) and European International Good Clinical Practices Guidelines (GCPG)] is that routine pharmacologic thromboprophylaxis is not currently recommended in cancer outpatients, based upon moderate evidence. Nevertheless, recent ASCO guidelines recommend considering primary LMWH prophylaxis based on a case-by-case basis in highly selected outpatients with solid tumors receiving chemotherapy [Citation5,Citation13,Citation14].
In view of the conflicting data from randomized clinical trials, and due to the different biological course and mechanism of certain malignancies, which may result in higher efficacy for LMWH in some subpopulations, we performed a systematic review of the literature and a meta-analysis of all randomized trials to evaluate the impact of LMWH primary prophylaxis on VTE incidence as well as survival in cancer patients. Moreover, we aimed to assess subpopulations of specific malignancies considered as high risk for developing VTE.
Methods
Data sources
We searched the Cochrane Central Register of Controlled Trials, published in The Cochrane Library, PubMed (1966–Oct 2013); the database of clinical trials in cancer patients; conference proceedings of the American Society of Clinical Oncology (1995–Oct 2013), American Society of Hematology (2006–Oct 2013), proceedings of the European Society of Medical Oncology (ESMO) (2006–Oct 2013) and the European Hematology Association (EHA) (2006–Oct 2013); and databases of ongoing and unpublished trials: http://www.clinicaltrials.gov and http://www.clinicaltrials.nci.nih.gov. The terms (tumor OR malign* OR carcinoma* OR cancer) AND (heparin OR low-molecular weight heparin OR enoxaparin OR dalteparin OR reviparin OR certoparin OR tinzaparin OR bemiparin OR nadroparin OR *parin) AND (thromboembolism) were cross-searched. The result was limited to randomized controlled trials using a highly sensitive filter [Citation15]. We scanned references of all included trials and reviews identified for additional studies.
Study selection
We included randomized controlled trials that compared the addition of LMWH to standard chemotherapy in ambulatory cancer patients, as primary thromboprophylaxis. We included trials regardless of publication status, date of publication, and language. Two authors (IBA and AG) independently inspected each reference title identified by the search and applied the inclusion criteria.
Data extraction and quality assessment
Trials that fulfilled the inclusion criteria were assessed for methodological quality by two authors (IBA and AG). Both reviewers independently assessed risk of bias in the included trials. We used the Cochrane Collaboration's tool for assessing risk of bias. We individually assessed the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data reporting and selective outcome reporting. We separately assessed each domain and graded it as low risk for bias, unclear risk (lack of information or uncertainty over the potential for bias), or high risk for bias according to the criteria specified in the Cochrane Handbook version 5.1.0 [Citation15]. The same two authors independently extracted the data from publications of included trials. The data extraction was discussed, decisions were documented, and, if necessary, the authors of the trials were contacted for clarification. Authors of included trials were contacted for all data relevant to the primary and secondary outcomes (VTE, survival and safety data) of the study and quality variables. In case of several publications for the same trial, the most updated one was extracted. Safety outcomes were pooled from the most updated publication of every trial.
Outcome measures
The primary outcome was symptomatic VTE, which was defined according to a unified definition in all included trials. Secondary outcomes were any VTE, PE, DVT, all-cause mortality, and toxicity (defined as grade 3 or 4 hematological and non-hematological adverse events). Regarding bleeding, we extracted data regarding major bleeding and clinically relevant bleeding (defined as major plus minor bleeding).
Data synthesis and statistical analysis
Risk ratios (RRs) and 95% confidence intervals (CIs) for dichotomous data were estimated using the Mantel-Haenszel method and pooled according to inverse of variance method [Review Manager (RevMan), version 5.1 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011)]. A RR less than 1.0 was in favor of LMWH therapy. We assessed heterogeneity of trial results by calculating a χ2-test of heterogeneity and the I2 measure of inconsistency. We chose a random-effects model and used the Der Simonian and Laird method for all analyses due to different types of cancer and therefore different VTE risks [Citation16]. Subgroup analyses were performed for lung cancer and pancreatic cancer, which are regarded as cancers with a high thrombogenic potential.
For calculating number needed to treat (NNT) in order to evaluate the additive effect of LMWH on the absolute risk for VTE, we retrieved the data on in each arm (LMWH vs. control). Risk was calculated by multiplying the absolute risk of the control arm by (1-RR for VTE/PE/DVT).
Results
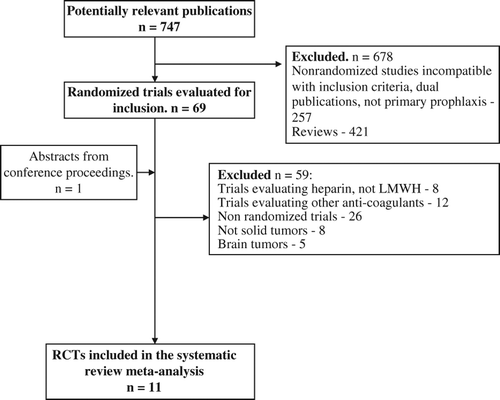
The literature search identified 747 trials up to October 2013, of which 69 were considered potentially relevant. Additional trials were identified by searching conference proceedings and electronic resources of ongoing trials. illustrates the process of study selection. Eleven trials were designed to evaluate the effect of primary LMWH prophylaxis on cancer-related VTE and fulfilled the inclusion criteria for published studies [17–27; including safety reports]. The TOPIC trial was analyzed separately for breast cancer patients and for lung cancer patients [Citation26,Citation27].
Studies characteristics
A total of 6942 patients were randomly assigned in the 11 trials included in the meta-analysis for VTE. Not all 11 reported the same outcomes, hence analysis has been performed upon specific outcome. One trial was in the form of an abstract (CONKO004, 22). Six studies [Citation17–20,Citation23,Citation24] were included in the all-cause mortality analysis (12 months) and evaluated 2550 patients.
Trial design
In three trials [Citation19,Citation21,Citation23] patients were randomly assigned to nadroparin in addition to standard-of-care therapy. Four trials used dalteparin [Citation17,Citation18,Citation20,Citation25], two trials evaluated certoparin [Citation26,Citation27], one trial used enoxaparin [Citation22] and one semuloparin [Citation24]; patient population varied as reflected in .
Table I. Characteristics of studies included in the meta-analysis.
Quality of trials
Allocation concealment was reported as adequate in seven trials [Citation18,Citation19,Citation21,Citation23,Citation25,Citation26] and was not reported in the other four trials. Few of the trials have been open-labeled. The quality assessment of the included trials is described in detail in Supplementary Table I (available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.934397). We appraised the rate of patients loss to follow-up and in the majority of the studies the rate was < 10%. In two trials the rate was higher (range 19–42%).
Venous thromboembolism
The numbers of randomly assigned and analyzed patients in each included trial are described in . Seven trials reported on the primary outcome of our meta-analysis, symptomatic VTE. LMWH significantly reduced symptomatic VTE [RR 0.46 (95% CI 0.32–0.67, I2–0.6%)] as presented in . Ten trials (6942 patients) were eligible for meta-analysis of any VTE [Citation17–19,Citation21–27]. LMWH significantly reduced any VTE [RR 0.56 (95% CI 0.38–0.81, I2 – 36%)]. We specifically appraised the risk for DVT and PE. LMWH significantly reduced symptomatic DVT [RR 0.35 (95% CI 0.21–0.61)] and PE [RR 0.49 (95% CI 0.29–0.84)] as presented in and . In a subgroup analysis of VTE in patients with lung cancer LMWH further reduced VTE [RR 0.42 (95% CI 0.25–0.71)], as depicted in . The striking effect of LMWH on VTE reduction has been documented in the subgroup of patients with pancreatic cancer [RR 0.31 (95% CI 0.18–0.55)]. VTE analyses are depicted in . Sensitivity analysis according to risk of bias, and specifically according to allocation concealment showed similar results for VTE reduction in both trials of low risk for bias (RR 0.67, 95% CI 0.49–0.93) and those of high risk (RR 0.33, 95% CI 0.21–0.52).
Figure 2. Forest plot of risk ratios (RRs) comparing (A) Symptomatic venous thromboembolism (VTE) (B) Deep vein thrombosis symptomatic (DVT) and (C) pulmonary embolism for patients who received LMWH in addition to standard therapy versus those who received standard therapy only. Risk ratios for each trial are represented by the squares, the size of the square represents the weight of the trial in the meta-analysis, and the horizontal line crossing the square represents the 95% confidence interval (CI). The diamonds represent the estimated overall effect based on the meta-analysis random effects of all trials.
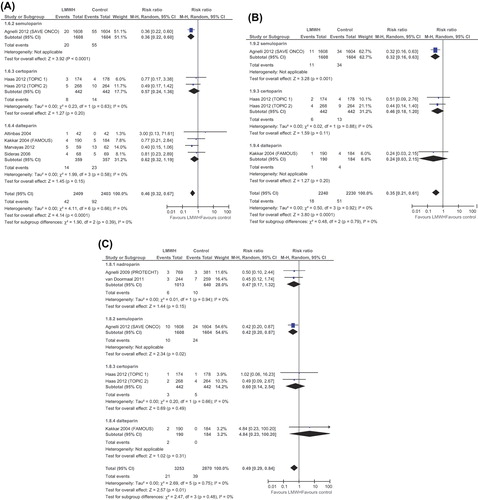
Figure 3. Forest plot of risk ratios (RRs) comparing (A) venous thromboembolism (VTE) in lung cancer patients and (B) in pancreatic cancer patients who received LMWH in addition to standard therapy versus those who received standard therapy only. Risk ratios for each trial are represented by the squares, the size of the square represents the weight of the trial in the meta-analysis, and the horizontal line crossing the square represents the 95% confidence interval (CI). The diamonds represent the estimated overall effect based on the meta-analysis random effects of all trials.
All-cause mortality
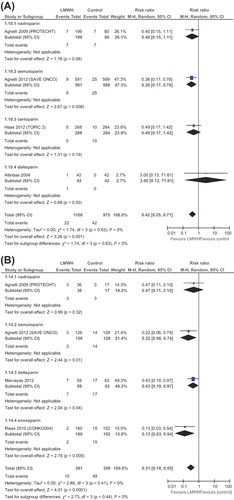
Six trials (2550 patients) reported on all-cause mortality. All trials reported mortality rates at 12 months of follow-up; five trials reported mortality at six months and four trials reported it also at 24 months. LMWH had no significant effect on survival (at 12 months [RR 0.93 (95% CI 0.83–1.04)]. RR for mortality at other time points resembled this figure. Survival RRs are depicted in . We lacked the mortality data for subgroup of lung cancer patients and pancreatic cancer patients.
Figure 4. Forest plot of risk ratios (RRs) comparing (A) mortality at 6 months or (B) 12 months for patients who received LMWH in addition to standard therapy versus those who received standard therapy only. Risk ratios for each trial are represented by the squares, the size of the square represents the weight of the trial in the meta-analysis, and the horizontal line crossing the square represents the 95% confidence interval (CI). The diamonds represent the estimated overall effect based on the meta-analysis random effect of all trials.
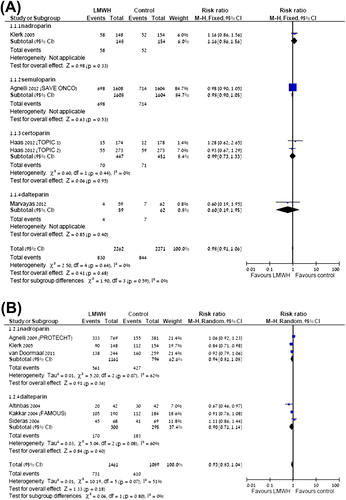
Type of LMWH
There were no significant variations in the effect of the different members of the LMWH utilized in the included trials in any of the outcomes.
Adverse events
The rate of grade 3 or 4 adverse events was reported in nine trials, evaluating 6595 patients. There was no significant increase in either the rate of clinically relevant bleeding [RR 1.29 (95% CI 0.95–1.77)], nor in major bleeding events [RR 1.28 (95% CI 0.84–1.95)]. There was no significant increase in thrombocytopenia [RR 1.05 (95% CI 0.76–1.45)].We could not conduct subgroup analysis for bleeding according to type of malignancy since data were not provided separately.
Discussion
The results of our meta-analysis indicate that administration of LMWH as primary thromboprophylaxis to cancer patients concomitantly with standard chemotherapy significantly reduces the risk for symptomatic VTE, any VTE and PE, while the risk for major bleeding is not significantly increased. In a subgroup analysis of pancreatic cancer and lung cancer LMWH reduced the RR for VTE even further [0.28 (95% CI 0.16–0.49) for pancreatic cancer] and [0.43 (95% CI 0.26–0.71) for lung cancer]. LMWH had no effect on survival in meta-analysis of all trials.
Based on our meta-analysis the NNT to prevent one symptomatic VTE is 50 (95% CI 33–100). Among lung cancer patients the NNT to prevent one VTE is 33 (95% CI 25–100) and in pancreatic cancer the NNT to prevent one VTE is 10 patients (95% CI 7–16), indicating a substantial benefit for LMWH in this subpopulation. The rate of serious adverse events was low, with the number needed to harm (NNH) being 100 (95% CI 50–very large number) for clinically relevant bleeding. The RR for major bleeding events was not greater compared with the control arm and neither the rate of thrombocytopenia. We could not infer based upon the analysis whether a specific LMWH exhibit superior results compared with other agents of this group, it is therefore probably safe to assume there is a class effect.
The rationale for primary thromboprophylaxis in cancer patients arises from the marked risk of cancer-associated VTE. Population-based case-control studies indicate a two-year cumulative incidence of 0.6–7.8%, depending on the population studied [Citation28,Citation29]. The risk for VTE depends profoundly on the primary site of cancer, whereas pancreatic, gastric and lung cancers confer the highest risk to develop VTE [Citation30]. Lung and cardiac comorbidities which are frequent in lung cancer patients increase the risk of VTE by 20% [Citation31,Citation32]. Former studies indicate that the incidence of VTE is highest within the first six months of commencing the anti-cancer treatment [Citation2].
Our meta-analysis did not show a survival advantage. Several studies have indicated that selected populations may gain a survival advantage from LMWH prophylaxis, whereas the LMWH benefit was most apparent among patients with a better prognosis. Some other considerations that may impact survival analysis include the short length of follow-up: in some of the studies in a subgroup analysis, the good-prognosis group of patients experienced a superior survival with LMWH [Citation18,Citation19]. Another determinant is the effect of LMWH in different tumor types and disease stages. The majority of studies included in the meta-analysis encompass a variety of tumor types whereas the biological role of LMWH may differ in distinctive cancers. Due to the main role of coagulation pathways in pancreatic and lung cancer, the potential benefit of concomitant administration of LMWH may be enhanced in these cancers, as we have shown in this meta-analysis in terms of VTE reduction in these entities.
Several limitations of this analysis must be acknowledged. The heterogeneity of cancer types and disease stages in some of the studies may attenuate the impact of LMWH, especially on survival outcomes. We could only conduct subgroup analysis for VTE reduction for lung and pancreatic cancers, but not other types of malignancies. Moreover, there were various LMWHs used and in different dosages. The short follow-up is a flaw of the included studies. As mentioned, post hoc analysis of longer follow-up in some of the studies revealed a survival benefit in specific populations, yet meta-analysis of these figures was not feasible.
Future research should focus on identifying risk factors that predispose patients to develop VTE in order to define the subgroup of patients that would benefit the most from the addition of LMWH to the treatment regimen, considering cancer type and stage. The type of LMWH preparation and the optimal prophylactic dosage should be further explored. Future study design should be powered to evaluate a survival endpoint, but also quality of life parameters in patients for whom expected survival is poor. In addition, nowadays new oral anticoagulants (NOAC) are available. They are both convenient due to the oral administration and safe. These agents have been extensively studied for prophylaxis of acute VTE, long-term anticoagulation for atrial fibrillation, and acute coronary syndromes [Citation33]. Future trials should assess their role in prophylaxis in ambulatory cancer patients.
Cost effectiveness analysis should be appraised as well, since most of the included studies did not report hospitalization rate and cost. It is important to investigate whether specific LMWHs are superior to other agents since there are data suggesting that some of the antitumor effects of LMWH are dependent on molecular weight.
In conclusion, our meta-analysis shows a significant reduction in all types of VTE, with no apparent increase in the incidence of major bleeding episodes. The reduction in VTE may translate into improved quality of life, less hospitalizations and consequently fewer delays in the administration of chemotherapy. Our analysis indicates an exceptionally marked benefit for patients with pancreatic cancer where NNT for VTE to prevent one VTE is 10 patients (95% CI 7–16) and in lung cancer patients [NNT 33 (95% CI 25–100)]. Therefore, our data suggest that practitioners should strongly consider the use of LMWH as primary thromboprophylaxis to significantly reduce the rate of VTE specifically in pancreatic cancer patients, and most possibly lung cancer patients.
http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.934397
Download PDF (22.2 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer 2007;15:2339–46.
- Khorana AA, Dalal M, Lin J, Connolly GC. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer 2013;119:648–55.
- Levitan N, Dowlati A, Remick SC, Tahsildar HI, Sivinski LD, Beyth R, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine (Baltimore) 1999;78:285–91.
- Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med 2006; 166:458–66.
- Lyman GH, Khorana AA, Kuderer NM, Lee AY, Arcelus JI, Balaban EP, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013;31:2189–204.
- Heit JA, Silverstein MD, Mohr DN, Petterson TM, Lohse CM, O’Fallon WM, et al. Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Arch Intern Med 2000;160:809–15.
- Haddad TC, Greeno EW. Chemotherapy-induced thrombosis. Thromb Res 2006;118:555–68.
- Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: A meta-analysis. JAMA 2008; 300:2277–85.
- Palumbo JS, Kombrinck KW, Drew AF, Grimes TS, Kiser JH, Degen JL, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood 2000;96:3302–9.
- Palumbo JS, Potter JM, Kaplan LS, Talmage K, Jackson DG, Degen JL. Spontaneous hematogenous and lymphatic metastasis, but not primary tumor growth or angiogenesis, is diminished in fibrinogen deficient mice. Cancer Res 2002; 62:6966–72.
- Sampson MT, Kakkar AK. Coagulation proteases and human cancer. Biochem Soc Trans 2002;30:201–7.
- Rickles FR, Patierno S, Fernandez PM. Tissue factor, thrombin, and cancer. Chest 2003;124:58S–68S.
- Khorana AA, Streiff MB, Farge D, Mandala M, Debourdeau P, Cajfinger F, et al. Venous thromboembolism prophylaxis and treatment in cancer: A consensus statement of major guidelines panels and call to action. J Clin Oncol 2009;27:4919–22.
- Debourdeau P, Farge D, Beckers M, Baglin C, Bauersachs RM, Brenner B, et al. International clinical practice guidelines for the treatment and prophylaxis of thrombosis associated with central venous catheters in patients with cancer. J Thromb Haemost 2013;11:71–8.
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions Version 5.0. The Cochrane Collaboration. [cited 2013 Aug 10]. Available from: www.cochrane-handbook.org. 2008.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88.
- Altinbas M, Coskun HS, Er O, Ozkan M, Eser B, Unal A, et al. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J Thromb Haemost 2004;2:1266–71.
- Kakkar AK, Levine MN, Kadziola Z, Lemoine NR, Low V, Patel HK, et al. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: The fragmin advanced malignancy outcome study (FAMOUS). J Clin Oncol 2004;22:1944–8.
- Klerk CPW, Smorenburg SM, Otten HM, Lensing AW, Prins MH, Piovella F, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol 2005;23:2130–5.
- Sideras K, Schaefer PL, Okuno SH, Sloan JA, Kutteh L, Fitch TR, et al. Low-molecular-weight heparin in patients with advanced cancer: A phase 3 clinical trial. Mayo Clin Proc 2006;81:758–67.
- Agnelli G, Gussoni G, Bianchini C, Verso M, Mandalà M, Cavanna L, et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: A randomised, placebo-controlled, double blind study. Lancet Oncol 2009;10:943–9.
- Riess H, Pelzer U, Deutschinoff G, Opitz M, Stauch P, Reitzig S, et al. A prospective, randomized trial of chemotherapy with or without the low molecular weight heparin (LMWH) enoxaparin in patients (pts) with advanced pancreatic cancer (APC): Results of the CONKO 004 trial. J Clin Oncol 2009;27:18S.
- van Doormaal FF, Di Nisio M, Otten HM, Richel DJ, Prins M, Buller HR. Randomized trial of the effect of the low molecular weight heparin nadroparin on survival in patients with cancer. J Clin Oncol 2011;29:2071–6.
- Agnelli G, George DJ, Kakkar AK, Fisher W, Lassen MR, Mismetti P, et al. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med 2012;366:601–9.
- Maraveyas A, Waters J, Roy R, Fyfe D, Propper D, Lofts F, et al. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur J Cancer 2012; 48:1283–92.
- Haas SK, Freund M, Heigener D, Heilmann L, Kemkes-Matthes B, von Tempelhoff GF, et al. Low-molecular-weight heparin versus placebo for the prevention of venous thromboembolism in metastatic breast cancer or stage III/IV lung cancer. Clin Appl Thromb Hemost 2012;18:159–65.
- Verso M, Gussoni G, Agnelli G. Prevention of venous thromboembolism in patients with advanced lung cancer receiving chemotherapy: A combined analysis of the PROTECHT and TOPIC-2 studies. J Thromb Haemost 2010;8:1649–51.
- Bick RL. Alterations of hemostasis associated with malignancy: Etiology, pathophysiology, diagnosis and management. Semin Thromb Hemost 1978;5:1–26.
- Johnson MJ, Sproule MW, Paul J. The prevalence and associated variables of deep venous thrombosis in patients with advanced cancer. Clin Oncol (R Coll Radiol) 1999;11:105–10.
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005;293:715–22.
- Alikhan R, Cohen AT, Combe S, Samama MM, Desjardins L, Eldor A, et al. Prevention of venous thromboembolism in medical patients with enoxaparin: A subgroup analysis of the MEDENOX study. Blood Coagul Fibrinolysis 2003;14:341–6.
- Noble SI, Shelley MD, Coles B, Williams SM, Wilcock A, Johnson MJ;Association for Palliative Medicine for Great Britain and Ireland. Management of venous thromboembolism in patients with advanced cancer: A systematic review and meta-analysis. Lancet Oncol 2008;9:577–84.
- Weitz JI, Eikelboom JW, Samama MM, American College of Chest Physicians. New antithrombotic drugs: Antithrombotic therapy and prevention of thrombosis, 9th ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e120–51S.