Abstract
Atherosclerotic peripheral arterial disease (PAD) is highly prevalent in the elderly and subjects with atherosclerotic risk factors such as smoking, diabetes mellitus, hypertension, and hyperlipidemia. Importantly, PAD is rarely an isolated condition, but rather a manifestation of systemic atherosclerosis. Hence, there is often coexisting disease in the coronary and cerebral arteries and, consequently, an increased risk of myocardial infarction and stroke.
Intermittent claudication is the classic symptom of PAD, yet up to 50% of patients are asymptomatic. Despite the availability of reliable, non-invasive screening tests, PAD is largely underdiagnosed and undertreated, mostly due to the paucity of symptoms and underutilization of screening tools. The ankle-brachial index (ABI), a simple, rapid, and inexpensive diagnostic tool, holds much prognostic value for PAD diagnosis and is ideal for implementation in the primary care physician’s office. The early detection of PAD with ABI screening and subsequent medical management represents a critical opportunity to prevent considerable vascular morbidity and mortality.
The management of PAD must address claudication symptoms (with cilostazol or pentoxifylline, or in severe cases endovascular or surgical revascularization) and modifiable atherosclerotic risk factors (with an aggressive global risk-reduction regimen involving lifestyle modifications, exercise, smoking cessation, and antiplatelet, lipid-lowering, and antihypertensive therapy).
Introduction
Peripheral arterial disease (PAD) is a progressive atherosclerotic disease resulting in arterial stenosis and occlusions in non-coronary or non-carotid arteries and typically manifests in at least one of the major vessels supplying the lower extremities. Peripheral arterial disease is commonly a clinical manifestation of systemic atherosclerosis but may also result from trauma, vasculitis, or an inflammatory process. In rare cases, peripheral ischemia can be caused by migraine medications containing ergotamine derivatives (Citation1). As atherosclerosis is central to the development of PAD, the key risk factors for PAD are similar to those for atherosclerosis, i.e. age >50 years, cigarette smoking, diabetes mellitus, dyslipidemia, and hypertension (2,3).
The prevalence of PAD in US adults is estimated to be 8–12 million affected individuals (Citation4,Citation5), with advancing age and the presence of diabetes associated with increased prevalence (Citation6,Citation7). Notably, the reported age- and sex-specific prevalence of PAD has varied depending largely on the diagnostic technique employed and the population studied. The prevalence of PAD as defined by >50% obstruction of at least one major lower extremity vessel is estimated to be 3-fold higher when sensitive, non-invasive diagnostic tests are utilized. In contrast, the use of claudication as a primary means of diagnosis is less sensitive and yields lower prevalences (Citation6,Citation8,Citation9).
Given the commonality of risk factors for ischemic atherothrombotic events, patients with asymptomatic and symptomatic PAD are at significant risk for cardiovascular and cerebrovascular morbidity and mortality. Importantly, PAD is considered a coronary disease risk equivalent as it confers equal risk for a major vascular event as a previous myocardial infarction (MI) (Citation10). In addition, the presence of extensive PAD correlates with overall atherosclerotic burden and is an indicator of multivessel involvement (Citation11).
Key messages
Peripheral arterial disease is associated with an increased risk of cardiovascular morbidity and mortality.
Peripheral arterial disease is easily diagnosed by assessing the ankle-brachial index, a rapid and simple non-invasive diagnostic technique.
Appropriate management of peripheral arterial disease includes treatment of claudication, lifestyle alterations, and antihypertensive, antiplatelet, and lipid-lowering therapies.
Early and accurate identification of this high-risk population with PAD, especially those patients who are asymptomatic, is a critical issue that is easily undertaken using simple and reliable non-invasive diagnostic tests such as the ankle-brachial pressure index (ABI) (Citation4,Citation11).
The purpose of this review is to provide an overview of PAD and the available diagnostic and therapeutic options that can be implemented in the primary care physician’s office.
Symptom progression in PAD and associated risks
Patients with PAD may be asymptomatic or present with a spectrum of symptoms including atypical leg pain, classic claudication, rest pain, or critical limb ischemia with gangrene. The natural history of PAD is depicted in (Citation3). More than 50% of patients identified as having PAD by ABI screening do not present with classic claudication symptoms but rather are asymptomatic or experience other types of exertional leg pain, thus highlighting the diverse clinical manifestations of PAD (Citation12).
Figure 1. The natural progression of symptoms in patients with peripheral arterial disease (PAD). (CLI = critical limb ischemia; CV = cardiovascular; MI = myocardial infarction.) Rreproduced with permission from Hirsch et al., J Am Coll Cardiol. 2006;47:1239–12 (3).
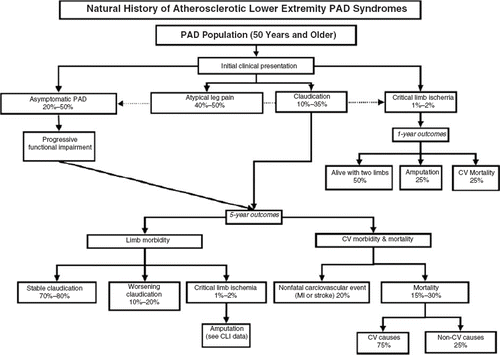
Intermittent claudication is defined as exercise-induced muscle discomfort (not joint fatigue) in one or both of the lower limbs that is continuous during walking/exertion and is a result of poor blood flow to the leg muscles. Although claudication is the classical manifestation of PAD, it occurs in only 10% to 35% of patients (Citation8,Citation13,Citation14). While frequently manifested as painful discomfort, claudication may also present as burning, tightness, or fatigue of the lower extremities. The limb discomfort is typically relieved within 10 minutes of rest (cessation of the exertion, which does not have to be a sitting position) as the blood flow normalizes. A stationary standing position generally provides faster relief of pain secondary to the effects of gravity on blood flow to the lower extremities. Leg discomfort improved by sitting is usually due to pseudoclaudication secondary to spinal stenosis. For patients with intermittent claudication, the risk of progressing to the severest form of the disease, critical leg ischemia, is less than 5% over 5 years; however, the level of functional disability may significantly compromise ambulatory activity and quality of life (Citation3).
A small proportion of patients with PAD (1%–2%) eventually develop critical limb ischemia as a result of severely compromised arterial blood supply. Critical limb ischemia is characterized by ischemic leg pain at rest, non-healing ulcers or gangrene, and may necessitate limb amputation. Affected patients often present with leg edema and dependent rubor, as they frequently sleep with their legs overhanging the bed in an effort to improve blood flow. These patients tend to have multilevel disease with atherosclerotic involvement of below-the-knee vessels. Evidently, the prognosis of patients with critical limb ischemia is worse than those with claudication, with an annual mortality rate as high as 25% (Citation15). The prognosis of patients with below-knee disease is dependent on the presence of co-morbid conditions, particularly diabetes. Aggressive glucose control can reduce the risk of amputation (Citation16); in addition, smoking cessation should be strongly encouraged to improve outcomes (Citation15). Although the majority, an estimated 50%, will require revascularization, 25% may require aggressive medical intervention and 25% primary amputation (Citation15).
Cardiovascular risk
One of the key concerns in PAD is the increased risk of cardiovascular ischemic events due to the systemic nature of the atherosclerotic process that may be associated with the development of concomitant cardiovascular and cerebrovascular disease. Coronary artery disease (CAD) is the predominant cause of death in patients with PAD (40%–60%), followed by cerebrovascular disease (10%–20%) (Citation15). In comparison, the risk of limb loss is relatively low () (Citation8,Citation17–19). It is estimated that patients with PAD are three times more likely to experience all-cause mortality and are at a 6-fold greater risk of death from cardiovascular disease compared with patients without PAD (Citation17,Citation18,Citation20). Furthermore, the 5-year mortality risk of PAD is greater than the 5-year mortality risk for breast cancer and many other malignancies (Citation21–23).
It has also been demonstrated that clinical PAD is a strong predictor for cardiovascular events, cardiovascular mortality, MI, and stroke (Citation24–26). In the first year of the large REACH (REduction of Atherothrombosis for Continued Health) Registry, the PAD cohort demonstrated the highest risk for cardiovascular mortality (2.5%), compared with patients with CAD (1.93%) and cerebrovascular disease (2.05%), and the rate increased with overlapping vascular bed disease (Citation26).
Asymptomatic PAD is also associated with poor lower limb functioning and increased cardiovascular morbidity and mortality (Citation20,Citation27,Citation28). In the Edinburgh Artery Study, a cross-sectional study of vascular events in 1592 primary care patients aged 55–74 years at enrollment, the relative risk of cardiovascular mortality was increased 2.7-fold in patients with claudication and 2.1-fold in asymptomatic patients (Citation28). Therefore, asymptomatic PAD should not be taken lightly because of a mistaken belief that it is more benign.
Non-invasive diagnosis of PAD
It is increasingly recognized that PAD confers a high risk for fatal and non-fatal cardiovascular ischemic events and that early detection is imperative to reduce cardiovascular morbidity and mortality. However, data from the international REACH Registry showed that although cardiovascular risk factors are consistent and common worldwide, diagnosis and treatment are suboptimal (Citation11). Furthermore, in primary practices throughout the United States in the PARTNERS (PAD Awareness, Risk, and Treatment: New Resources for Survival) program, PAD was highly prevalent (29%), yet over 85% of subjects were undiagnosed as they were asymptomatic or had atypical symptoms, and physician awareness of PAD was relatively low. Furthermore, a patient’s vascular history and a thorough examination of peripheral pulses are rarely obtained (Citation4). Moreover, even when diagnostic evaluation of PAD is undertaken, there may be discrepancies in the method of evaluation that could negatively affect the correct diagnosis, referral for vascular surgery, and, importantly, patient outcomes (Citation29).
Differential diagnosis
Atherosclerosis is not the sole cause of lower extremity PAD, which may have a range of underlying causes including atherosclerotic, thromboembolic, inflammatory, or traumatic etiologies. For example, young male smokers may present with severe distal PAD secondary to Buerger’s disease, and female patients with collagen vascular disease may present with peripheral vascular disease affecting the upper extremities associated with Raynaud’s disease (Citation3). Thus, consideration of a broad differential diagnosis is pertinent. It is particularly important to differentiate between non-vascular and vascular etiologies to determine an accurate diagnosis in order to initiate pharmacological, endovascular, surgical, or rehabilitative care.
Although intermittent claudication is the classic symptom of PAD, patients may present with pain on walking that stems from other unrelated conditions such as musculoskeletal disorders, venous claudication, nerve root compression, hip or foot/ankle arthritis, or spinal stenosis. Clinical clues frequently facilitate correct diagnosis. Importantly, in PAD, claudication distance is always very predictable and unlikely to change from day to day. In addition, claudication affects muscle groups, not joints, and does not require sitting for relief (Citation15).
Peripheral vascular disease may also be associated with non-healing ulcers, and the nature of these ulcers can also provide diagnostic clues. Peripheral arterial disease ulcers are painful, yet rarely bleed. In contrast, venous ulcers are usually malleolar in location, are painless unless infected, tend to bleed profusely, and are associated with skin darkening. Neuropathic ulcers affect areas of pressure, are non-painful, and are generally surrounded by callus.
Initial examination and history
A clinical evaluation of PAD may be reliably determined in the primary care physician’s office using non-invasive diagnostic techniques. A systematic approach to the diagnosis of PAD may predict the location and severity of the disease in most patients, beginning with a detailed history and physical examination focusing on the peripheral vasculature (Citation15).
All pulses should be carefully palpated starting with the temporal pulses. Carotid disease frequently affects the bulb, and a delayed or absent temporal pulse may indicate the presence of significant carotid disease. Both radial pulses should be palpated simultaneously; a delayed radial pulse indicates the presence of subclavian stenosis. Blood pressure (BP) should be measured on both arms, noting that BP in the right arm is frequently higher secondary to the size of the innominate artery; however, an inter-arm difference of >10 mmHg suggests stenosis of the subclavian artery (Citation30). Femoral, popliteal, dorsalis pedis, and posterior tibial pulses should be palpated. A brisk popliteal pulse may indicate the presence of popliteal artery aneurysm and should prompt immediate ultrasound of the popliteal arteries and possibly vascular surgical consultation. The dorsalis pedis pulse cannot be detected on the dorsum of the foot in some patients because their anterior tibial artery branches at the ankle; a viable alternative in this scenario is to assess the distal anterior tibial artery pulse at the ankle. An absent or abnormal posterior tibial pulse is the best predictor of PAD.
Auscultation of the carotids, abdominal aorta, and femoral arteries is also paramount during vascular evaluation. An abdominal bruit is four times more common in patients with renal artery stenosis than in patients with essential hypertension and is a significant predictor of renal artery stenosis (Citation31).
Limited exercise performance and walking ability is characteristic of PAD. A history of intermittent claudication is a marker for PAD but does not indicate the true prevalence of the disease. Pulse examination alone may miss up to 50% of PAD cases, and pulse abnormality (absent or diminished) significantly overestimates the true prevalence of PAD (Citation15).
Questionnaires
Questionnaires are subjective measures available to primary care physicians to assess the degree of claudication or walking impairment. Common assessment measures include the Medical Outcomes Study 36-Item Short Form (SF-36), which is a broad functional status questionnaire useful in clinical and outcome trials; the Walking Impairment Questionnaire (WIQ), which grades walking ability through the use of questions on walking distance, walking speed, and stair climbing; and the new disease-specific health status measure, the Peripheral Artery Questionnaire (PAQ), recently developed by Spertus (Citation32).
Diagnosis of PAD using questionnaires is not always accurate and does not necessarily assess the severity of the disease. Thus, as diagnostic tools for PAD, questionnaires are of limited value, especially for asymptomatic PAD. Questionnaires appear most useful in patients without classic claudication, but who have walking impairment nonetheless.
Ankle-brachial index
The ABI, a primary non-invasive screening test for PAD, is an objective measure and a risk-assessment tool with a level of sensitivity that suggests that the method may have greater utility than questionnaires and other non-invasive tools for evaluating PAD (Citation33). There are a number of types of ABI that have different diagnostic purposes (), but the principal type for initial examination is the single-level ABI. Unless otherwise specified, the term ABI is used to denote single-level ABI in this paper.
Table I. Types of ankle-brachial index (ABI) assessments and their diagnostic function.
The American College of Cardiology/American Heart Association (ACC/AHA) guidelines recommend that resting ABI should be used to establish PAD diagnosis in patients with suspected PAD (subjects with exertional leg symptoms), with non-healing wounds, and in those aged ≥70 years or ≥50 years with a history of smoking or diabetes (Citation3). For patients with atypical leg pain or asymptomatic PAD, the ABI is a simple, rapid (on average requiring ≤15 minutes to complete), inexpensive, and non-invasive method for identifying and monitoring the disease.
The ABI is essentially a ratio of Doppler-recorded systolic BP in the lower and upper extremities and can be easily calculated. Systolic BP is measured using a Doppler stethoscope and BP cuffs on each arm and each ankle (Citation34). The ACC/AHA guidelines recommend selecting the higher of the two arm pressures (brachial) and the higher of the two ankle pressures (anterior tibial/dorsalis pedis or posterior tibial) to calculate the ABI (Citation3). Thus, the right ABI = higher right ankle pressure/higher arm pressure; the left ABI = higher left ankle pressure/higher arm pressure. The index leg is generally defined as the leg with the lower ABI. It is important to note that because this technique for ABI calculation uses the higher pressure in the lower extremity instead of the lower pressure, it may potentially miss distal disease, thus underestimating the severity and prevalence of PAD.
In addition to its utility as a screening tool for PAD, where a normal ABI falls in the range of 0.91–1.30, a low ABI at rest (≤0.90) indicates a high risk of PAD and provides significant evaluative and prognostic information on cardiovascular risk (Citation15). There is an inverse relationship between the ABI and cardiovascular mortality and risk, thus providing valuable data for risk stratification (Citation22,Citation35–38). In a 6-year follow-up of 154 patients aged >40 years with established PAD (initial resting ABI ≤0.90), the relative risk of mortality was 3.1 per 0.50 reduction in resting ABI (P ≤ 0.03); the relative risk of mortality or a vascular event was 3.3 per 0.50 reduction in resting ABI (P ≤ 0.001) (Citation39). Cumulative patient survival after 5 years was greater for patients with higher compared to lower ABIs (91% versus 63%). Lower ABI values thus correlate with multivessel disease and increased atherosclerotic burden. In addition, the HOPE (Heart Outcomes Prevention Evaluation) study demonstrated that the ABI was a strong predictor for cardiovascular events and mortality (Citation25,Citation40). Further, several studies conducted in diverse clinical practice settings have shown that measuring the ABI improves cardiovascular risk stratification (Citation35,Citation41,Citation42). These observations suggest that wider implementation of the ABI in primary prevention settings would be valuable because it would help identify patients with an elevated cardiovascular risk who would benefit from aggressive treatment with risk-reducing therapies (Citation43).
Recently, several studies have reported the association between different methods of measuring ABI and the estimated prevalence of PAD and the risk of cardiovascular events. In an analysis of getABI, in which five different methods of determining ABI were compared, the lowest PAD prevalence estimates were observed when the highest ankle systolic BP was used to assess ABI (18.0%) (Citation44). When the lowest ankle systolic BP was used, the PAD prevalence was almost doubled (34.5%). Notably, although the mode of ABI assessment impacted the prevalence of ABI, all methods assessed had virtually equal sensitivity and specificity for predicting cardiovascular events (Citation44). Based on these observations, the getABI investigators concluded that the highest ankle pressure should be used when calculating the ABI. In contrast, the AtheroGene investigators concluded that the lower ankle pressure should be used when calculating the ABI because they observed that compared with use of the lower ankle pressure, the higher pressure underestimated the risk of cardiovascular events (Citation45).
In patients with asymptomatic or atypical symptoms, a reduced ABI correlates with reduced limb function, i.e. reduced walking speed and/or distance during a timed 6-minute walk. Furthermore, the ABI correlates with symptom severity; patients with claudication have ABIs >0.5, subjects with rest pain frequently have ABI values in the range of 0.4–0.5, and non-healing ulcers are associated with ABIs <0.3. Stress ABIs are indicated for patients with symptoms of claudication but with a normal resting ABI. In these patients, remeasuring the ABI after a 5-minute treadmill walk will lead to a drop in systolic ankle pressure and recurrence of symptoms.
The ABI has been validated by angiography to determine its sensitivity, specificity, and accuracy as a diagnostic tool for PAD. When correctly assessed, the ABI was shown to be accurate and reproducible such that a change of 0.15 in a measurement, or >0.10 if accompanied by a change in clinical status, is considered clinically relevant (Citation3,Citation46). An ABI <0.9 is 95% sensitive and 99% specific in detecting angiogram-positive PAD (Citation33). However, the sensitivity and specificity of the ABI in predicting PAD may be limited by the inaccuracy of ABI-related measurements and calculations frequently observed in primary care (Citation47). Observations such as these suggest that clinicians, and subsequently their patients, would benefit from focused training on the correct techniques to measure systolic BP and calculate the ABI.
The ABI may be used as a tool for differential diagnosis; patients with exercise-related leg pain of non-vascular origin will have a normal stress ABI (Citation15). In asymptomatic patients with normal or borderline ABI, especially those with co-morbid disease, such as diabetes, repeat ABI measurements may be indicated over subsequent months to monitor vascular function. Repeat ABI measurements are recommended during follow-up post-vascular surgical procedures to evaluate for recurrence or progression of claudication (Citation3).
Clearly, the identification of high-risk asymptomatic () or symptomatic patients with PAD () by primary care physicians may facilitate the rapid implementation of a therapeutic management plan to reduce the risk of atherothrombotic events, impede disease progression, and improve quality of life. However, the ABI may not be accurate when systolic BP cannot be abolished by BP cuff inflation, i.e. in the elderly or patients with diabetes, renal insufficiency, or other diseases that result in arterial calcification. Non-compressible arteries result in unusually high ABI measurements, higher than in the normal range (>1.5) (Citation15,Citation48). In addition, patients with collateral arterial networks at sites of high-grade aortoiliac stenoses may provide a normal ABI value. It is therefore recommended that in cases where lower extremity PAD is strongly suspected, yet the ABI is high or normal, an alternative diagnostic test is utilized.
Figure 2. a: Eevaluation of patients in whom peripheral arterial disease (PAD) is suspected; b: evaluation and treatment of patients with established PAD. (LDL = low-density lipoprotein; MWD = maximal walking distance; PFWD = pain-free walking distance; SF-36 = Medical Outcomes Study 36-Item Short Form; WIQ = Walking Impairment Questionnaire.) Rreproduced with permission from Hiatt, N Eengl J Med. 2001;344:1608–21 (34).
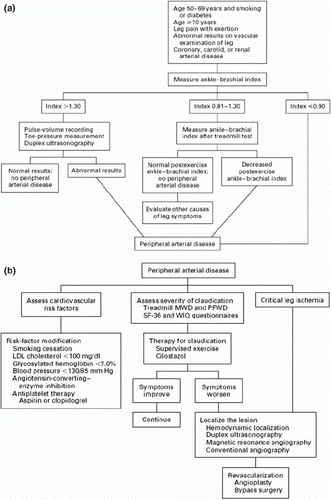
Other non-invasive tests
Alternative non-invasive vascular diagnostic techniques for the detection of PAD include segmental pressure measurements, pulse volume recordings, Doppler waveform analysis, duplex ultrasound, and exercise testing (Citation3). Segmental limb pressure measurement facilitates the determination of the level and degree of obstruction but is limited in the presence of calcification and non-compressible vessels. Pulse volume recordings are less likely to be influenced by vessel calcification. Duplex ultrasound, which may be useful in assessing the patency of an artery and can accurately detect the site of artery stenosis and the degree of obstruction, is the preferred method of screening for graft patency. Exercise or stress ABIs may aid in the identification of symptomatic patients with normal resting ABIs.
Some of these tests may provide anatomical data and, if required, may be supplemented by the use of magnetic resonance angiography (to characterize arterial wall and atherosclerotic lesions), computed tomographic angiography, or catheter-based angiographic techniques. Catheter angiography should be reserved for patients in whom intervention is planned. In contrast to ABI, segmental pressure measurements (with plethysmographic cuffs placed sequentially along the limb at various levels) accurately determine the location of individual stenosis (Citation3).
Finally, urgent referral to a vascular specialist is appropriate for patients with severe symptoms, rest pain, ischemic ulceration or gangrene, and in instances where a diagnosis cannot be confidently made, or there is significant carotid stenosis or aneurysmal disease. Tests for tissue oxygenation, such as transcutaneous oxygen pressure (TcPO2) or laser oximetry, are indicated in patients with ulceration or gangrene; TcPO2 of <20 mmHg (2.66 kPa) is predictive of wound failure and is accurate in 85%–90% of cases (Citation49).
For most other patients, medical management may be implemented in the primary care setting to reduce cardiovascular risk and to provide symptom alleviation.
Medical management of PAD
The ABI is instrumental in establishing a diagnosis of PAD, especially asymptomatic, stratifying disease severity and cardiovascular risk. A management plan for patients with PAD may be tailored according to whether patients require aggressive management for cardiovascular risk reduction to prevent secondary ischemic events, claudication symptom alleviation, and/or surgery. In addition, the ABI may be used to monitor the short- and long-term efficacy of a chosen therapeutic regime. A summary of the ACC/AHA guidelines for the management of patients with PAD is presented in (Citation3).
Table II. ACC/AHA guidelines for the management of peripheral arterial disease (PAD) (3).
Cardiovascular risk factor modification
Aggressive management of the modifiable risk factors for PAD through lifestyle changes, such as smoking cessation, weight loss, and exercise (walking programs), as well as control of underlying co- morbidities such as diabetes, hyperlipidemia, and hypertension, is the mainstay of preventing disease progression (Citation2,Citation3).
Smoking is a preventable cause of cardiovascular risk in patients with PAD. Accumulated data from observational studies in patients with PAD have demonstrated that, compared with continuing to smoke, smoking cessation markedly reduces the risk of death, MI, and amputation, and increases exercise time, accompanied by a modest improvement in ABI values (Citation3).
Patients with PAD and diabetes represent a particularly high-risk population for cardiovascular morbidity and mortality yet are generally undertreated (Citation50), and the risk of developing foot ulcers as a result of pre-existing diabetic sensory neuropathy is increased. Although no prospective trials have demonstrated the effect of improved glycemic control on cardiovascular risk in diabetic patients with PAD, previous studies have shown that, compared with conventional therapy, intensive glucose control non-significantly reduces the risk of lower extremity claudication, peripheral revascularization, or amputation (Citation51), predominantly due to significant reductions in microvascular (nephropathy, retinopathy), not macrovascular, events (Citation51,Citation52).
The management of elevated low-density lipoprotein (LDL)-cholesterol levels with hydroxymethyl glutaryl coenzyme-A reductase inhibitors (statins) is recommended for most patients with PAD (Citation3) to reduce the incidence of major cardiovascular events and alleviate symptoms (Citation53). A complete lipid profile is also worthwhile in patients with atherosclerosis in other vascular beds to identify other potentially damaging lipoprotein abnormalities such as low levels of high-density lipoprotein (HDL)-cholesterol levels or elevated triglycerides. Data in patients with CAD indicate that treating these conditions, even in patients with normal LDL-cholesterol, can improve outcomes and stabilize atherosclerotic plaque (Citation54,Citation55). Although similar studies have not been conducted in patients with PAD, these data suggest that aggressive lipid risk factor management is likely to be worthwhile in patients with PAD with a history or high risk of CAD.
Acute BP reduction has been hypothesized to exacerbate intermittent claudication; however, this has not been validated by clinical data. Existing data do not show an adverse effect of beta-blockers on walking distance in patients with claudication (Citation56). However, because the overall data pool is limited and beta-blockers are not considered first-line antihypertensive agents, a combined alpha-beta blocker may be preferred. The use of angiotensin-converting enzyme (ACE) inhibitors is also recommended for symptomatic PAD (Citation3), based on clinical data showing reductions in the risk of MI, stroke, or vascular death by approximately 25% in symptomatic patients with PAD, with a protective effect independent of BP control (Citation25). Angiotensin-converting enzyme inhibitors have also been shown to improve maximum and pain-free walking distance in patients with PAD (Citation57), by reducing arterial stiffness (Citation58), thereby providing symptomatic benefits as well as risk reduction.
Patients with atherosclerotic lower extremity PAD are at increased risk of ischemic cardiovascular events due to an enhanced prothrombotic state secondary to platelet activation (Citation34). Hence, antiplatelet therapy is recommended to reduce the risk of MI, stroke, or vascular death in patients with symptomatic PAD or an ABI ≤0.9, and based on best evidence and tolerability data to date aspirin and clopidogrel are currently the only recommended such agents (Citation3). A large meta-analysis unequivocally showed that compared with control, antiplatelet therapy (predominantly aspirin) significantly reduced the risk of serious vascular events by approximately 23%, and similar benefits were seen in patients with intermittent claudication (Citation59).
Additional supportive evidence comes from the large CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events) trial. In the entire study population (patients with recent ischemic stroke, recent MI, or symptomatic PAD), there was a statistically significant 8.7% reduction in the risk of major vascular events with clopidogrel as compared to aspirin (P = 0.043). Subgroup analysis of the PAD cohort (n = 6452) showed that clopidogrel significantly reduced this risk by 24% (P = 0.0028) (Citation60). Additionally, clopidogrel and aspirin had similar safety profiles, including a similar risk of any bleeding complication (9.27% with clopidogrel versus 9.28% with aspirin).
Dual antiplatelet therapy with clopidogrel and aspirin has demonstrated greater protection from thrombotic complications than aspirin alone in the setting of acute coronary syndrome and coronary stenting (61–63). These results prompted an evaluation of this combination in a broad population of high-risk patients, including those with prior MI, ischemic stroke, or symptomatic PAD, or those with three or more atherothrombotic risk factors, in the CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance) trial. Although no significant benefit was seen with dual antiplatelet therapy over aspirin monotherapy in the overall study population (Citation64) (the asymptomatic patients (Citation64), or the cohort of patients with PAD (Citation65)), the overall symptomatic population did show a significant benefit with dual antiplatelet therapy. While there was no significant difference in the rate of severe bleeding with combination therapy, moderate bleeding was significantly increased compared with placebo (2.0% versus 1.3%; P = 0.004) (Citation65). The increased bleeding risk associated with combination therapy can be minimized by decreasing the dosage of aspirin. Additionally, antiplatelet therapy should be used with caution in patients taking cilostazol due to an increased risk for bleeding. Overall, further study regarding the benefits and risks of combination antiplatelet therapy in secondary prevention is necessary.
Of note, the ACC/AHA guidelines do not indicate the use of oral anticoagulation therapy with warfarin for cardiovascular risk reduction in patients with PAD (Citation3,Citation66). Furthermore, it has recently been demonstrated that in patients with PAD, the combination of an oral anticoagulant and antiplatelet therapy was not more effective than antiplatelet therapy alone in preventing major cardiovascular complications and was associated with an increased risk of serious bleeding (Citation67).
Symptom alleviation
Risk factor modification through medical management in PAD targets the reduction of cardiovascular risk; however, there is minimal symptom alleviation with these interventions, and, depending on the level of symptom severity, revascularization procedures may be required () (Citation3).
A considerable body of evidence demonstrates that a supervised exercise program improves walking ability and reduces claudication symptoms (Citation68,Citation69). Exercise training is effective in improving exercise performance, walking ability, and physical functioning, is generally safe with no demonstrable morbidity and mortality risk, and has beneficial effects on lipid profile, BP, and overall metabolic health (Citation70). However, exercise has some major limitations including the fact that most patients lack the motivation to exercise. Exercise programs are not readily available, and significant improvement in claudication distance requires patients to be committed to exercise at least 5 days a week for 6 months.
The currently recommended pharmacotherapies for improvement of functional capacity in patients with claudication include the phosphodiesterase type-3 inhibitor, cilostazol, and the methylxanthine derivative, pentoxifylline () (Citation3). Studies have shown that cilostazol improves maximal walking distance by 40% to 60% (Citation71–74) and is significantly better than pentoxifylline for treating moderate to severe claudication (Citation75). Cilostazol has been shown to improve ABI (Citation76) and also has other salutary effects resulting from its vasodilatory properties, its inhibitory effect on smooth muscle proliferation and platelets, and its beneficial effect on lipid profiles (Citation77). However, cilostazol is contraindicated in patients with congestive heart failure and has been shown to interact significantly with certain cardiovascular drugs, including diltiazem (Citation77), limiting its use (Citation3). Pentoxifylline has modest clinical benefits relative to placebo (Citation78) and is no longer widely used.
Conclusions
Patients with PAD are at increased risk of major coronary and cerebrovascular events, including MI, stroke, and death, secondary to systemic atherothrombosis. Furthermore, they may have coexisting progressive atherosclerosis in other vascular beds including coronary and carotid arteries.
Although these patients are at increased risk of cardiovascular morbidity and mortality, and non-invasive and simple diagnostic tests are readily available, the disease remains significantly underdiagnosed and undertreated in clinical practice (Citation4,Citation11). Moreover, patients with PAD are less intensively treated relative to those with cardiovascular disease (Citation4), and management of cardiovascular risk factors is suboptimal (Citation79).
The lack of awareness among physicians contributes to lower rates of treatment for atherosclerotic risk factors in patients with PAD compared with those with CAD, such that there is a lower rate of treatment with antiplatelet agents, and the threshold LDL-cholesterol level at which lipid-lowering therapy is initiated is higher (Citation80).
Increasing primary care giver awareness and adherence to guideline recommendations for the management of PAD may reduce the high rates of cardiovascular morbidity and mortality. As pivotal primary care providers in the medical community, primary care physicians are well placed to address this issue. Primary care physicians may play a central role in increasing the awareness of PAD as a silent disease state and improving the rate of early detection of asymptomatic patients using the simple, inexpensive, non-invasive ABI test, a prognostic marker for vascular morbidity and mortality.
Acknowledgements
The authors wish to thank Christopher Radel, PhD, and Melanie Leiby, PhD, for their editorial support in the preparation of this manuscript. This support was funded by the Bristol-Myers Squibb/Sanofi Pharmaceutical Partnership. The authors did not receive any compensation for this work.
Declaration of interest: The authors report no conflicts of interest. The authors wrote and directed the development of the paper from outline through submission.
References
- Zavaleta EG, Fernandez BB, Grove MK, Kaye MD. St. Anthony’s fire (ergotamine induced leg ischemia)—a case report and review of the literature. Angiology. 2001;52: 349–56.
- Meijer WT, Grobbee DE, Hunink MG, Hofman A, Hoes AW. Determinants of peripheral arterial disease in the elderly: The Rotterdam study. Arch Intern Med. 2000;160: 2934–8.
- Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, . ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): Executive summary A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47: 1239–312.
- Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, . Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–24.
- Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, . Heart disease and stroke statistics—2007 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171.
- Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71: 510–5.
- Kannel WB. The demographics of claudication and the aging of the American population. Vasc Med. 1996;1:60–4.
- Weitz JI, Byrne J, Clagett GP, Farkouh ME, Porter JM, Sackett DL, . Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: A critical review. Circulation. 1996;94:3026–49.
- Meijer WT, Hoes AW, Rutgers D, Bots ML, Hofman A, Grobbee DE. Peripheral arterial disease in the elderly: The Rotterdam Study. Arterioscler Thromb Vasc Biol. 1998;18:185–92.
- National Cholesterol Education Program Expert Panel. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421.
- Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, . International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–9.
- McDermott MM, Mehta S, Liu K, Guralnik JM, Martin GJ, Criqui MH, . Leg symptoms, the ankle-brachial index, and walking ability in patients with peripheral arterial disease. J Gen Intern Med. 1999;14:173–81.
- McDermott MM, Mehta S, Greenland P. Exertional leg symptoms other than intermittent claudication are common in peripheral arterial disease. Arch Intern Med. 1999;159: 387–92.
- McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, . Leg symptoms in peripheral arterial disease: Associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–606.
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, . Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33 Suppl 1:S1–75.
- Collins TC, Beyth RJ, Nelson DB, Petersen NJ, Suarez-Almazor ME, Bush RL, . Process of care and outcomes in patients with peripheral arterial disease. J Gen Intern Med. 2007;22:942–8.
- Newman AB, Sutton-Tyrrell K, Vogt MT, Kuller LH. Morbidity and mortality in hypertensive adults with a low ankle/arm blood pressure index. JAMA. 1993;270:487–9.
- Criqui MH, Denenberg JO. The generalized nature of atherosclerosis: How peripheral arterial disease may predict adverse events from coronary artery disease. Vasc Med. 1998;3:241–5.
- Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: Morbidity and mortality implications. Circulation. 2006;114:688–99.
- Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, . Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–6.
- Criqui MH. Peripheral arterial disease—epidemiological aspects. Vasc Med. 2001;6:3–7.
- McKenna M, Wolfson S, Kuller L. The ratio of ankle and arm arterial pressure as an independent predictor of mortality. Atherosclerosis. 1991;87:119–28.
- Beaglehole R, Irwin A, Prentice T. The world health report 2004—changing history. Geneva, Switzerland: World Health Organization. 2004.
- Hebert K, Lopez B, Macedo FY, Gomes CR, Urena J, Arcement LM. Peripheral vascular disease and erectile dysfunction as predictors of mortality in heart failure patients. J Sex Med. 2009;6:1999–2007.
- Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–53.
- Steg PG, Bhatt DL, Wilson PW, D’Agostino R Sr, Ohman EM, Rother J, . One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–206.
- McDermott MM, Guralnik JM, Ferrucci L, Tian L, Liu K, Liao Y, . Asymptomatic peripheral arterial disease is associated with more adverse lower extremity characteristics than intermittent claudication. Circulation. 2008;117: 2484–91.
- Leng GC, Lee AJ, Fowkes FGR, Whiteman M, Dunbar J, Housely E, . Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1996;25:1172–81.
- McLafferty RB, Dunnington GL, Mattos MA, Markwell SJ, Ramsey DE, Henretta JP, . Factors affecting the diagnosis of peripheral vascular disease before vascular surgery referral. J Vasc Surg. 2000;31:870–9.
- English JA, Carell ES, Guidera SA, Tripp HF. Angiographic prevalence and clinical predictors of left subclavian stenosis in patients undergoing diagnostic cardiac catheterization. Catheter Cardiovasc Interv. 2001;54:8–11.
- Krijnen P, van Jaarsveld BC, Steyerberg EW, Man in ‘t Veld AJ, Schalekamp MA, Habbema JD. A clinical prediction rule for renal artery stenosis. Ann Intern Med. 1998;129:705–11.
- Spertus J, Jones P, Poler S, Rocha-Singh K. The peripheral artery questionnaire: A new disease-specific health status measure for patients with peripheral arterial disease. Am Heart J. 2004;147:301–8.
- Fowkes FG. The measurement of atherosclerotic peripheral arterial disease in epidemiological surveys. Int J Epidemiol. 1988;17:248–54.
- Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344:1608–21.
- Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, . Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: A meta-analysis. JAMA. 2008;300: 197–208.
- Newman AB, Siscovick DS, Manolio TA, Polak J, Fried LP, Borhani NO, . Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993;88:837–45.
- Vogt MT, Cauley JA, Newman AB, Kuller LH, Hulley SB. Decreased ankle/arm blood pressure index and mortality in elderly women. JAMA. 1993;270:465–9.
- Zheng ZJ, Sharrett AR, Chambless LE, Rosamond WD, Nieto FJ, Sheps DS, . Associations of ankle-brachial index with clinical coronary heart disease, stroke and preclinical carotid and popliteal atherosclerosis: The Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 1997;131:115–25.
- Sikkink CJ, van Asten WN, van ‘t Hof MA, van Langen H, van der Vliet JA. Decreased ankle/brachial indices in relation to morbidity and mortality in patients with peripheral arterial disease. Vasc Med. 1997;2:169–73.
- Ostergren J, Sleight P, Dagenais G, Danisa K, Bosch J, Qilong Y, . Impact of ramipril in patients with evidence of clinical or subclinical peripheral arterial disease. Eur Heart J. 2004;25:17–24.
- Morillas P, Cordero A, Bertomeu V, Gonzalez-Juanatey JR, Quiles J, Guindo J, . Prognostic value of low ankle-brachial index in patients with hypertension and acute coronary syndromes. J Hypertens. 2009;27:341–7.
- Ramos R, Quesada M, Solanas P, Subirana I, Sala J, Vila J, . Prevalence of symptomatic and asymptomatic peripheral arterial disease and the value of the ankle-brachial index to stratify cardiovascular risk. Eur J Vasc Endovasc Surg. 2009;38:305–11.
- Cacoub P, Cambou JP, Kownator S, Belliard JP, Beregi JP, Branchereau A, . Prevalence of peripheral arterial disease in high-risk patients using ankle-brachial index in general practice: A cross-sectional study. Int J Clin Pract. 2009; 63:63–70.
- Lange SF, Trampisch HJ, Pittrow D, Darius H, Mahn M, Allenberg JR, . Profound influence of different methods for determination of the ankle brachial index on the prevalence estimate of peripheral arterial disease. BMC Public Health. 2007;7:147.
- Espinola-Klein C, Rupprecht HJ, Bickel C, Lackner K, Savvidis S, Messow CM, . Different calculations of ankle-brachial index and their impact on cardiovascular risk prediction. Circulation. 2008;118:961–7.
- Fowkes FG, Housley E, Macintyre CC, Prescott RJ, Ruckley CV. Variability of ankle and brachial systolic pressures in the measurement of atherosclerotic peripheral arterial disease. J Epidemiol Community Health. 1988;42:128–33.
- Nicolaï SP, Kruidenier LM, Rouwet EV, Bartelink ML, Prins MH, Teijink JA. Ankle brachial index measurement in primary care: Are we doing it right? Br J Gen Pract. 2009;59: 422–7.
- Resnick HE, Lindsay RS, McDermott MM, Devereux RB, Jones KL, Fabsitz RR, . Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: The Strong Heart Study. Circulation. 2004;109: 733–9.
- Wütschert R, Bounameaux H. Determination of amputation level in ischemic limbs. Reappraisal of the measurement of TcPo2. Diabetes Care. 1997;20:1315–8.
- Brown LC, Johnson JA, Majumdar SR, Tsuyuki RT, McAlister FA. Evidence of suboptimal management of cardiovascular risk in patients with type 2 diabetes mellitus and symptomatic atherosclerosis. CMAJ. 2004;171:1189–92.
- The DCCT Research Group. Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol. 1995;75:894–903.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53.
- Mohler ER 3rd, Hiatt WR, Creager MA. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation. 2003;108: 1481–6.
- Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, . Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–92.
- Miller M, Cannon CP, Murphy SA, Qin J, Ray KK, Braunwald E. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. 2008;51:724–30.
- Paravastu SC, Mendonca D, Da Silva A. Beta blockers for peripheral arterial disease. Cochrane Database Syst Rev. 2008;CD005508.
- Ahimastos AA, Lawler A, Reid CM, Blombery PA, Kingwell BA. Brief communication: Ramipril markedly improves walking ability in patients with peripheral arterial disease: A randomized trial. Ann Intern Med. 2006;144:660–4.
- Ahimastos AA, Dart AM, Lawler A, Blombery PA, Kingwell BA. Reduced arterial stiffness may contribute to angiotensin-converting enzyme inhibitor induced improvements in walking time in peripheral arterial disease patients. J Hypertens. 2008;26:1037–42.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86.
- A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329–39.
- Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, . Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: The PCI-CURE study. Lancet. 2001;358:527–33.
- Steinhubl SR, Berger PB, Mann JT 3rd, Fry ET, DeLago A, Wilmer C, . Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: A randomized controlled trial. JAMA. 2002;288:2411–20.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502.
- Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, . Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–17.
- Bhatt DL, Flather MD, Hacke W, Berger PB, Black HR, Boden WE, . Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol. 2007;49: 1982–8.
- Anand SS, Yusuf S. Oral anticoagulants in patients with coronary artery disease. J Am Coll Cardiol. 2003; 41: 62S–9S.
- Anand S, Yusuf S, Xie C, Pogue J, Eikelboom J, Budaj A, . Oral anticoagulant and antiplatelet therapy and peripheral arterial disease. N Engl J Med. 2007;357:217–27.
- Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA. 1995;274:975–80.
- Leng GC, Fowler B, Ernst E. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2000;CD000990.
- Milani RV, Lavie CJ. The role of exercise training in peripheral arterial disease. Vasc Med. 2007;12:351–8.
- Beebe HG, Dawson DL, Cutler BS, Herd JA, Strandness DE Jr, Bortey EB, . A new pharmacological treatment for intermittent claudication: Results of a randomized, multicenter trial. Arch Intern Med. 1999;159:2041–50.
- Dawson DL, Cutler BS, Meissner MH, Strandness DE Jr. Cilostazol has beneficial effects in treatment of intermittent claudication: Results from a multicenter, randomized, prospective, double-blind trial. Circulation. 1998;98: 678–86.
- Money SR, Herd JA, Isaacsohn JL, Davidson M, Cutler B, Heckman J, . Effect of cilostazol on walking distances in patients with intermittent claudication caused by peripheral vascular disease. J Vasc Surg. 1998;27:267–74; discussion 74–5.
- Strandness DE Jr, Dalman RL, Panian S, Rendell MS, Comp PC, Zhang P, . Effect of cilostazol in patients with intermittent claudication: A randomized, double-blind, placebo-controlled study. Vasc Endovascular Surg. 2002; 36:83–91.
- Dawson DL, Cutler BS, Hiatt WR, Hobson RW 2nd, Martin JD, Bortey EB, . A comparison of cilostazol and pentoxifylline for treating intermittent claudication. Am J Med. 2000;109:523–30.
- Mohler ER 3rd, Beebe HG, Salles-Cuhna S, Zimet R, Zhang P, Heckman J, . Effects of cilostazol on resting ankle pressures and exercise-induced ischemia in patients with intermittent claudication. Vasc Med. 2001;6:151–6.
- Chapman TM, Goa KL. Cilostazol: A review of its use in intermittent claudication. Am J Cardiovasc Drugs. 2003;3: 117–38.
- Girolami B, Bernardi E, Prins MH, Ten Cate JW, Hettiarachchi R, Prandoni P, . Treatment of intermittent claudication with physical training, smoking cessation, pentoxifylline, or nafronyl: A meta-analysis. Arch Intern Med. 1999; 159:337–45.
- Bianchi C, Montalvo V, Ou HW, Bishop V, Abou-Zamzam AM Jr. Pharmacologic risk factor treatment of peripheral arterial disease is lacking and requires vascular surgeon participation. Ann Vasc Surg. 2007;21:163–6.
- McDermott MM, Hahn EA, Greenland P, Cella D, Ockene JK, Brogan D, . Atherosclerotic risk factor reduction in peripheral arterial disease: Results of a national physician survey. J Gen Intern Med. 2002;17:895–904.
