Abstract
Objective. To assess whether serum magnesium concentration can predict risk of short-term outcome of acute ischemic stroke. Methods. Between January 1, 2006 and December 31, 2008, 1493 patients with acute ischemic stroke were recruited from four hospitals in Shandong province, P.R China. Data on demographic characteristics, life style risk factors, history of cardiovascular disease, admission blood pressure and other clinical characteristics were collected from all subjects. The short-term outcome was defined as neurological deficiency NIHSS ≥ 10 or death (NIHSS ≥ 10/death). The Cox proportion hazard regression model was used to evaluate the association between serum magnesium concentration and risk of short-term outcome of acute ischemic stroke. Results. Serum magnesium concentration in subjects with NIHSS ≥ 10/death was lower than those with NIHSS < 10 (p < 0.05). When comparing the highest quartile of serum magnesium concentration with the lowest quartile in an unadjusted model, there was a decreased risk of NIHSS ≥ 10/death in individuals with the highest quartile; the risk ratio (RR) was 0.47 (p < 0.05). However, after adjustment for age, sex, serum calcium concentration, serum potassium concentration and other covariates, the fourth and the third quartiles of serum magnesium concentration were associated with decreased risks of NIHSS ≥ 10/death; the RRs were 0.40 and 0.56 (all p < 0.05), respectively. The dose–response relationship between serum magnesium concentration and risk of NIHSS ≥ 10/death was not materially altered after adjustment for other covariates (p-value for trend = 0.002). Conclusion. Higher serum magnesium concentration was associated with lower risk of NIHSS ≥ 10/death; there was a dose–response relationship between serum magnesium concentration and risk of NIHSS ≥ 10/death.
Introduction
Stroke is the third highest cause of death and long-term disability worldwide (Citation1). Stroke accounted for 21.6% of total mortality in males and 20.8% of total mortality in females in China (Citation2), and the incidence of stroke is likely to increase in the next two decades (Citation3). Acute ischemic stroke accounted for 60% of total stroke (Citation4,Citation5), and due to the higher mortality and disability rate (Citation6), the prognosis of acute ischemic stroke remains an important issue. A potential neuroprotective effect of magnesium has been discussed (Citation7,Citation8), and an earlier case–control study suggested that maintaining high level of dietary magnesium may be beneficial in preventing ischemic stroke in southern Chinese adults (Citation9). Furthermore, a meta-analysis of seven prospective studies (Citation10) showed a modest but statistically significant inverse relationship between magnesium intake and risk of acute ischemic stroke. Recently, a prospective study found that serum magnesium concentration was inversely associated with ischemic stroke incidence (Citation11). In respect to the ongoing debate on serum magnesium concentration and ischemic stroke incidence, we wanted to assess the relationship between serum magnesium concentration and the prognosis of acute ischemic stroke in Chinese population. We used the data from Shandong province in Northern China to examine the influence of serum magnesium concentration on the short-term outcome among acute ischemic stroke patients.
Materials and Methods
Study participants
A total of 1686 patients with a clinical diagnosis of acute ischemic stroke admitted to the four hospitals (the Center Hospital of TaiAn, the 88 People's Liberation Hospital of TaiAn, the 89 People's Liberation Hospital of WeiFang and the 148 People's Liberation Hospital of ZiBo) in Shandong province were recruited from January 1, 2006 to December 31, 2008. One hundred and ninety-three patients were excluded from our analysis due to lack of serum magnesium concentration measurements. Written informed consent was obtained from all the patients. This study was approved by the Radiation Medicine and Public Health Ethics Committee of Soochow University.
Data collection
Baseline data were collected within the first 24 h after admission by in-person interviews with patients or their family members (if patients were not able to communicate). Data on demographic characteristics, lifestyle risk factors, medical history, clinical laboratory tests and imaging data (CT and MRI) were obtained through a standard questionnaire. Cigarette smokers were defined as having smoked at least one cigarette per day for 1 year or more. The amount and type of alcohol consumed during the past year were collected. Alcohol consumption was defined as consuming one or more alcoholic drinks per day during the last year. Blood pressure (BP) measurements were performed in the first 72 h (one measurement for every 8 h) after admission while the study participants were in the supine position using a standard mercury sphygmomanometer according to a standard protocol. The first and fifth Korotkoff sounds were recorded as systolic (SBP) and diastolic BP (DBP), respectively. The neurological deficiency of all patients was assessed by trained neurologists at discharge using NIH stroke scale (NIHSS) (Citation12). Study outcome was defined as death during hospitalization or neurological deficiency (NIHSS ≥ 10) at discharge (NIHSS ≥ 10/death).
Acute ischemic stroke was confirmed by imaging (CT scan or MRI) according to the acute ischemic stroke diagnosis and treatment guidelines in China (Citation13). The criterion of dyslipidemia was as follows: total cholesterol ≥ 6.22 mmol/l (high TC); triglyceride ≥ 2.26 mmol/l (high TG); low-density lipoprotein- cholesterol ≥ 4.14 mmol/l (high LDL-C); high-density lipoprotein-cholesterol < 1.04 mmol/l (low HDL-C) (Citation14). The hyperglycemia was defined as fasting plasma glucose ≥ 6.1 mmol/l (Citation15).
Plasma glucose was measured by a modified hexokinase enzymatic method. TC, HDL-C, TG, LDL-C, serum magnesium concentration, serum calcium concentration and serum potassium concentration were analyzed on a Beckman Synchron CX5 Delta Clinical System (Beckman Coulter, Inc., Fullerton, California, USA) using commercial reagents.
Statistical analysis
Patients were divided into two groups, NIHSS ≥ 10/death and NIHSS < 10. The mean and standard deviation of continuous variables and proportion of categorical variables were calculated and compared between two groups. Serum magnesium concentration was divided into four levels according to quartiles (< 0.83 mmol/l, 0.84–0.88 mmol/l, 0.89–0.97 mmol/l, ≥ 0.98 mmol/l; < 0.83 mmol/l is the lowest quartile and ≥ 0.98 mmol/l is the highest quartile). The Cox's proportional hazard regression model was used to examine the relationship between serum magnesium levels and short-term outcome of acute ischemic stroke. Statistical analyses were conducted using SAS statistical software (version 9.1; SAS Institute Inc, Cary, North Carolina, USA).
Results
presents the demographic and clinical characteristics at admission in patients with NIHSS ≥ 10/death or NIHSS < 10. A total of 1493 acute ischemic stroke were included in our analysis. Patients with NIHSS < 10 were more likely to have higher serum magnesium concentration and hospitalize days, whereas patients with NIHSS ≥ 10/death were more likely to have higher admission SBP and fasting plasma glucose, higher proportion of high LDL-C, hyperglycemia and history of atrial fibrillation. There were no significant differences in age, sex, serum calcium concentration, serum potassium concentration, admission DBP, TC, TG, LDL-C and HDL-C, proportion of cigarette smokers, alcohol consumption, high TC, high TG, low HDL-C, history of hypertension, history of diabetes, history of coronary heart disease, history of rheumatic heart disease, family history of stroke and family history of hypertension.
Table I. Baseline characteristics of acute ischemic stroke patients with NIHSS ≥ 10/death or NIHSS < 10.
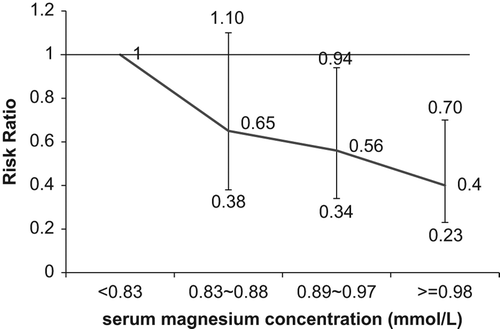
There was an inverse and significant association between serum magnesium concentration and risk of NIHSS ≥ 10/death (). When comparing with the lowest quartile of serum magnesium concentration, patients in the highest quartile had decreased risk of NIHSS ≥ 10/death; the risk ratio (RR) was 0.47 (p < 0.05). After adjustment for age, sex, serum calcium concentration, serum potassium concentration and other important covariates, the highest and the third quartile were associated with decreased risks of NIHSS ≥ 10/death; the RRs were 0.40 and 0.56 (all p < 0.05), respectively. The dose–response relationship between serum magnesium concentration and risk of NIHSS ≥ 10/death was not materially altered after adjustment for other covariates (p-value for trend = 0.002). illustrates the multiple-adjusted risk ratio and 95% confidence interval of NIHSS ≥ 10/death by quartiles of serum magnesium concentration among patients with acute ischemic stroke.
Table II. Risk ratio (RR) and 95% confidence interval (CI) of NIHSS ≥ 10/death by quartiles of serum magnesium concentration.
Discussion
Our study found an inverse and significant association between serum magnesium concentration and short-term outcome. The cross-sectional study by Ma et al. (Citation16) suggested that low serum magnesium concentration may be related to cardiovascular disease. Amighi et al.'s cohort study (Citation17) found that low serum magnesium level predicted neurological events, mainly ischemic stroke, in patients with advanced atherosclerosis. The study by Ohira et al. (Citation11) showed that low serum magnesium level was associated with increased risk of ischemic stroke; however, the association was not significant after adjustment for hypertension and diabetes. The association still existed after adjustment for hypertension and diabetes in our study. A prospective study in male subjects (Citation18) showed that dietary magnesium was not associated with cardiovascular mortality but hypertension and other covariates were not taken into account. Reffelmann's prospective study (Citation19) suggested that low serum magnesium level was associated with higher all-cause mortality and cardiovascular mortality, and this association remained statistically significant after adjustment for various cardiovascular risk factors, such as arterial hypertension and antihypertensive therapies. This conclusion was consistent with ours.
The mechanisms by which high serum magnesium concentration may decrease risk of NIHSS ≥ 10/death among acute ischemic stroke patients have not yet been fully elucidated. Firstly, low level of magnesium concentration may accelerate atherosclerosis by promoting inflammation and oxidative modification. Magnesium deficiency could be associated with the onset of an inflammatory response leading to increase circulating levels of cytokines, which triggers oxidative responses in endothelial cells (Citation20,Citation21). This may be the basis of a potential neuroprotective effect. Secondly, magnesium deficiency could be associated with the risk of thrombus formation, which may exacerbate ischemic injury. Some studies have shown that platelet-dependent thrombosis was significantly increased in stable coronary artery disease patients with low intracellular magnesium level (Citation22). Thirdly, BP is a risk factor of acute ischemic stroke and can also influence the prognosis. Magnesium may lower BP by acting like a natural calcium channel blocker. Specifically, magnesium competes with sodium for binding sites on vascular smooth muscle cells, binding to potassium in a cooperative manner, to induce endothelial-dependent vasodilation and BP reduction (Citation23–25). Fourthly, serum magnesium can bind to adenosine triphosphate, increase intracellular energy stores and thus promote survival of ischemic neurons (Citation26). In addition, serum magnesium concentration was inversely associated with von Willebrand factor, and a previous study indicated that von Willebrand factor was associated with worse clinical course after stroke (Citation27). Finally, magnesium can reduce the ischemic injury through enhancing the reuptake of catecholamines by Mg2+–ATP enzyme system. Norepinephrine is one of catecholamines family of neurotransmitters derived from the amino acid tyrosine (Citation28), and increased blood norepinephrine concentration within 48 h of hemispheric infarction independently predicted poor outcome at 1 year (Citation29).
Magnesium in serum accounts for less than 1% of the whole-body magnesium concentration, and serum magnesium concentration may thus not reflect magnesium concentration accurately in other tissues. However, serum magnesium level has shown to be correlated with the intracellular free magnesium level (r = 0.54, p < 0.05) (Citation16, Citation30). Thus serum magnesium can equally reflect the activity of intracellular free magnesium. Other studies found that serum calcium and potassium concentration may be confounding factors (Citation31,Citation32); however after adjustment for the above factors, the inverse and significant association between serum magnesium concentration and short-term outcome remained significant in our study.
Potential limitations of this study merit consideration. Treatment of hypertension with diuretics may lower serum magnesium concentration (Citation33). In addition, nutrients or dietary components that are related to serum magnesium concentration were not available, which would most likely to lead to an underestimation of the true relationship.
In conclusion, the present study suggested that higher serum magnesium concentration was associated with lower risk of NIHSS ≥ 10/death among patients with acute ischemic stroke in Northern Chinese population.
Acknowledgement
We are deeply appreciative of the participants in this study, and thank all staffs for their support and assistance. This work was supported by a Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Conflict of interest: The authors declare no financial or other conflict of interests.
References
- WHO. Global Burden of Disease 2002: Deaths by age, sex and cause for the year 2002. Geneva: World Health Organization; 2003.
- He J, Gu D, Wu X, Reynolds K, Duan X, Yao C, et al. Major causes of death among men and women in China. N Engl J Med. 2005;353:1124–1134.
- Meairs S, Wahlgren N, Dirnagl U, Lindvall O, Rothwell P, Baron JC, et al. Stroke research priorities for the next decade – A representative view of the European scientific community. Cerebrovasc Dis. 2006;22:75–82.
- Jiang B, Wang WZ, Chen H, Hong Z, Yang QD, Wu SP, et al. Incidence and trends of stroke and its subtypes in China: Results from three large cities. Stroke. 2006;37:63–68.
- Liang W, Huang R, Lee AH, Hu D, Binns CW. Hospitalizations for incident stroke in Shunde District, Foshan, South China. Neuroepidemiology. 2008;30:101–104.
- Zhang Y, Reilly KH, Tong W, Xu T, Chen J, Bazzano LA, et al. Blood pressure and clinical outcome among patients with acute stroke in Inner Mongolia, China. J Hypertens. 2008; 26:1446–1452.
- Stewart D, Marder VJ, Starkman S, Saver JL. Magnesium sulfate neither potentiates nor inhibits tissue plasminogen activator- induced thrombolysis. J Thromb Haemost. 2006;4:1575–1579.
- Muir KW. Magnesium for neuroprotection in ischaemic stroke: Rationale for use and evidence of effectiveness. CNS Drugs. 2001;15:921–930.
- Liang W, Lee AH, Binns CW. Dietary intake of minerals and the risk of ischemic stroke in Guangdong Province, China, 2007–2008. Prev Chronic Dis. 2011;8:A38.
- Larsson SC, Orsini N, Wolk A. Dietary magnesium intake and risk of stroke: A meta-analysis of prospective studies. Am J Clin Nutr. 2012;95:362–366.
- Ohira T, Peacock JM, Iso H, Chambless LE, Rosamond WD, Folsom AR. Serum and dietary magnesium and risk of ischemic stroke: The Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2009;169:1437–1344.
- Shafqat S, Kvedar JC, Guanci MM, Chang Y, Schwamm LH. Role for telemedicine in acute stroke. Feasibility and reliability of remote administration of the NIH stroke scale. Stroke. 1999;30:2141–2145.
- The writing group of acute ischemic stroke diagnosis and treatment guideline in Chinese Medical Academy of Neurology. Acute ischemic stroke diagnosis and treatment guideline in China. Chin J Neurol. 2010;43:146–152.
- Prevention guide of dyslipidemia in chinese adults. Chin J Cardiol. 2007;35:390–419.
- Alberti K, Zimmet P. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med. 1998;15:539–553.
- Ma J, Folsom AR, Melnick SL, Eckfeldt JH, Sharrett AR, Nabulsi AA, et al. Associations of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: The ARIC study. Atherosclerosis Risk in Communities Study. J Clin Epidemiol. 1995;48:927–940.
- Amighi J, Sabeti S, Schlager O, Mlekusch W, Exner M, Lalouschek W, et al. Low serum magnesium predicts neurological events in patients with advanced atherosclerosis. Stroke. 2004;35:22–27.
- Kaluza J, Orsini N, Levitan EB, Brzozowska A, Roszkowski W, Wolk A. Dietary calcium and magnesium intake and mortality: A prospective study of men. Am J Epidemiol. 2010; 171:801–807.
- Reffelmann T, Ittermann T, Dörr M, Völzke H, Reinthaler M, Petersmann A, et al. Low serum magnesium concentrations predict cardiovascular and all-cause mortality. Atherosclerosis. 2011;219:280–284.
- Ferrè S, Mazur A, Maier JA. Low-magnesium induces senescent features in cultured human endothelial cells. Magnes Res. 2007;20:66–71.
- Wolf FI, Trapani V, Simonacci M, Ferré S, Maier JA. Magnesium deficiency and endothelial dysfunction: Is oxidative stress involved?Magnes Res. 2008;21:58–64.
- Shechter M, Merz CN, Rude RK, Paul Labrador MJ, Meisel SR, Shah PK, et al. Low intracellular magnesium levels promote platelet-dependent thrombosis in patients with coronary artery disease. Am Heart J. 2000;140: 212–218.
- Jee SH, Miller ER 3rd,Guallar E, Singh VK, Appel LJ, Klag MJ. The effect of magnesium supplementation on blood pressure: A meta-analysis of randomized clinical trials. Am J Hypertens. 2002;15:691–696.
- Bo S, Pisu E. Role of dietary magnesium in cardiovascular disease prevention, insulin sensitivity and diabetes. Curr Opin Lipidol. 2008;19:50–56.
- Uzui H, Lee JD. Role of magnesium in hypertension therapy. Clin Calcium. 2005;15:117–122.
- Schanne FA, Gupta RK, Stanton PK. 31P-NMR study of transient ischemia in rat hippocampal slices in vitro. Biochim Biophys Acta. 1993;28:1158:257–263.
- Audebert HJ, Pellkofer TS, Wimmer ML, Haberl RL. Progression in lacunar stroke is related to elevated acute phase parameters. Eur Neurol. 2004;51:125–131.
- Katan M, Elkind MS. Inflammatory and neuroendocrine biomarkers of prognosis after ischemic stroke. Expert Rev Neurother. 2011;11:225–239.
- Sander D, Winbeck K, Klingelhöfer J, Etgen T, Conrad B. Prognostic relevance of pathological sympathetic activation after acute thromboembolic stroke. Neurology. 2001,11: 833–838.
- Ryzen E, Servis KL, DeRusso P, Kershaw A, Stephen T, Rude RK. Determination of intracellular free magnesium by nuclear magnetic resonance in human magnesium deficiency. J Am Coll Nutr. 1989;8:580–587.
- Appel SA, Molshatzki N, Schwammenthal Y, Merzeliak O, Toashi M, Sela BA, et al. Serum calcium levels and long-term mortality in patients with acute stroke. Cerebrovasc Dis. 2011;31:93–99.
- Ascherio A, Rimm EB, Hernán MA, Giovannucci EL, Kawachi I, Stampfer MJ, et al. Intake of potassium, magnesium, calcium, and fiber and risk of stroke among US men. Circulation. 1998;98:1198–1204.
- Musso CG. Magnesium metabolism in health and disease. Int Urol Nephrol. 2009;41:357–362.