Abstract
Background. Abnormal variations in night-time hypertension such as “non-dipping” type (< 10% decrease in nocturnal systolic blood pressure [SBP] from daytime SBP) are a risk factor for cardiovascular events independent of 24-h BP. Methods. As part of a randomized, double-blind study of azilsartan (20–40 mg once daily) and candesartan (8–12 mg once daily) in Japanese patients with essential hypertension, an exploratory analysis was performed using ambulatory BP monitoring (ABPM) at baseline and Week 14. Effects of study drugs on nocturnal BP variations according to patients’ nocturnal SBP dipping status were evaluated. Results. ABPM data were available for 273 patients treated with azilsartan and 275 with candesartan. In the dipping group (≥ 10% decrease from daytime SBP), azilsartan produced a greater reduction from baseline in daytime than in night-time SBP (− 14.1 and − 10.9 mmHg, respectively), and the change in daytime SBP was significantly greater with azilsartan than with candesartan (p = 0.0077). In the non-dipping group, azilsartan produced a greater reduction from baseline in night-time than in daytime SBP (− 20.2 and − 9.9 mmHg, respectively), and reductions in both night-time SBP (p = 0.02) and daytime SBP (p = 0.0042) were significantly greater with azilsartan than with candesartan. Conclusions. Once-daily azilsartan improved non-dipping night-time SBP to a greater extent than candesartan in Japanese patients with grade I–II essential hypertension.
Introduction
It is well known that abnormal patterns of nocturnal blood pressure (BP) variation are associated with increased cardiovascular risk. In normotensive subjects, BP decreases during sleep by 10% or more from the level recorded while awake (dipper) and increases promptly on waking. This normal nocturnal BP variation pattern is usually preserved in hypertensive patients, particularly when there is no target organ damage. Among abnormal nocturnal BP variation patterns, a non-dipper type (nocturnal decrease in BP < 10%) has been described which can result in nocturnal hypertension and early morning hypertension. As cardiovascular events, including stroke, frequently occur in the period from early morning to noon, effective control of night-time and early morning BP is important to help prevent such events (Citation1,Citation2).
The nocturnal non-dipper type is of particular interest because it is a risk factor for hypertensive target organ damage and cardiovascular events independent of the 24-h BP level (Citation3–9). Ohkubo et al. (Citation10) analyzed the relationship between risk of cardiovascular mortality and the nocturnal fall in BP and found that a 5% decrease in the nocturnal fall in SBP was associated with an 18% increase in the risk of cardiovascular mortality. For these reasons, alleviating nocturnal BP variation is important for preventing cardiovascular events. Only recently has sufficient evidence been accumulated about the lowering of night-time BP to enable recommendations for treatment. The core of such treatment is to use drugs with a long-lasting BP-lowering effect that is sustained throughout the night.
Azilsartan is a new angiotensin receptor blocker (ARB) that selectively inhibits the binding of angiotensin II (AII) to AII type 1 (AT1) receptors. In an in vitro study, azilsartan was shown to have higher affinity for and slower dissociation from AT1 receptors than other ARBs including olmesartan, telmisartan, valsartan and irbesartan (Citation11); it is thus expected to exert a more potent and sustained BP-lowering effect than these ARBs. In a previously-reported randomized, double-blind comparative study, we observed that once-daily administration of azilsartan produced more potent 24-h sustained antihypertensive activity than candesartan in Japanese patients with grade I–II essential hypertension, and had an equivalent level of safety (Citation12). Analysis of ambulatory blood pressure monitoring (ABPM) data in this same study showed that azilsartan provided greater BP reduction than candesartan over the entire 24-h monitoring period as well as during the specific daytime, night-time and early morning periods. The current exploratory analysis was planned as part of this comparative study in order to analyze the ABPM data in more detail according to patients’ nocturnal BP dipping status, with the goal of comparing the effects of azilsartan and candesartan on nocturnal BP variation.
Methods
The above-mentioned study which compared azilsartan with candesartan in Japanese patients was conducted over a period of 16 weeks at 33 centers in Japan between May 2009 and June 2010 (Clinical Trial Identifier: JapicCTI-090762). The study procedures, patient inclusion/exclusion criteria, dosage regimens, efficacy and safety endpoints, statistical analysis and ethical provisions have been reported previously (Citation12), and will be described only briefly in this exploratory analysis report.
In this study, patients received either azilsartan 20 mg daily for the first 8 weeks followed by 40 mg daily for the second 8 weeks, or candesartan cilexetil (candesartan) 8 mg daily for the first 8 weeks followed by 12 mg daily for the second 8 weeks. ABPM was conducted over a period of ≥ 26 h using an oscillometric monitor (TM-2431; A&D Co., Inc.) at baseline (Week 0) and at Week 14 of the study, during which time BP was measured at 30-min intervals starting at 10:00 h (± 1 h). For ABPM at Week 14, patients took their assigned study drug ≥ 1 h after the start of measurements in the morning and after completion of measurements on the following day. During the period of ABPM, patients were instructed to avoid taking a bath, taking an afternoon nap, performing exercise or consuming alcohol or caffeine-containing food/drinks. For ABPM data to be acceptable for analysis, a minimum of 80% of the BP readings recorded during a 24-h period were required, there could be no more than 2 non-consecutive hours with < 1 valid reading, and there could be no patient behaviors that would have seriously affected BP (e.g. afternoon naps, drinking alcohol).
Analysis of ABPM data by dipping status of nocturnal BP
Patients who underwent ABPM at baseline were subsequently classified by dipping status according to the degree of their nocturnal fall in SBP. Nocturnal SBP fall (%) was calculated as 100×[1 − mean night-time SBP/mean daytime SBP]. Night-time BP was defined as the average of BP measurements from bedtime to 1 min before waking; daytime BP was defined as the average of BP measurements from midday on Day 1 of ABPM measurements to 1 min before bedtime, and from wake-up time to 11:59 h on Day 2. On this basis, patients were classified into either dipping (SBP fall ≥ 10%) or non-dipping (SBP fall < 10%) groups.
Statistical analysis
The 24-h time-course changes in SBP, diastolic BP (DBP) and heart rate in each of the two treatment groups were analyzed by calculating summary statistics at each evaluation time point (every hour from 12:00 h on Day 1 to 12:00 h on Day 2 of measurement). The change from baseline to Week 14 was analyzed for the daytime average and night-time average of SBP and DBP by calculating summary statistics in each treatment group, followed by a one-sample t-test. For each parameter except heart rate, analysis of covariance (ANCOVA) was carried out for the two dipping patterns by using a model in which plasma renin activity (< 0.5 or ≥ 0.5 ng/ml/h) at Week ‐2 and treatment group served as independent variables and baseline values served as covariates.
Results
Patient characteristics
ABPM data, which enabled evaluation at both baseline and Week 14, were available for 548 patients, 273 of whom were randomized to receive azilsartan and 275 to candesartan. With regard to patients’ baseline characteristics (), the percentage of males was slightly lower in the azilsartan dipping group than in the candesartan dipping group (55.9% vs 66%) and was slightly higher in the azilsartan non-dipping group than in the candesartan non-dipping group (61.7% vs 57.5%). Otherwise, there were no notable differences in mean age, body mass index (BMI) or BP values between treatment groups at baseline when compared by corresponding dipping type (i.e. dipping vs dipping; non-dipping vs non-dipping). Treatment compliance ranged between 90% and 100% in 99% of patients in both groups.
Table I. Patient characteristics at baseline (Week 0 unless indicated otherwise).
BP-lowering effects by dipping status
The 24-h SBP time profiles at baseline and at Week 14 in both treatment groups according to dipping status are shown in . The change from baseline in mean daytime and night-time SBP is shown in .
Figure 1. Twenty-four-hour time-course changes in mean systolic blood pressure (SBP) at baseline (Week 0) and Week 14 according to dipping status in patients with essential hypertension treated with azilsartan or candesartan: (a) non-dipping group; (b) dipping group.
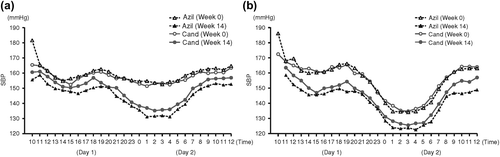
Figure 2. Changes from baseline (Week 0) to Week 14 in mean systolic blood pressure (SBP) according to dipping status in patients with essential hypertension treated with azilsartan or candesartan: (a) non-dipping group; (b) dipping group.
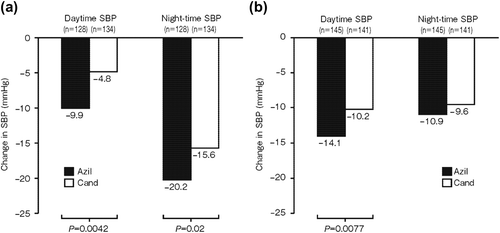
Azilsartan produced significant reductions from baseline to Week 14 in daytime SBP and night-time SBP in the non-dipping group (− 9.9 and − 20.2 mmHg, respectively; p < 0.001) () and in the dipping group (− 14.1 and − 10.9 mmHg, respectively; p < 0.001) (). In the non-dipping group, the reduction in night-time SBP was greater than that in daytime SBP, whereas in the dipping group, the reduction in daytime SBP was greater than that in night-time SBP.
Candesartan produced significant reductions from baseline to Week 14 in daytime SBP and night-time SBP in the non-dipping group (− 4.8 and − 15.6 mmHg, respectively; p < 0.001) () and in the dipping group (− 10.2 and − 9.6 mmHg, respectively; p < 0.001) (). In the non- dipping group, the reduction in night-time SBP was greater than that in daytime SBP, whereas in the dipping group, the reductions in night-time SBP and daytime SBP were of a similar magnitude.
Comparison of BP-lowering effect between treatment groups
The mean changes in SBP from baseline to Week 14 in both treatment groups according to dipping status are provided in and shown in .
Table II. Changes in mean systolic blood pressure (SBP, mmHg) from baseline to Week 14 according to baseline dipping pattern in patients with essential hypertension receiving azilsartan or candesartan.
In the non-dipping group (), the reductions in both daytime SBP (p = 0.0042) and night-time SBP (p = 0.02) were significantly greater with azilsartan than with candesartan. In the dipping group (), the reduction in daytime SBP was significantly greater with azilsartan than with candesartan (p = 0.0077), while the reduction in night-time SBP was not significantly different between the treatment groups.
Changes in DBP followed a similar pattern as those observed for SBP in both the dipping and non-dipping groups ().
Table III. Changes in mean diastolic blood pressure (DBP, mmHg) from baseline to Week 14 according to baseline dipping pattern in patients with essential hypertension receiving azilsartan or candesartan.
Discussion
Alleviating nocturnal BP variation is important for preventing cardiovascular events. A rational approach is to use drugs with a long-lasting BP-lowering effect that is sustained throughout the night. Among ARBs, telmisartan and valsartan have been reported to shift the non-dipping pattern to the dipping pattern if administered at bedtime (Citation13,Citation14), whereas olmesartan has been shown to reduce mean 24-h SBP and DBP to a similar extent irrespective of whether it is administered in the morning or evening (Citation15).
We have previously reported that once-daily azilsartan produced greater reductions than candesartan in clinic BP, 24-h BP and BP during the daytime, night-time and early morning while offering comparable safety in Japanese patients with grade I–II essential hypertension (Citation12). In this exploratory analysis, we analyzed ABPM data according to patients’ dipping status at baseline, with the goal of comparing the effects of azilsartan and candesartan on nocturnal BP variations. Overall, azilsartan was found to exert better antihypertensive activity than candesartan for both the dipping and non-dipping patterns of nocturnal hypertension. In particular, in the non-dipping group (i.e. those with persistent hypertension during night-time and a propensity toward early morning hypertension), azilsartan produced a remarkable reduction in night-time SBP, and the magnitude of the reduction was significantly greater than that with candesartan. On the basis of the greater reductions in BP with azilsartan compared with candesartan reported previously, and the greater reduction in night-time hypertension in the non-dipping group produced by azilsartan versus candesartan in this exploratory analysis, it might be expected that azilsartan would provide greater protection against cardiovascular events in patients with essential hypertension. This is supported by the association of non-dipping patterns of nocturnal hypertension as an apparent risk factor for cardiovascular events (Citation3–10).
The causes and mechanisms of abnormal circadian BP variations are diverse; cause-and-effect relationships between factors of circadian BP variation, organ dysfunction and cardiovascular risk are not clear. Previous studies have suggested a number of possible causes/mechanisms of non-dipping patterns including enhanced-pressure natriuresis at night due to a renal defect in daytime sodium excretory capability (Citation16); disturbed reduction of nocturnal sympathetic nervous activity (Citation17–19); and insulin resistance (Citation20,Citation21). It is well known that AII stimulates tubular sodium reabsorption at various segments from the proximal to the distal tubules, and that ARBs suppress tubular sodium reabsorption in vitro (Citation22). Fukuda et al. (Citation23,Citation24) recently reported that nocturnal BP elevation due to sodium retention was suppressed by olmesartan through inhibition of tubular sodium reabsorption, resulting in a shift of circadian BP variation from the non-dipping to the dipping type in patients with chronic kidney disease. In other studies, it has been reported that administration of an α-adrenergic blocker at bedtime produced significant reductions in night-time BP only in patients showing the non-dipping pattern (Citation19). AII has also been shown to potentiate the activity of rostral ventrolateral medulla neurons, a sympathetic nervous center, whereas an ARB (candesartan) reduced rostral ventrolateral medulla activities in spontaneously hypertensive rats (Citation25). Furthermore, pioglitazone, which acts as an insulin sensitizer, restored nocturnal BP decreases in parallel with a reduction in the homeostasis model assessment index in non-dippers (but not dippers) with type 2 diabetes mellitus (Citation26).
In vitro studies have shown that azilsartan has more powerful inhibition and slower detachment from the AT1 receptor than other ARBs (Citation11). In a previous report of our study involving Japanese patients with grade I or II essential hypertension, once-daily azilsartan exerted a more potent 24-h sustained antihypertensive effect compared with candesartan (Citation12) and, in this analysis, it was shown to produce a greater reduction in nocturnal hypertension in the non-dipping group compared with candesartan. It seems likely, therefore, that the effect of azilsartan in normalizing circadian BP variation depends on potent, 24-h sustained antihypertensive activity, including suppression of sodium reabsorption and abnormal nocturnal sympathetic nervous activity due to its potent and sustained AT1 receptor inhibitory effect on the renin–angiotensin–aldosterone system. Since azilsartan greatly improved glucose intolerance and insulin sensitivity compared with candesartan in type 2 diabetic KK-A(y) mice (Citation27), it is possible that azilsartan may also normalize circadian BP variation in part by improving insulin resistance.
Limitations of the present study include possible issues with the reproducibility of ABPM data. Although ABPM was carried out at two time points in our study (one session at baseline and another at Week 14), issues regarding the reproducibility of classifying patients into dipping and non-dipping status have previously been described. In an ABPM study conducted over 2 days, about 70% of patients were classified with the same type of variation (i.e. either dipping or non-dipping) on Day 1 and Day 2 of the ABPM session, whereas the dipping profile differed between session days in the remaining 30% of patients (Citation28). Classification of dipping patterns ideally needs to be based on two or more sessions of ABPM. A further limitation relates to the short study duration, which prevented demonstration of a relationship between improvement in nocturnal BP variation and cardiovascular events. Indeed, the effect on cardiovascular risk of normalizing circadian BP variation has not, as yet, been fully evaluated. However, independent of the 24-h BP level, the non- dipping type of nocturnal BP variation is clearly a risk factor for cardiovascular events and cardiovascular mortality (Citation6,Citation10). Furthermore, the MAPEC (Ambulatory Blood Pressure Monitoring and Cardiovascular Events) study has indicated that an increase in sleep-time relative BP decline toward a more normal dipping pattern is associated with a decrease in cardiovascular risk, whereas a decrease in nocturnal BP fall is associated with an increase in morbidity and mortality (Citation29). In light of these collective findings, evaluation of the potential decrease in cardiovascular risk from normalization of circadian BP variation beyond the reduction of BP levels warrants further prospective investigation.
In conclusion, this study has shown that once-daily administration of azilsartan improved the non-dipping pattern in nocturnal hypertension and was significantly more effective than candesartan in this regard. Although it remains to be confirmed in long-term studies, it is possible that azilsartan may provide cardioprotection not only through its potent and sustained antihypertensive activity but also through its effect on improving nocturnal BP variation.
Acknowledgements
The authors would like to thank all patients, physicians and medical staff who supported this study, and ContentEd Net for editorial assistance with the manuscript, which was funded by Takeda Pharmaceutical Company, Limited.
Conflict of interest
HR and KK served as medical experts for this study. HR received honoraria from Takeda Pharmaceutical Company for lectures he gave during the study period. KE, MI and YI are all employees of Takeda Pharmaceutical Company. All authors state that they have no conflicts of interest regarding the content of this article other than those stated above.
Source of funding
The study was sponsored by Takeda Pharmaceutical Company, Limited.
References
- Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation. 1989;79:733–743.
- Kario K. Time for focus on morning hypertension: Pitfall of current antihypertensive medication. Am J Hypertens. 2005;18:149–151.
- Staessen JA, Thijs L, Fagard R, O’Brien ET, Clement D, de Leeuw PW, et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. JAMA. 1999;282:539–546.
- Metoki H, Ohkubo T, Kikuya M, Asayama K, Obara T, Hashimoto J, et al. Prognostic significance for stroke of a morning pressor surge and a nocturnal blood pressure decline: The Ohasama study. Hypertension. 2006;47:149–154.
- Kikuya M, Ohkubo T, Asayama K, Metoki H, Obara T, Saito S, et al. Ambulatory blood pressure and 10-year risk of cardiovascular and noncardiovascular mortality: The Ohasama study. Hypertension. 2005;45:240–245.
- Hoshide S, Kario K, Hoshide Y, Umeda Y, Hashimoto T, Kunii O, et al. Associations between nondipping of nocturnal blood pressure decrease and cardiovascular target organ damage in strictly selected community-dwelling normotensives. Am J Hypertens. 2003;16:434–438.
- Ohkubo T, Imai Y, Tsuji I, Nagai K, Watanabe N, Minami N, et al. Relation between nocturnal decline in blood pressure and mortality. The Ohasama Study. Am J Hypertens. 1997;10:1201–1207.
- Kario K, Matsuno T, Kobayashi H, Imiya M, Matsuo M, Shimada K. Nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensive patients. Advanced silent cerebrovascular damage in extreme dippers. Hypertension.1996;27:130–135.
- Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension 2001; 38:852–857.
- Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: The Ohasama study. J Hypertens. 2002; 20:2183–2189.
- Ojima M, Igata H, Tanaka M, Sakamoto H, Kuroita T, Kohara Y, et al. In vitro antagonistic properties of a new angiotensin type 1 receptor blocker, azilsartan, in receptor binding and function studies. J Pharmacol Exp Ther. 2011;336:801–808.
- Rakugi H, Enya K, Sugiura K, Ikeda Y. Comparison of the efficacy and safety of azilsartan with that of candesartan cilexetil in Japanese patients with grade I–II essential hypertension: A randomized, double-blind clinical study. Hypertens Res. 2012;35:552–558.
- Hermida RC, Ayala DE, Fernandez JR, Calvo C. Comparison of the efficacy of morning versus evening administration of telmisartan in essential hypertension. Hypertension. 2007;50:715–722.
- Hermida RC, Ayala DE, Calvo C. Optimal timing for antihypertensive dosing: Focus on valsartan. Ther Clin Risk Manag. 2007;3:119–131.
- Smolensky MH, Hermida RC, Portaluppi F. Comparison of the efficacy of morning versus evening administration of olmesartan in uncomplicated essential hypertension. Chronobiol Int. 2007;24:171–181.
- Uzu T, Kimura G. Diuretics shift circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1999;100:1635–1638.
- Sherwood A, Steffen PR, Blumenthal JA, Kuhn C, Hinderliter AL. Night-time blood pressure dipping: The role of the sympathetic nervous system. Am J Hypertens. 2002; 15:111–118.
- Grassi G, Seravalle G, Quarti-Trevano F, Dell’Oro R, Bombelli M, Cuspidi C, et al. Adrenergic, metabolic, and reflex abnormalities in reverse and extreme dipper hypertensives. Hypertension. 2008;52:925–931.
- Kario K, Schwartz JE, Pickering TG. Changes of nocturnal blood pressure dipping status in hypertensives by night-time dosing of α-adrenergic blocker, doxazosin: Results from the HALT study. Hypertension. 2000;35:787–794.
- Anan F, Takahashi N, Ooie T, Yufu K, Saikawa T, Yoshimatsu H. Role of insulin resistance in nondipper essential hypertensive patients. Hypertens Res. 2003;26: 669–676.
- Della Mea P, Lupia M, Bandolin V, Guzzon S, Sonino N, Vettor R, et al. Adiponectin, insulin resistance, and left ventricular structure in dipper and nondipper essential hypertensive patients. Am J Hypertens. 2005;18:30–35.
- Coltamai L, Maillard M, Simon A, Vogt B, Burnier M. Comparative vascular and renal tubular effects of angiotensin II receptor blockers combined with a thiazide diuretic in humans. J Hypertens. 2010;28:520–526.
- Fukuda M, Yamanaka T, Mizuno M, Motokawa M, Shirasawa Y, Miyagi S, et al. Angiotensin II type 1 receptor blocker, olmesartan, restores nocturnal blood pressure decline by enhancing daytime natriuresis. J Hypertens. 2008;26: 583–588.
- Fukuda M, Wakamatsu-Yamanaka T, Mizuno M, Miura T, Tomonari T, Kato Y, et al. Angiotensin receptor blockers shift the circadian rhythm of blood pressure by suppressing tubular sodium reabsorption. Am J Physiol Renal Physiol. 2011;301:F953–F957.
- Kumagai H, Oshima N, Matsuura T, Iigaya K, Imai M, Onimaru H, et al. Importance of rostral ventrolateral medulla neurons in determining efferent sympathetic nerve activity and blood pressure. Hypertens Res. 2012;35:132–141.
- Anan F, Masaki T, Fukunaga N, Teshima Y, Iwao T, Kaneda K, et al. Pioglitazone shift circadian rhythm of blood pressure from non-dipper to dipper type in type 2 diabetes mellitus. Eur J Clin Invest. 2007;37:709–714.
- Iwai M, Chen R, Imura Y, Horiuchi M. TAK–536, a new AT1 receptor blocker, improves glucose intolerance and adipocyte differentiation. Am J Hypertens. 2007; 20:579–586.
- Mochizuki Y, Okutani M, Donfeng Y, Iwasaki H, Takusagawa M, Kohno I, et al. Limited reproducibility of circadian variation in blood pressure dippers and nondippers. Am J Hypertens. 1998,11:403–409.
- Portaluppi F, Smolensky MH. Perspectives on the chronotherapy of hypertension based on the results of the MAPEC study. Chronobiol Int. 2010;27:1652–1667.
