Abstract
Psoriasis is a hereditary, chronic inflammatory disorder of the skin. Generally, the psoriatic process is limited to the skin; however, internal organs such as the kidneys may be involved in the course. Several glomerular diseases have been distinguished due to renal histological findings of psoriatic patients to date. The underlying pathogenetic mechanisms of these associations remain unclear because of the limited number of cases. We report a case of primary membranoproliferative glomerulonephritis (MPGN) in a psoriatic patient. This is the first reported case that demonstrates the coexistence of MPGN and psoriasis.
INTRODUCTION
Psoriasis is a hereditary, chronic inflammatory disorder of the skin characterized by epidermal hyperplasia, altered keratinocyte differentiation, and vascular hyperplasia with typical hyperkeratotic and erythematous plaques, papules, or patches covered with silvery white squames.Citation[1] The disease affects 1.5–2.0 percent of the population in western countries.Citation[2] It is generally accepted that the psoriatic process is limited to the skin; however, internal organs such as the kidneys may be involved with similar vascular lesions.Citation[3] Indeed, an increase in urinary albumin excretion rate has been demonstrated in patients affected with diffuse psoriasis, which is regarded as an early marker of glomerular dysfunction.Citation[4] Although renal involvement is rare during the course of psoriasis, certain glomerular diseases including secondary renal amyloidosis, Ig A nephropathy, and membranous glomerulopathy were proposed to be more common in psoriatic patients.Citation[5–7] In this paper, we describe a patient with psoriasis who developed membranoproliferative glomerulonephritis (MPGN). This is the first reported case that demonstrates the coexistence of MPGN and psoriasis.
CASE REPORT
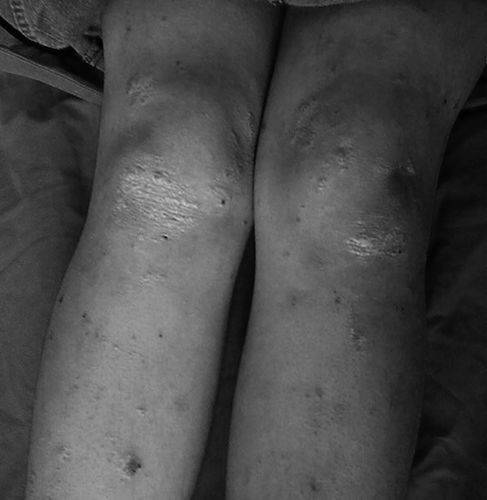
An 18-year-old woman was referred to our clinic for nephrotic range proteinuria. She presented with fatigue and swelling of lower limbs for three months. The medical history revealed that she was diagnosed as having psoriasis two years ago. She was on topical therapy for psoriasis. No oral steroid therapy, methotrexate, cyclosporin A, or long-term nonsteroidal anti-inflammatory drugs were used since the diagnosis was made. She had no history of smoking, alcohol consumption, or illicit drug use. There was no other considerable morbidity in her medical history. Upon admission, the patient's vital signs were within normal limits with a blood pressure reading of 130/80 mmHg. Upon physical examination, multiple erythematous, hyperkeratotic plaques and papules with silvery squames were observed on the extensor surfaces of elbows, knees, and scalp (see ). A moderate edema involved the lower part of extremities bilaterally. There were no signs of arthritis on joint examination.
Figure 1. Erythematous, hyperkeratotic plaques with silvery squames on the extansor surfaces of knees.
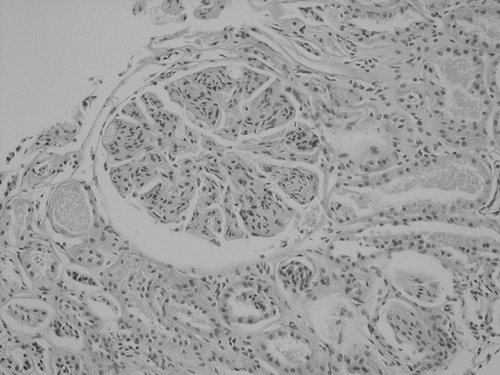
Upon admission, her hemoglobin level was 8.7 g/dL with a mean corpuscular volume of 68.7 fL. White blood cell count and platelet count were within normal range. Peripheral smear examination revealed microcytosis, hypochromia, anisocytosis, and poikilocytosis. Laboratory measurements for serum iron parameters were consistent with iron deficiency anemia. Fecal occult blood testing was negative all three times. Liver enzyme tests, serum bilirubin, alkaline phosphatase, calcium, phosphorus, and parathormone levels were within normal range, and there were no electrolyte disorders. Serum albumin level was 2.0 g/dL with serum urea and creatinine concentration of 39 and 0.64 mg/dL, respectively. Urine analysis revealed mild hematuria and proteinuria with granular casts. Spot urine sampling revealed a protein level of 500 mg/dL. Proteinuria in 24-h urine was 5.6 g/day. Antinuclear antibody (ANA) titer and anti-ds DNA antibodies were negative. Serum complement 3 (C3) level was 0.91 g/L (normal ranges, 0.90–1.80) and serum complement 4 level (C4) was 0.09 g/L (normal ranges, 0.10–0.40). C-reactive protein (CRP) was within normal range. Serological markers of hepatitis B and C viruses were negative. Serum protein electrophoresis was unremarkable. Abdominal ultrasound did not show organomegaly or any renal abnormalities. Skin biopsy was consistent with psoriasis. Because the patient had nephrotic range proteinuria, an ultrasound-guided renal biopsy was performed. Renal biopsy revealed glomerulonephritis with prominent mesangial and endocapillary proliferation accompanied by peripheral capillary loop thickening in the glomerulus, which were characteristic for membranoproliferative morphology (see ). Systemic diseases in association with membranoproliferative glomerulonephritis such as connective tissue diseases, chronic infections, malignancy, liver diseases, sarcoidosis, thrombotic microangiopathies, and heroin use were excluded. Thus, diagnosis of membranoproliferative glomerulonephritis with psoriasis was made. A treatment protocol including prednisone 1 mg/kg/day and cyclosporine A 3 mg/kg/day was started. She was discharged on the fourth day of the hospitalization and was to come for regular hospital visits. After 12 weeks of treatment, her skin lesions completely resolved. Serum albumin level was 3.5 g/dL with serum urea and creatinine concentration of 43 and 0.6 mg/dL, respectively. The 24-h urinary protein was 2.7 g/day, and urinalysis revealed a protein level of 30 mg/dL without hematuria or granular casts. The follow-up examination 10 months after the diagnosis showed a serum albumin level of 4.1 g/dL with serum creatinine concentration of 0.7 mg/dL. The urinalysis was completely normal, and 24-h urinary protein was only 960 mg/day.
DISCUSSION
Renal glomerular disease in psoriatic patients has been demonstrated in several reports.Citation[5–7] The presentation of these patients may range from asymptomatic proteinuria and hematuria to clinically significant generalized edema, hypertension, and high levels of proteinuria accompanied by elevated creatinine and urea levels.Citation[8]
Generally, renal glomerular disease in psoriatic patients is extremely rare. Some authors suggest no higher prevalence of renal disease in psoriasis. Furthermore, it has been proposed that glomerular diseases in psoriatic patients may be seen coincidentally without any common pathogenetic factors with the exception of secondary renal amyloidosis and poststreptococcal glomerulonephritis.Citation[15,Citation16] On the other hand, recent reports have suggested that glomerular disease may be a common accompaniment of psoriasis.Citation[6,Citation7,Citation15] This concept is named psoriatic nephropathy, though a consensus has not been established yet. The main reason of disagreement at this point is the lack of data on pathogenetic mechanisms to demonstrate the exact association between psoriasis and glomerular diseases.
Various studies have been undertaken in an effort to clarify the pathogenetic mechanisms of glomerular diseases associated with psoriasis. They commonly emphasized the role of underlying genetic and immunologic mechanisms. Increased serum levels of IgG, IgA, and IgM immune complexes have been found, and a defect in suppressor T cell function has been defined.Citation[7,Citation8] Elevations in inflammatory markers such as CRP have also been associated with renal involvement.Citation[10,Citation17] However, these studies have not been confirmed through further investigations.
Several glomerular diseases have been distinguished due to renal histological findings of psoriatic patients to date. IgA nephropathy has been recognized as the most common glomerulonephritis among them.Citation[5,Citation6] An immune mechanism was proposed to explain the close association between psoriasis and IgA nephropathy due to the fact that both disease have been mediated by various immunologic mechanisms.Citation[9] However, pathological evidences supporting any immunologic mechanisms common for these two entities have not been demonstrated yet.Citation[10] Other glomerular lesions including membranous glomerulopathy and nephropathies due to nephrotoxic effects of systemically active medications administered for the treatment of psoriasis are quite rare in psoriatic patients.Citation[11–14] The exact pathogenetic mechanisms of these entities remain unclear because of the limited number of cases. Drug-induced nephrotoxicity probably depends on dose-related toxicity of NSAI drugs and interstitial fibrosis, tubular atrophy, and glomerulosclerosis due to cyclosporine A.Citation[18,Citation19] Methotrexate may also play a role in nephrotoxicity. The drug may lead to diminished renal perfusion due to the vasoconstrictive effects and direct tubular epithelial damage due to intratubular precipitation.Citation[20] Unlike glomerulonephritis, secondary renal amyloidosis is a relatively common entity seen in the course of psoriasis. Renal involvement is mostly associated with psoriatic arthritis and pustular psoriasis. The main pathogenetic mechanism proposed to explain the increased incidence of amyloidosis is the presence of chronic inflammation in these settings.Citation[21]
In this report, we demonstrated MPGN in a patient suffering psoriasis for two years. She did not use any of the systemic medications for treatment of the disease, nor did she have any other systemic diseases in association with membranoproliferative glomerulonephritis. Thus, her renal pathology should be an idiopathic form rather than secondary to drug effects or systemic diseases.
Primary MPGN is an uncommon glomerular disease with a slowly progressive course. However, if left untreated, end stage renal disease develops in 50% of the patients. Poor prognostic signs include severe proteinuria, renal insufficiency, hypertension, and existence of glomerular crescents.Citation[22] To date, clinical trials have not provided convincing evidence for the effectiveness of any treatment for idiopathic MPGN in adult patients.
The treatment strategy, in the present case, targeted both MPGN and psoriasis. Thus, a treatment protocol including a corticosteroid and cyclosporine A was preferred. As for other cytotoxic drugs, cyclosporine treatment has not been proven to be effective in MPGN. However, a limited number of clinical trials and case reports suggest that cyclosporine therapy may be useful in adult patients with primary MPGN.Citation[23–26] In this case, combination therapy with cyclosporin A and prednisone provided complete remission of skin disease and more than 75% reduction of initial proteinuria while preserving renal functions.
CONCLUSION
We reported a case of primary MPGN in a psoriatic patient. To the best of our knowledge, this is the first report describing the coexistence of MPGN and psoriasis in the literature. However, we cannot clearly suggest that MPGN demonstrated in this case should be considered as psoriatic nephropathy. More cases are needed for evaluation of clinical and histopathological data to consider a true association between psoriasis and glomerular diseases. Until then, psoriatic patients should be followed up by close monitoring of urinalysis and renal functions for early diagnosis and treatment of coexisting glomerular lesions.
ACKNOWLEDGMENTS
The authors report no conflicts of interest.
REFERENCES
- Gudjonsson JE, Elder JT. Psoriasis: Epidemiology. Clin Dermatol. 2007;25(6):535–546.
- Whittam LR, McGibbon DH. The management of psoriasis. Int J Clin Pract. 1998;52(7):487–491.
- Szepietowski JC, Szepietowski T. Is renal function altered in patients with psoriasis vulgaris? J Dermatol. 2000;27(9): 569–572.
- Cecchi R, Seghieri G, Gironi A, Tuci F, Giomi A. Relation between urinary albumin excretion and skin involvement in patients with psoriasis. Dermatology. 1992;185(2):93–95.
- Ahuja TS, Funtanilla M, de Groot JJ, Velasco A, Badalamenti J, Wilson S. IgA nephropathy in psoriasis. Am J Nephrol. 1998;18(5):425–429.
- Panasiuk NN, Mukhin NA, Varshavskii VA, . The clinico-morphological characteristics of psoriatic nephropathy. Ter Arkh. 1990;62(6):99–103.
- Singh NP, Prakash A, Kubba S, . Psoriatic nephropathy—does an entity exist? Ren Fail. 2005;27(1):123–127.
- Kim M, Ko Y, Yeo UC, Kim Y, Oh H. Psoriasis and glomerulonephritis. Clin Exp Dermatol. 1998;23(6):295–296.
- Yamamoto M, Yorioka T, Kawada M, . A case of IgA nephropathy associated with psoriasis vulgaris. Nippon Jinzo Gakkai Shi. 1994;36(6):779–783.
- Jiao Y, Xu H, Li H, Li X. Mesangial proliferative glomerulonephritis with or without IgA deposits: The morphological characters in psoriasis vulgaris. Nephron Clin Pract. 2008; 108(3):c221–c225.
- Sakemi T, Hayashida R, Ikeda Y, Baba N, Nishihara G, Kohda H. Membranous glomerulonephropathy associated with psoriasis vulgaris. Nephron. 1996;72(2):351–352.
- Kaji T, Tsukada Y, Shimada A, . Membranous nephropathy associated with psoriasis vulgaris. Clin Nephrol. 1994; 42(1):63–64.
- Powles AV, Cook T, Hulme B, . Renal function and biopsy findings after 5 years’ treatment with low-dose cyclosporin for psoriasis. Br J Dermatol. 1993;128(2):159–165.
- Grcevska L, Polenaković M, Ferluga D, Vizjak A, Stavrić G. Membranous nephropathy with severe tubulointerstitial and vascular changes in a patient with psoriatic arthritis treated with non-steroidal anti-inflammatory drugs. Clin Nephrol. 1993;39(5):250–253.
- Zadrazil J, Tichý T, Horák P, . IgA nephropathy associated with psoriasis vulgaris: A contribution to the entity of “psoriatic nephropathy.” J Nephrol. 2006;19(3):382–386.
- Chalmers RJ, Ead RD, Whale K, Ive FA. Guttate psoriasis, glomerulonephritis and streptococcal infection. Arch Dermatol. 1983;119(12):956.
- Alenius GM, Stegmayr BG, Dahlqvist SR. Renal abnormalities in a population of patients with psoriatic arthritis. Scand J Rheumatol. 2001;30(5):271–274.
- Heuvels J, Maximus A, Bosmans JL, Lambert J, De Broe ME. Renal abnormalities in psoriatic patients: A review. Nephron. 1999;82(1):1–6.
- Markham T, Watson A, Rogers S. Adverse effects with long-term cyclosporin for severe psoriasis. Clin Exp Dermatol. 2002;27(2):111–114.
- Weinblatt ME, Coblyn JS, Fox DA, . Efficacy of low-dose methotrexate in rheumatoid arthritis. N Engl J Med. 1985; 312(13):818–822.
- Wittenberg GP, Oursler JR, Peters MS. Secondary amyloidosis complicating psoriasis. J Am Acad Dermatol. 1995;32(3): 465–468.
- Klein M, Radhakrishnan J, Appel G. Cyclosporine treatment of glomerular diseases. Annu Rev Med. 1999;50:1–15.
- Zelichowski G, Nowosławski A, Wawer Z, Wańkowicz Z. Evaluation of cyclosporin A efficacy in the treatment of steroid-resistant nephrotic syndrome in the course of membranoproliferative glomerulonephritis. Pol Arch Med Wewn. 2001;106(5):1065–1069.
- Matsumoto H, Shibasaki T, Ohno I, . Effect of cyclosporin monotherapy on proteinuria in a patient with membranoproliferative glomerulonephritis. Nippon Jinzo Gakkai Shi. 1995;37(4):258–262.
- Erbay B, Karatan O, Duman N, Ertuğ AE. The effect of cyclosporine in idiopathic nephrotic syndrome resistant to immunosuppressive therapy. Transplant Proc. 1988;20(3 Suppl. 4):289–292.
- Bagheri N, Nemati E, Rahbar K, Nobakht A, Einollahi B, Taheri S. Cyclosporine in the treatment of membranoproliferative glomerulonephritis. Arch Iran Med. 2008;11(1):26–29.