Abstract
Introduction: It has been demonstrated that peroxynitrite accompanies acute renal ischemia and contributes to the pathophysiology of renal damage. Therefore, we aimed to investigate the roles of N-acetylcysteine (NAC), a well-known powerful antioxidant, and ebselen (E), a scavenger of peroxynitrite, on renal injury induced by renal ischemia/reperfusion injury (IRI) of rat kidney. Materials and methods: Forty male Sprague–Dawley rats were divided into five groups: sham, renal IRI, renal IRI+NAC, renal IRI+E, and renal IRI+NAC+E. IR injury was induced by 60 min of bilateral renal ischemia followed by 6 h of reperfusion. After reperfusion, kidneys and blood samples were obtained for histopathological and biochemical evaluations. Results: Renal IR resulted in increased malondialdehyde and nitrite/nitrate levels suggesting increased lipid peroxidation and peroxynitrite production and decreased superoxide dismutase and glutathione peroxidase activities. Both NAC and E alone significantly decreased malondialdehyde and nitrite/nitrate levels and increased superoxide dismutase and glutathione peroxidase activities. Additionally in the renal IRI+NAC+E group, all biochemical results were quite close to those of sham group. Histopathologically, the kidney injury in rats treated with combination of NAC and E was found significantly less than the other groups. Conclusions: Both NAC and E are able to ameliorate IRI of the kidney by decreasing oxidative and nitrosative stresses and increasing free radical scavenger properties. Additionally, combination of NAC and E prevents kidney damage more than when each drug is used alone, suggesting that scavenging peroxynitrite nearby antioxidant activity is important in preventing renal IRI.
INTRODUCTION
Cessation of kidney blood supply leads to acute renal failure (ARF) causing failure of the kidneys over a period of hours or days.Citation1,2 The causes of ARF are often multifactorial, but depending on the cause, the prerenal ARF, in which the kidney fails to receive an adequate blood supply, is the most common. In clinical practice, ischemic ARF is often caused by hypotension followed by resuscitation which is simulated by animal models of renal ischemia reperfusion injury (IRI).Citation1,3,4 Ischemia of one or both kidneys is also a common problem experienced during aortic surgery, renal transplantation, or cardiovascular anesthesia leading to renal dysfunction and injury. Despite our current knowledge of the pathophysiology underlying the development of renal IRI, the cellular mechanisms involved are complex and yet to be fully understood.Citation4,5 Unfortunately, dialysis and transplantation are the most applied therapeutic interventions for clinical ARF following renal IRI.Citation2
Kidney subjected to a period of ischemia undergoes morphological and functional damages, which increase during the reperfusion phase. Reperfusion of kidney increases the effects of early ischemic injury by release of oxygen-derived reactive oxygen species (ROS), such as superoxide , hydrogen peroxide (H2O2), and hydroxyl radical (•OH) or nitrogen-derived reactive nitrogen species (RNS), such as nitric oxide (NO) and peroxynitrite (ONOO−).Citation1,4,6–8 The consequences of oxidative and nitrosative stresses are various and include lipid peroxidation, resulting in destruction of membrane lipids, and oxidative DNA damage, collectively leading to the loss of cell viability, either via necrotic or apoptotic pathways.Citation2 Some in vivo and in vitro investigations have also demonstrated that decrease in production or scavenging of ROS and RNS reduces the renal IRI.Citation2,3,9,10 It is well documented in several studies that scavenging of ROS from kidney would protect it from IRI, while RNS might continue to cause cellular damage.Citation4,11 Therefore, scavenging of both ROS and RNS in IRI may be more beneficial.
Based on the mentioned facts, we hypothesized that combination of N-acetylcysteine (NAC), as a well-known strong antioxidant,Citation10,12 and ebselen (E), as a scavenger of peroxynitrite,Citation13,14 may be more efficient than when used alone on kidney damage induced by renal ischemia/reperfusion.
MATERIALS AND METHODS
Animals and Surgery Procedure
The project was approved by the local Experimental Ethics Committee, and the National Institute of Health’s Guide for the Care and Use of Laboratory Animals was followed.
Forty male Sprague–Dawley rats were randomly divided into five groups: sham, renal IRI, renal IRI+NAC, renal IRI+E, and renal IRI+NAC+E. NAC (300 mg/kg – intraperitoneally) and E (20 mg/kg – via intragastric tube) were administered to rats 24 h before the operation and at the beginning of reperfusion period.
Following a 12 h fasting period, animals were anesthetized with an intraperitoneal injection of ketamine hydrochloride (50 mg/kg) and xylazine (10 mg/kg). A midline incision was made, the renal pedicle observed, and the arteries bilaterally occluded with an atraumatic microvascular clamp (Bulldog Artery Clamp, Harvard Apparatus, Holliston, MA, USA) for 60 min. After 60 min of renal ischemia, clamps were removed and the kidneys were inspected for restoration of blood flow and the abdomen was closed. Sham group rats underwent the same surgical procedure without clamp application. Following 6 h of reperfusion period, animals were killed by cervical dislocation. At the time of death, blood samples were collected by heart puncture for biochemical analysis. Both kidneys were harvested for histopathological evaluation and biochemical examination.
Biochemical Analysis
Serum samples were used for measurement of blood urea nitrogen (BUN) and creatinine (SCr) levels, which were used as indicators of impaired glomerular function, and aspartate aminotransferase (AST), which was used as an indicator of renal IRI.Citation9 BUN, SCr, and AST were determined with the Olympus AU-2700 autoanalyzer (Olympus, Hamburg, Germany) using original commercial kits.
All tissues were homogenized in phosphate buffer (pH 7.4) by means of a homogenizator (Heidolph Diax 900; Heidolph Elektro GmbH, Kelhaim, Germany) with ice cubes. The supernatant was aliquoted into two to three separate tubes and stored at −70°C until analysis. First, the protein content of tissue homogenates was measured by the method of Lowry et al. Efficacy of treatment was assessed by tissue levels of malondialdehyde (MDA) using the method of Ohkawa et al., superoxide dismutase (SOD) using the method of Sun et al., and glutathione peroxidase (GSH-Px) using the method of Paglia and Valentine. Nitrate/nitrite (NOx) levels, end products of NO degradation, were measured using the method described by Miranda et al., as we described in our previous studies.Citation4,5,11
Histopathological Evaluation
Kidneys of each animal were taken for histopathological evaluation. In all groups, samples were placed in formalin and processed through paraffin. They were subsequently sectioned at 5 μm and stained with hematoxyline–eosine. The sections were scored with a previously described semiquantitative scale designed to evaluate the degree of renal damage (tubular cell necrosis, cytoplasmic vacuole formation, hemorrhage, and tubular dilatation).Citation15 A minimum of 10 fields for each kidney slide were examined and assigned for severity of changes. The scoring system used was 0, absent; 1, present; and 2, marked. Total histopathological injury score per kidney was calculated by addition of all scores. Blind analysis of the histological samples was performed by two independent experts.
Statistical Analysis
Biochemical data are expressed as mean ± standard deviations (SD). Statistical analyses were carried out using SPSS statistical software (SPSS for Windows, Version 15.0, Chicago, IL, USA). Differences in measured parameters among the three groups were analyzed by Kruskal–Wallis test. Dual comparisons between groups that present significant values were evaluated by Mann–Whitney U-test. Statistical significance was accepted at a value of p < 0.05.
RESULTS
Serum biochemical results for each group are shown in There was a significant increase in the SCr and BUN levels in the renal IRI group. The serum AST activity was also found significantly increased in the renal IRI group (p < 0.05). Administration of NAC and E decreased the SCr and BUN levels and AST activities (p < 0.05; renal IRI vs. renal IRI+NAC, renal IRI+E). In addition, all biochemical results were found quite close to those of sham in the renal IRI+NAC+E group (p < 0.05).
Table 1. Biochemical evaluation of serum for each group.
Table 2. Biochemical evaluation of kidney for each group.
The SOD and GSH-Px enzyme activities are shown in . Antioxidant enzyme activities were significantly lower in the renal IRI group than in any other group (p < 0.01; renal IRI vs. the others). Administration of NAC and E significantly increased SOD and GSH-Px activities in renal IRI+NAC and renal IRI+E groups when compared to renal IRI group (p < 0.05; renal IRI+NAC and renal IRI+E vs. renal IRI). SOD and GSH-Px activities were significantly higher in the groups treated with the combination of NAC and E than in the groups treated with either NAC or E (p < 0.05; renal IRI+NAC+E vs. renal IRI+NAC, renal IRI+E).
The renal tissue MDA and NOx levels are shown in . The rats subjected to renal IRI revealed a sharp increase in the tissue MDA and NOx levels, suggesting increased lipid peroxidation and peroxynitrite production. The MDA and NOx levels in all of the treatment groups were significantly lower than those in the renal IRI group (p < 0.05; treatment groups vs. renal IRI). The MDA and NOx levels in the renal tissue of the groups treated with the combination of NAC and E were almost the same as those in the sham group.
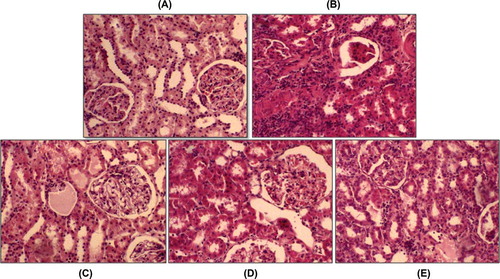
Histopathologically, interstitial inflammation, tubules, and glomeruli were evaluated for grading of renal injury. Sham groups were normal. There was strong interstitial inflammation in IRI group, and the total injury was significantly high in the renal IRI group compared to all treatment groups (p < 0.05). In treated groups, injuries were similar. In the renal IRI+NAC+E group, injury was found almost the same as in sham group (p > 0.05). Representative histological samples from all the groups are shown in .
Figure 1 Representative histological photographs of kidney tissues from (A) sham group, (B) renal IRI, (C) renal IRI+NAC, (D) renal IRI+E, and (E) renal IRI+NAC+E. Sham rats show normal histological characteristic of glomeruli and tubules. Rats in the renal IRI group show marked necrosis with tubular dilation, swelling, luminal congestion, and medullar hemorrhage. Renal IRI+NAC and renal IRI+E groups show moderate kidney damage and moderate dilatation of the tubular structure. Kidney section in the renal IRI+NAC+E group shows minimal tubular dilatation and preservation of tissue histology of the kidney (hematoxyline–eosine).
DISCUSSION
In this experiment, we evaluated the effects of NAC, a disulphide antioxidant, and E, a molecule with peroxynitrite-scavenging capability, in renal IRI. The outcomes of the present study demonstrate that administration of NAC and E prior to IRI clearly protects the kidneys. Each agent has ameliorative effect on both oxidative and nitrosative status and histopathological changes. We also found that combination of these two agents has more beneficial effect than when each of them was used alone.
Increased SCr and BUN levels in renal IRI indicate significant impairment of glomerular function. Renal IRI also caused a significant increase in serum AST activity, used as a marker of renal IRI. Renal IRI causes both glomerular and tubular dysfunctions. Tubular injury was shown by histological examination.
Generation of ROS and/or the decline of antioxidant defense lead to oxidative stress, which plays an important role in development of renal IRI.Citation2,7,16 Under physiological conditions, ROS plays an important role in intracellular and cell-to-cell communications.Citation17 However, in oxidative stress situations such as renal IRI, sepsis, and inflammatory bowel disease, excessive ROS generation occurs.Citation18–20 On the other hand, some studies have shown the ameliorative effects of therapeutic agents via preventing ROS production, inhibiting enzymes responsible for ROS generation, and administration of antioxidant enzymes and scavenging of ROS.Citation2–5,10–12,21
In this study, we evaluated the possible beneficial effects of NAC as a free radical scavenger antioxidant. We found that administration of NAC prior to injury showed considerable improvement in renal IRI by decreasing lipid peroxidation and increasing antioxidant enzyme activity. There are some possible explanations for NAC’s beneficial effects: NAC has the ability to interact with signaling oxidants (hydroperoxides)Citation22; NAC acts as a precursor of GSH, which is important in conditions of extreme GSH depletionCitation12,23,24; NAC might reduce the oxidative stress by improving the thiol redox status and inhibiting neutrophils and monocyte chemotaxis.Citation23,25 Some experimental and clinical investigations have shown that NAC might ameliorate IRI in myocardial, pulmonary, hepatic, kidney, and intestinal tissues.Citation10,24,25 In addition, it is shown that NAC is effective in preventing contrast agent-induced nephrotoxicity.Citation26 Therefore, it is likely that NAC would be a beneficial agent for treatment of renal IRI.
In this study, we also demonstrate that E decreased lipid peroxidation and increased antioxidant enzyme activities in the kidneys. It may be the most possible explanation for decreased lipid peroxidation in that E has specificity for H2O2, smaller organic hydroperoxides, and membrane-bound phospholipid and cholesterylester hydroperoxides.Citation13,27 In addition, E quickly reacts with H2O2 to form water, and the ebselen selenic acid spontaneously releases another molecule of water to regenerate E.Citation28 E’s ability to mimic GSH-Px is the most likely explanation for increased SOD and especially GSH-Px activity.Citation13,29 It has also been shown that E rapidly reacts with ONOO–. In many studies, E is investigated. It has been shown that E could protect organs such as intestine, neurons, lungs, and myocardium against IRI.Citation6,30–32 Taken together, E plays an important role in protection of kidneys against IRI experimentally. It may be a good candidate to prevent IRI in kidney transplantations but further studies should be conducted to evaluate its safety and efficacy in humans. Meotti et al.Citation33 has investigated E-enhanced urea levels suggesting renal toxicity in rats. But just higher urea levels do not indicate renal dysfunction.
Rodríguez et al.Citation34 demonstrated that pretreatment with the antioxidants NAC or E abolished sex differences in ONOO–, nitrotyrosine, and GFR, suggesting that a greater oxidative and nitrosative stress worsens renal damage in males. Females suffer a less severe ischemic ARF than males, apparently because of higher NO bioavailability and/or lower levels of oxidative stress.Citation34 In our study, we used male rats so we did not evaluate the effects of E and NAC on sex differences.
NO is one of the most important mediators in pathophysiological changes of tissues. Under physiological conditions, NO maintains vascular tone and inhibits aggregation and adhesion of neutrophils and platelets to vascular endothelium; these are beneficial aspects of NO function.Citation8,16,35 NO-induced IRI may also be a result of production of ONOO–, since NO couples with , which is also increased in response to ischemia. Production of ONOO– (nitrosative stress) occurs instantaneously and causes the nitration of cellular proteins with subsequent loss of protein structure and function.Citation16,31 NO and ONOO– are eventually converted to nitrite and/or nitrate. NOx has been used as an indirect but reliable indicator for NOCitation4,5,21 and ONOO– formation in vivo.Citation4,36 Therefore, the reduction of ONOO– level and increase in the scavenging of ROS activity may be a potential mechanism to protect kidneys against IRI. Based on this hypothesis, we administered the combination of NAC and E to rats. And, we found that NOx and MDA levels and antioxidant enzyme activities in the renal IRI+NAC+E group were similar to those of the sham group. E is a well-known ONOO– scavenger and it rapidly reacts with ONOO–.Citation14 ONOO– is reduced to nitrite by E; the resulting selenoxide is subsequently reduced by glutathione establishing a catalytic cycle so that the defense can be maintained in an ONOO– reductase reaction.Citation13,37 We have displayed that the combination of NAC and E presented a superior beneficial effect against renal IRI compared to each drug used separately. With NAC alone, ROS was scavenged from the environment; however, RNS (including ONOO–) might continue to cause tissue damage, which scavenged with E. Thus, the combination of NAC and E indicates that it may be more beneficial to use both agents together against renal IRI.
In conclusion, both NAC and E are able to ameliorate IRI of the kidneys by decreasing oxidative and nitrosative stress. Additionally, the combination of NAC and E prevents kidney damage more than when each drug is used alone suggesting that scavenging peroxynitrite nearby antioxidant activity is important in preventing renal IRI. However, further studies are required to better understand the interaction between scavenging of peroxynitrite and renal IRI.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Sheridan AM, Bonventre JV. Pathophysiology of ischemic acute renal failure. Contrib Nephrol. 2001;132:7–21.
- Chatterjee PK. Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: A comprehensive review. Naunyn Schmiedebergs Arch Pharmacol. 2007;376:1–43.
- Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med. 2000;109:665–678.
- Guven A, Uysal B, Akgul O, . Scavenging of peroxynitrite reduces renal ischemia/reperfusion injury. Ren Fail. 2008;30:747–754.
- Yanarates O, Guven A, Sizlan A, . Ameliorative effects of proanthocyanidin on renal ischemia/reperfusion injury. Ren Fail. 2008;30:931–938.
- Guven A, Tunc T, Topal T, . Alpha-lipoic acid and ebselen prevent ischemia/reperfusion injury in the rat intestine. Surg Today. 2008;38:1029–1035.
- Greene EL, Paller MS. Oxygen free radicals in acute renal failure. Miner Electrolyte Metab. 1991;17:124–132.
- Noiri E, Nakao A, Uchida K, . Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol. 2001;281:F948–F957.
- Chatterjee PK, Patel NS, Kvale EO, . Inhibition of inducible nitric oxide synthase reduces renal ischemia/reperfusion injury. Kidney Int. 2002;61:862–871.
- Di Giorno C, Pinheiro HS, Heinke T, . Beneficial effect of N-acetyl-cysteine on renal injury triggered by ischemia and reperfusion. Transplant Proc. 2006;38:2774–2776.
- Ersoz N, Guven A, Cayci T, . Comparison of the efficacy of melatonin and 1400W on renal ischemia/reperfusion injury: A role for inhibiting iNOS. Ren Fail. 2009;31:704–710.
- Zafarullah M, Li WQ, Sylvester J, . Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6–20.
- Klotz LO, Sies H. Defenses against peroxynitrite: Selenocompounds and flavonoids. Toxicol Lett. 2003;140–141:125–132.
- Daiber A, Zou MH, Bachschmid M, . Ebselen as a peroxynitrite scavenger in vitro and ex vivo. Biochem Pharmacol. 2000;59:153–160.
- Rabb H, Ramirez G, Saba SR, . Renal ischemic-reperfusion injury in L-selectin-deficient mice. Am J Physiol. 1996;271:F408–F413.
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437.
- Nose K. Role of reactive oxygen species in the regulation of physiological functions. Biol Pharm Bull. 2000;23:897–903.
- Williams P, Lopez H, Britt D, . Characterization of renal ischemia-reperfusion injury in rats. J Pharmacol Toxicol Methods. 1997;37:1–7.
- Yaman H, Cayci T, Seyrek M, . Effects of vitamin A and C and melatonin on 3-nitrotyrosine formation in guinea pig heart under lipopolysaccharide-induced stress. Turk J Med Sci. 2010;40:715–721.
- Scaldaferri F, Fiocchi C. Inflammatory bowel disease: Progress and current concepts of etiopathogenesis. J Dig Dis. 2007;8:171–178.
- Oztas E, Guven A, Turk E, 3-Aminobenzamide, a poly ADP ribose polymerase inhibitor, attenuates renal ischemia/reperfusion injury. Ren Fail. 2009;31:393–399.
- Aruoma OI, Halliwell B, Hoey BM, . The antioxidant action of N-acetylcysteine: Its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6:593–597.
- Grinberg L, Fibach E, Amer J, . N-acetylcysteine amide, a novel cell-permeating thiol, restores cellular glutathione and protects human red blood cells from oxidative stress. Free Radic Biol Med. 2005;38:136–145.
- Tunc T, Oter S, Guven A, . Protective effect of sulfhydryl-containing antioxidants against ischemia/reperfusion injury of prepubertal rat intestine. J Gastroenterol Hepatol. 2009;24:681–687.
- Dodd S, Dean O, Copolov DL, . N-acetylcysteine for antioxidant therapy: Pharmacology and clinical utility. Expert Opin Biol Ther. 2008;8:1955–1962.
- Briguori C, Colombo A, Violante A, . Standard vs double dose of N-acetylcysteine to prevent contrast agent associated nephrotoxicity. Eur Heart J. 2004;25:206–211.
- Muller A, Gabriel H, Sies H, . A novel biologically active selenooorganic compound–VII. Biotransformation of ebselen in perfused rat liver. Biochem Pharmacol. 1988;37:1103–1109.
- Zhao R, Masayasu H, Holmgren A. Ebselen: A substrate for human thioredoxin reductase strongly stimulating its hydroperoxide reductase activity and a superfast thioredoxin oxidant. Proc Natl Acad Sci U S A. 2002;99:8579–8584.
- Ozaki M, Nakamura M, Teraoka S, . Ebselen, a novel anti-oxidant compound, protects the rat liver from ischemia-reperfusion injury. Transpl Int. 1997;10:96–102.
- Seo JY, Lee CH, Cho JH, . Neuroprotection of ebselen against ischemia/reperfusion injury involves GABA shunt enzymes. J Neurol Sci. 2009;285:88–94.
- Tunc T, Uysal B, Atabek C, . Erdosteine and ebselen as useful agents in intestinal ischemia/reperfusion injury. J Surg Res. 2009;155:210–216.
- Baljinnyam E, Hasebe N, Morihira M, . Oral pretreatment with ebselen enhances heat shock protein 72 expression and reduces myocardial infarct size. Hypertens Res. 2006;29:905–913.
- Meotti FC, Borges VC, Zeni G, . Potential renal and hepatic toxicity of diphenyl diselenide, diphenyl ditelluride and Ebselen for rats and mice. Toxicol Lett. 2003;143(1):9–16.
- Rodríguez F, Nieto-Cerón S, Fenoy FJ, . Sex differences in nitrosative stress during renal ischemia. Am J Physiol Regul Integr Comp Physiol. 2010;299(5):R1387–R1395.
- Guven A, Gundogdu G, Vurucu S, . Medical ozone therapy reduces oxidative stress and intestinal damage in an experimental model of necrotizing enterocolitis in neonatal rats. J Pediatr Surg. 2009;44:1730–1735.
- Guven A, Gundogdu G, Uysal B, . Hyperbaric oxygen therapy reduces the severity of necrotizing enterocolitis in a neonatal rat model. J Pediatr Surg. 2009;44:534–540.
- Briviba K, Tamler R, Klotz LO, . Protection by organotellurium compounds against peroxynitrite-mediated oxidation and nitration reactions. Biochem Pharmacol. 1998;55:817–823.
