Abstract
Background and aim: Omentin-1 is suggested to affect inversely atherosclerosis (AS). Data about omentin-1 is limited to chronic kidney disease (CKD). Our aim was to examine omentin-1 in non-diabetic CKD patients who are not dialyzed and investigate its relationships with inflammation and carotid AS. Materials and Methods: We performed a cross-sectional study in 55 non-diabetic CKD patients and 30 healthy controls. Baseline clinical and laboratory data were obtained for all participants. Serum omentin-1 and interleukin-6 (IL-6) levels were measured according to the manufacturer’s instructions. Carotic plaque and intima-media thickness (IMT) were assessed by carotid ultrasonography. The homeostasis model assessment of insulin resistance index (HOMA-IR) was used to assess IR. Results: Omentin-1 and IL-6 levels in the patient group were found to be higher than the control group; the differences were statistically significant (p = 0.01 and p = 0.04, respectively). Carotid IMT(mean) was significantly higher in the patient group (p = 0.01). Omentin-1 did not correlate with IL-6 and IMT in the patient group (p = 0.51 and p = 0.76, respectively). In subgroup analysis, omentin-1 levels in patients with carotid plaque were lower than those without carotid plaque (179.5 ± 88.1 ng/ml and 185.9 ± 67.8 ng/ml, respectively). However, the difference was not statistically significant (p = 0.47). Conclusion: We conclude that omentin-1 is higher in not dialyzed non-diabetic CKD and there is no correlation between omentin-1 and IL-6 or carotid IMT(mean).
Introduction
Chronic kidney disease (CKD), a global public health problem, is associated with increased morbidity and mortality of cardiovascular disease (CVD).Citation1 Although traditional cardiovascular risk factors including diabetes mellitus and hypertension are highly prevalent in patients with CKD, they cannot fully explain the increased risk for CVD.Citation2,Citation3 Therefore, a number of novel CVD risk factors have increasingly been studied as nontraditional risk factors.Citation4,Citation5
Adipose tissue is suggested to affect the cardiovascular system as an active endocrine organ secreting a variety of bioactive proteins and adipokines. Several inflammatory molecules derived from adipose tissue, such as leptin, resistin, tumor necrosis factor (TNF)-α, and interleukin-6 (IL-6), have been suggested to exacerbate vascular diseases. On the other hand, adiponectin is known to protect the vascular system.Citation6–9
Omentin-1 is a novel visceral fat depot-specific secretory protein prefentially synthesized by the visceral stromal vascular cells. It has been shown that omentin-1 increases insulin-stimulated glucose uptake and Akt phosphorylation in human adipocytes,Citation10 plays an anti-inflammatory role in vascular smooth cells,Citation11 and suppresses arterial calcification.Citation12
Omentin-1 levels are decreased in obesity, diabetes mellitus, and insulin resistance (IR). Also, it is negatively correlated with metabolic risk factors and inflammation.Citation13–19 Recent reports have usually demonstrated an inverse association between omentin-1 and atherosclerosis (AS).Citation18,Citation20–23 Little is known about omentin-1 in CKD. It may be implicated in the pathogenesis of CVD in these patients. There is only one study investigating omentin-1 in CKD patients on hemodialysis.Citation24 There is no study evaluating omentin-1 levels and its relationship with carotid AS and inflammation in non-diabetic CKD patients, not on dialysis. Therefore, we aim to examine the hypothesis that omentin-1 levels are reduced in these patients and are inversely associated with carotid AS, which is mediated by inflammation.
Patients and methods
Study population
This study was conducted with a cross-sectional design. We consecutively enrolled 55 outpatients with stage 3–4 CKD attending the department of nephrology and 30 healthy controls who visited the department of internal medicine for routine check-up at Kocaeli Derince Education and Research Hospital between October 2012 and January 2013. Exclusion criteria were: stage 5 CKD, a history of ischemic cardiovascular disease (myocardial infarction, stroke, peripheral artery disease, cardiovascular revascularization), severe heart failure, uncontrolled hypertension, corticosteroid therapy, severe hepatic disease, smoking, inflammatory disease, acute infectious disease, and malignancy. Diabetes mellitus was defined according to the American Diabetes Association criteria.Citation25 Therefore, patients with high fasting plasma glucose (>7.0 mmol/L), hemoglobin A1c ≥6.5, and patients on oral antidiabetics or insulin therapy were excluded from the study.
Medical history and medicine were obtained from all patients by a standardized questionnaire.
The study protocol was reviewed and approved by the ethics committee of Kocaeli University. It was conducted according to the Declaration of Helsinki. Written consent was obtained from all participants after the purpose of the study was explained to them. The study was registered at clinicaltrials.gov with the reference number NCT01701830.
Methods
Body mass index (BMI) was estimated by body weight in kilograms divided by height in meter squared. Blood pressure (BP) was measured using an appropriate cuff with a mercury sphygmomanometer. After at least 10 min of rest in a sitting position, the average of three consecutive measurements was taken as BP. Korotkoff’s first sound was recorded as systolic BP and Korotkoff’s fifth sound was recorded as diastolic BP.
Blood and urine samples were analyzed by the Biochemistry Laboratory of Kocaeli Derince Education and Research Hospital. Patients were informed about 24-h urine collection. Venous blood samples were collected in the morning after an overnight fast. Serum samples for omentin-1 and IL-6 were centrifuged within 30 min and stored at −80 °C until assayed. Blood urea nitrogen (BUN), creatinine, fasting glucose, insulin, hemoglobinA1c (HbA1c), total cholesterol, triglyceride, high density lipoprotein cholesterol (HDL), calcium, phosphate, parathyroid hormone (PTH), hemoglobin, C-reactive protein (CRP), and 24-h urinary protein excretion were analyzed following the manufacturer’s instructions. Low density lipoprotein cholesterol (LDL) was calculated using the Friedewald formula. IR was examined with the homeostasis model assessment of insulin resistance index (HOMA-IR) by using the following formula = [fasting insulin (μIU/mL) × fasting glucose (mmol/L)/22.5].Citation26 Glomerular filtration rate (GFR) was calculated according to the abbreviated Modification of Diet in Renal Disease (MDRD) formulaCitation27; estimated GFR (eGFR) = 186 × serum creatinine−1.154 × age−0.203 × 0.742 (if female) × (1.212 [if patient is black]).
Serum IL-6 concentrations were measured using manual IL-6 (human) detection kit (a solid phase Enzyme Amplified Sensitivity Immunoassay) (DIAsource Immunoassays S.A., Belgium). Serum samples were assayed according to the manufacturer’s instructions. Intra- and interassay coefficients of variation were between 4% and 6%. The detection limit of the assay was 2 pg/mL. The antibodies used in this detection kit are specific for measurement of monoclonal antibodies (MAbs) directed against distinct epitopes of IL-6.
Serum omentin-1 concentrations were measured using manual omentin-1 (human) detection kit (Sandwich ELISA) (BIOVENDOR R&D, Czech Republic). Serum samples were diluted (1/40) and assayed according to the manufacturer’s instructions. Intra- and inter assay coefficients of variation were between 3% and 5%. The detection limit of the assay was 0.5 ng/mL. The antibodies used in this detection kit are specific for measurement of polyclonal anti-human omentin-1.
Ultrasonography of carotid artery
Ultrasonographic images of the right and left common carotid artery (CCA) of each subject at the lower 1/3 cervical region proximally and 1 cm above the carotid bulb distally in longitudinal plane were obtained using a sonography device with a high definition L12-5 linear wide band probe (Toshiba Aplio 500, Tokyo, Japan). CCA intima-media thickness (IMT) measurements of the proximal and distal CCA posterior wall were done manually by the provided distance measurement system of the sonography device after magnification of the images. A focal structure protruding into the arterial lumen with a thickness ≥1.3 mm was defined as carotid plaque.Citation28 The maximal IMT was the value at the maximal point of the region. The mean IMT was calculated as the mean of the maximal left and right IMT values. To avoid interobserver variance, all measurements were done by the same radiologist who was blind to the anthropometric and laboratory data.
Statistical analysis
All analyses were performed using Statistical Package for Social Sciences (SPSS) version 15.0 for Windows. Continuous variables are expressed as mean ± standard deviations (SD), or median and minimal-maximal value. Categorical variables are reported as number and percentages. One-sample Kolmogorov–Smirnov test was performed to prove the normality of data distributions. Demographic, laboratory and ultrasonographic findings of the groups were compared by independent sample t-test or Mann–Whitney U test. Pearson’s correlation test was used to identify whether there was any correlation between omentin-1 and continous variables or not. Categorical variables were analyzed using Chi-Square test. Probability values were two-tailed, and a p value of less than 0.05 was considered statistically significant.
Results
The present study was performed on fifty-five patients (33 female, 22 male) and thirty controls (21 female, 9 male). They were age- and sex-matched. The etiology of CKD was as follows: chronic glomerulonephritis (n = 16), hypertension (n = 15), nephrolithiasis (n = 7), polycystic kidney disease (n = 6), vesico-ureteral reflux (n = 5), pyelonephritis (n = 3), Alport syndrome (n = 1), and unknown (n = 2).
Of the patients, 61% were on an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker alone or on a combination with other antihypertensive drugs. Fifty-one percent of patients were on calcium channel blockers and/or beta blockers, and 33% were on diuretics. Furthermore, 7% were taking statin, 22% were taking an oral phosphorus binding drug, 7% were taking oral vitamin D, and 11% were taking oral iron therapy.
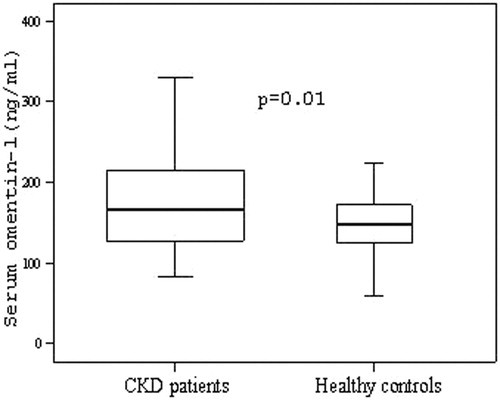
Characteristics of the patients and controls are shown in . Serum omentin-1 levels in the patient group were found to be higher than control group; the difference was statistically significant (p = 0.01) (). Systolic and diastolic BP were significantly elevated in the patient group (p = 0.003; p = 0.02, respectively). The differences in BMI, glucose, HOMA-IR, HbA1c, LDL, calcium, and phosphate levels between the groups were not statistically significant. BUN, creatinine, total cholesterol, triglyceride, PTH, IL-6, and CRP levels in the patient group were significantly higher compared with control group (p < 0.001; p < 0.001; p = 0.01; p < 0.001; p < 0.001; p = 0.04; p = 0.006, respectively). eGFR, HDL, and hemoglobin levels in patients were significantly lower compared with controls (p < 0.001; p = 0.001, respectively). Carotid IMT(mean) was significantly increased in the patient group (p = 0.01). The proportion of carotid plaque was 38% in the patient and 27% in control groups (p = 0.28). Serum omentin-1 level in patients with and without carotid plaque was 179.5 ± 88.1 and 185.9 ± 67.8 ng/mL, respectively. However, the difference was not statistically significant (p = 0.47).
Table 1. Baseline characteristics of patient and control groups.
In correlation analysis, we did not find any correlation between serum omentin-1 and other variables in the patient group, except for LDL (r = 0.277, p = 0.04) ().
Table 2. Correlation of omentin-1 with other variables in patient group.
Discussion
The present study demonstrated that (1) Serum omentin-1 levels in non-diabetic CKD patients not on dialysis were significantly elevated compared with healthy controls, (2) IL-6 and CRP in the patient group were markedly increased compared with control subjects, but omentin-1 was not correlated with IL-6 and CRP, (3) HOMA-IR was not associated with omentin-1 in patients, and (4) Omentin-1 levels did not correlate with carotid IMT in our patients. In subgroup analysis, omentin-1 levels in patients with carotid plaque were lower than those without carotid plaque, but not statistically significant.
Omentin, also known intellection, is a novel visceral fat depot-specific secretory protein prefentially synthesized by the visceral stromal vascular cells. It has two isoforms (1 and 2). Omentin-1 is the major circulating form in human blood. It increases insulin sensitivity, attenuates inflammation, and induces vasodilatation.Citation10,Citation11,Citation29 Insulin and glucose are suggested as two major factors affecting omentin-1 levels. Tan BK et al.Citation17 showed that prolonged insulin and glucose infusion caused a significant decrease in omentin-1 levels. Recent studies have shown that omentin-1 levels are reduced in obesity and obesity-related states including IR, metabolic syndrome, diabetes mellitus, and polycystic ovarian syndrome (PCOS). Furthermore, the levels of omentin-1 are decreased in patients with atherosclerotic vascular disease.Citation14,Citation20–22,Citation30 Taking into consideration the above associations, the effect of insulin sensitizing drugs and weight loss on omentin-1 have been studied and increases in omentin-1 levels are found after metformin treatment and weight loss.Citation31,Citation32
The other important factor regulating omentin-1 is inflammation. IL-6 was negatively associated with omentin-1 in type 2 diabetes mellitus.Citation19 Moreover, the change in high-sensitivity CRP levels were found to be the only variable negatively correlated with serum omentin-1 levels after metformin treatment in PCOS women.Citation33
CKD is linked to higher risk of CVD and mortality because of a variety of complex deleterious alterations in physiologic and metabolic functions such as IR, inflammation, malnutrition, anemia, and vitamin D deficiency along with classical risk factors.Citation34 As a consequence, it may be expected that omentin-1 is especially inhibited in CKD.
The majority of previous studies on omentin-1 have been mainly performed in patients with obesity, diabetes mellitus, and some other endocrine disease. To the best of our knowledge, this is the first study investigating omentin-1 levels in non-diabetic CKD not on dialysis and its relationships with inflammation and carotid AS.
We showed that omentin-1 levels were significantly higher in our patients compared with healthy controls. A similar result was reported from hemodialysis patients by Alcelik et al.Citation24 They suggested that this adipokine might be increased owing to defective degradation and excretion in hemodialysis patients. It is possible that the same mechanisms contribute to increased omentin-1 levels in non-dialyzed patients. Though significant elevations of IL-6 and CRP were found in patients, they did not correlate with omentin-1. We consider that IL-6 and CRP might have lost their statistical significance due to increased omentin-1 levels.
The other finding of this study was that the difference in HOMA-IR between the two groups was not significant and there was no correlation between HOMA-IR and omentin-1 level in patients, contrary to our expectations. This may be due to the fact that our study groups were similar in terms of fasting glucose, HOMA-IR, and HbA1c. Studies have shown that omentin-1 levels are usually reduced in patients with IR, but there are some different findings in the literature. Yilmaz et al.Citation35 reported that omentin-1 was significantly increased in patients with nonalcoholic fatty liver disease in which IR is highly prevalent. Furthermore, treatment with metformin and pioglitazone, insulin sensitizing drugs, reduced the levels of omentin-1 in patients with newly diagnosed diabetes in spite of the fact that a significant improvement in HOMA-IR were found in another study.Citation36
A low omentin-1 level is known as a risk factor for atherosclerotic vascular disease. Omentin-1 may be involved in vascular disease due to its effect on endothelial function, vasodilatation, arterial compliance and calcification, and inflammation.Citation11,Citation12,Citation21,Citation22 We did not find any correlation between omentin-1 and carotid IMT(mean). In subgroup analysis, omentin-1 levels were lower in patients with carotid plaque than without carotid plaque. However, the difference did not reach significance level. This result may be due to the small sample size in each patient subgroup and the potential effect of CKD on omentin-1 levels.
In the present study, we determined a positive correlation between omentin-1 and LDL. One report showed a positive correlation between HDL and omentin-1,Citation15 but others did not find any correlation between lipid profile and omentin-1.Citation16,Citation18
Finally, omentin-1 levels did not correlate with others factors including BMI, systolic and diastolic BP, BUN, creatinine, eGFR, proteinuria, hemoglobin, calcium, phosphate, and PTH.
In conclusion, we revealed that omentin-1 levels were significantly increased in non-diabetic CKD patients not dialyzed. Although not significant, omentin-1 was lower in patients with carotid plaque than without carotid plaque.
The major limitations of our study are the relatively small number of subjects and its cross-sectional design. We believe that further prospective studies are needed to clarify the level of omentin-1 and its functional significance in terms of CKD.
Declaration of interest
This study was supported by research grants from Kocaeli Derince Education and Research Hospital (Grant number:2012/2).
References
- Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147
- Locatelli F, Pozzoni P, Tentori F, del Vecchio L. Epidemiology of cardiovascular risk in patients with chronic kidney disease. Nephrol Dial Transplant. 2003;18:vii2–9
- Zoccali C. Cardiovascular risk in uraemic patients – is it fully explained by classical risk factors? Nephrol Dial Transplant. 2000;15:454–457
- Liao MT, Sung CC, Hung KC, Wu CC, Lo L, Lu KC. Insulin resistance in patients with chronic kidney disease. J Biomed Biotechnol. 2012;2012:691369
- Stenvinkel P, Herzog CA. Cardiovascular disease in chronic kidney disease. In Floege J, Johnson RJ, Feehally J, eds. Comprehensive Clinical Nephrology. 4th ed. Missouri: Elsevier Saunders; 2010:935–950
- Wang Z, Nakayama T. Inflammation, a link between obesity and cardiovascular disease. Mediators Inflamm. 2010;2010:535918
- Calabro P, Samudio I, Willerson JT, Yeh ET. Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation. 2004;110:3335–3340
- Yamawaki H. Vascular effects of novel adipocytokines: focus on vascular contractility and inflammatory responses. Biol Pharm Bull. 2011;34:307–310
- Hansen T, Ahlström H, Söderberg S, et al. Visceral adipose tissue, adiponectin levels and insulin resistance are related to atherosclerosis as assessed by whole-body magnetic resonance angiography in an elderly population. Atherosclerosis. 2009;205:163–167
- Yang RZ, Lee MJ, Hu H, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290:E1253–E1261
- Kazama K, Usui T, Okada M, Hara Y, Yamawaki H. Omentin plays an anti-inflammatory role through inhibition of TNF-α-induced superoxide production in vascular smooth muscle cells. Eur J Pharmacol. 2012;686:116–123
- Xie H, Xie PL, Wu XP, et al. Omentin-1 attenuates arterial calcification and bone loss in osteoprotegerin-deficient mice by inhibition of RANKL expression. Cardiovasc Res. 2011;92:296–306
- Shibata R, Ouchi N, Takahashi R, et al. Omentin as a novel biomarker of metabolic risk factors. Diabetol Metab Syndr. 2012;4:37
- Auguet T, Quintero Y, Riesco D, et al. New adipokines vaspin and omentin. Circulating levels and gene expression in adipose tissue from morbidly obese women. BMC Med Genet. 2011;12:60
- de Souza Batista CM, Yang RZ, Lee MJ, et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56:1655–1661
- Pan HY, Guo L, Li Q. Changes of serum omentin-1 levels in normal subjects and in patients with impaired glucose regulation and with newly diagnosed and untreated type 2 diabetes. Diabetes Res Clin Pract. 2010;88:29–33
- Tan BK, Adya R, Farhatullah S, et al. Omentin-1, a novel adipokine, is decreased in overweight insulin-resistant women with polycystic ovary syndrome: ex vivo and in vivo regulation of omentin-1 by insulin and glucose. Diabetes. 2008;57:801–808
- Moreno-Navarrete JM, Ortega F, Castro A, Sabater M, Ricart W, Fernández-Real JM. Circulating omentin as a novel biomarker of endothelial dysfunction. Obesity. 2011;19:1552–1559
- El-Mesallamy HO, El-Derany MO, Hamdy NM. Serum omentin-1 and chemerin levels are interrelated in patients with Type 2 diabetes mellitus with or without ischemic heart disease. Diabet Med. 2011;28:1194–1200
- Yoo HJ, Hwang SY, Hong HC, et al. Association of circulating omentin-1 level with arterial stiffness and carotid plaque in type 2 diabetes. Cardiovasc Diabetol. 2011;10:103
- Liu R, Wang X, Bu P. Omentin-1 is associated with carotid atherosclerosis in patients with metabolic syndrome. Diabetes Res Clin Pract. 2011;93:21–25
- Zhong X, Zhang HY, Tan H, et al. Association of serum omentin-1 levels with coronary artery disease. Acta Pharmacol Sin. 2011;32:873–878
- Shibata R, Takahashi R, Kataoka Y, et al. Association of a fat-derived plasma protein omentin with carotid artery intima-media thickness in apparently healthy men. Hypertens Res. 2011;34:1309–1312
- Alcelik A, Tosun M, Ozlu MF, et al. Serum levels of omentin in end-stage renal disease patients. Kidney Blood Press Res. 2012;35:511–516
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35:S64–S71
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470
- Kablak-Ziembicka A, Przewlocki T, Tracz W, et al. Prognostic value of carotid intima-media thickness in detection of coronary atherosclerosis in patients with calcified aortic valve stenosis. J Ultrasound Med. 2005;24:461–467
- Yamawaki H, Tsubaki N, Mukohda M, Okada M, Hara Y. Omentin, a novel adipokine, induces vasodilation in rat isolated blood vessels. Biochem Biophys Res Commun. 2010;393:668–672
- Choi JH, Rhee EJ, Kim KH, Woo HY, Lee WY, Sung KC. Plasma omentin-1 levels are reduced in non-obese women with normal glucose tolerance and polycystic ovary syndrome. Eur J Endocrinol. 2011;165:789–796
- Shaker M, Mashhadani ZI, Mehdi AA. Effect of treatment with metformin on omentin-1, ghrelin and other biochemical, clinical features in PCOS patients. Oman Med J. 2010;25:289–293
- Moreno-Navarrete JM, Catalán V, Ortega F, et al. Circulating omentin concentration increases after weight loss. Nutr Metab (Lond). 2010;7:27
- Tan BK, Adya R, Farhatullah S, Chen J, Lehnert H, Randeva HS. Metformin treatment may increase omentin-1 levels in women with polycystic ovary syndrome. Diabetes. 2010;59:3023–3031
- Slee AD. Exploring metabolic dysfunction in chronic kidney disease. Nutr Metab (Lond). 2012;9:36
- Yilmaz Y, Yonal O, Kurt R, et al. Serum levels of omentin, chemerin and adipsin in patients with biopsy-proven nonalcoholic fatty liver disease. Scand J Gastroenterol. 2011;46:91–97
- Esteghamati A, Noshad S, Rabizadeh S, Ghavami M, Zandieh A, Nakhjavani M. Comparative effects of metformin and pioglitazone on omentin and leptin concentrations in patients with newly diagnosed diabetes: a randomized clinical trial. Regul Pept. 2013;182:1–6