Abstract
Context Earlier we reported cardioprotective, antihyperlipidemic, and in vitro antioxidant activity of flax lignan concentrate (FLC) obtained from the seeds of Linum usitatissimum L. (Linaceae).
Objective To investigate the effect of FLC in deoxycorticosterone acetate (DOCA)-salt induced experimental renal hypertension in rats. Materials and methods Hypertension was induced in uninephrectomized (UNTZD) male Wistar rats (230–280 g) by injecting DOCA (25 mg/kg, subcutaneously, twice weekly) and supplementing 1% NaCl in drinking water for 5 weeks. The rats were divided in six groups. Captopril (30 mg/kg, p.o.) and FLC (200, 400 and 800 mg/kg, p.o.) were administered daily to the rats of groups III–VI, respectively, for 5 weeks. Various hemodynamic and biochemical parameters were investigated as well as histology of kidney and heart were carried out. Results In this study, the FLC (400 and 800 mg/kg) significantly (p < 0.01, p < 0.001) decreased the systolic blood pressure, diastolic blood pressure, and mean arterial blood pressure. It also significantly (p < 0.01, p < 0.001) decreased elevated end diastolic pressure (EDP), dP/dt max and dP/dt min, organs weights (kidney and heart) and activities of hepatic, renal and cardiac marker enzymes in the serum. Furthermore, FLC (400 and 800 mg/kg) significantly (p < 0.01, p < 0.001) restored altered antioxidant status, serum electrolyte level, lipid profile values, and histological abnormalities. Captopril (30 mg/kg) showed maximum antihypertensive effect but low dose of FLC (200 mg/kg) was not enough to show the antihypertensive activity. Conclusion FLC possessed antihypertensive effect via modulation of endogenous enzymes in DOCA-salt induced renal hypertension in rats.
Introduction
Hypertension is currently the most common cardiovascular disease and a major public health issue in developed as well as developing countries resulting in considerable death and disability worldwide.Citation1,Citation2 It accounted for 9.4 million deaths and 7% of disability adjusted life years in 2010.Citation3 The world’s adult population with hypertension is projected to increase from 1 billion in 2000 to 1.56 billion by 2025.Citation4
In India, hypertension is the leading non-communicable disease risk and it is estimated to be attributable for nearly 10% of all deaths.Citation5–7 It is estimated that in India 16% of ischemic heart disease, 21% of peripheral vascular disease, 24% of acute myocardial infarctions, and 29% of strokes are associated with hypertension. The prevention and control of hypertension with effective pharmacotherapy is very important to decrease the burden of cardiovascular disease.Citation8 A long-term use of synthetic antihypertensive drugs is associated with serious side effects. Therefore, it is necessary to find safer, innovative, and economical therapy for the prevention and cure of hypertension through the widespread research and development.Citation9
In human, the salt retention by the kidneys leads to hypertension. It can be achieved rapidly in rats by removing one kidney and administration of mineralocorticoid.Citation10 Mineralocorticoids like DOCA with sodium chloride (NaCl) are responsible for increase in blood pressure mainly due to the retention of sodium and decrease in potassium.Citation11 Recent studies suggested that several other mechanisms are also involved in salt sensitive DOCA hypertension models like local renal renin–angiotensin systemCitation12 associated with decrease in the bioactivity of nitric oxide (NO), simultaneous endothelial dysfunction and end-organ damage.Citation13 Several other studies reported that DOCA-salt hypertension involved the vascular oxidative stress induced activation of the endothelin system via ETA receptors and endothelin-1Citation7,Citation14 as well as the vascular superoxide producing nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) pathway.Citation15 Pharmaceutical companies have shown that natural product still represent an extremely valuable source for production of new chemical entities for the treatment of hypertension. About 65–80% of world’s population in developing countries do not have access to the modern medicine because of poverty and they totally depend on plants for their primary health care.Citation16,Citation17 The molecules derived from food chain raw materials with the established safety and physiological activity and documented evidence of use by a sizeable population will be safe for long-term use.
Linum usitatissimum Linn. (Linaceae) is commonly known as linseed or flaxseed. It is a popular traditional food and remedy in India, playing important role in the field of diet and disease research. Secoisolariciresinol digucoside (SDG) is a major lignan found in flaxseed (16.4 mg/g).Citation18 It exhibits various pharmacological activities like antidiabetic, antihyperlipidemic, cardioprotective, and renoprotective activity.Citation19–23 It is also useful in the treatments of hypercholesterolemic menopause,Citation24 hypertriglyceridemia, and atherosclerosis.Citation25,Citation26 Recently, it is reported that flaxseeds have therapeutic potential as antioxidant, hydroxyl radical scavenger, anticancer, antiviral, bactericidal, and anti-inflammatory drug.Citation20,Citation27–30 However, currently very few studies have reported about the antihypertensive potential of FLC. Therefore, the objective of this study was to evaluate the dose-dependent effect of FLC in DOCA-salt induced renal hypertension in Wistar rats.
Materials and methods
Experimental animals and protocol approval
Male Wistar rats weighing (230–280 g) were purchased from National Toxicology Center, Pune, India. They were maintained at temperature of 25 ± 1 °C and relative humidity of 45–55% under 12 h light/dark cycle. The animals had access to food pellets (manufactured by Pranav Agro Industries Ltd., Sangli, India) and water ad libitum. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) constituted as per guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), at Poona College of Pharmacy, Pune, India. The IAEC approval number is CPCSEA/69/2014.
Drugs and chemicals
Captopril, DOCA-salt, and dimethyl formamide were purchased from Sigma-Aldrich chemical company (St. Louis, MO). Absolute alcohol (Changshu Yangyuan Chemicals, China) was purchased from the respective vendors. n-Hexane, hydrochloric acid, sodium hydroxide, and sodium chloride of analytical grade were purchased from Qualigens Fine-Chemicals, Mumbai, India. Standard SDG was purchased from Chromadex Inc. (Irvine, CA). All solvents used were of HPLC grade. Acetonitrile (HPLC grade, Merck Chemicals, Atlanta, GA) and formic acid (LC-MS grade, Fluka Analytical, Bellefonte, PA) were purchased from respective vendor.
Collection and authentication of plant seeds
Seeds of Linum usitatissimum (Flaxseeds) were obtained from Punjabrao Deshmukh Krushi Vidyapeeth, College of Agriculture, Nagpur, India. After the authentication of the seeds, a voucher specimen was deposited at our institute. The flaxseeds were stored in cold room before processing for oil extraction at our Real World Nutrition Lab, Bharati Vidyapeeth Deemed University, Pune, India.
Preparation of flax lignan concentrate
Preparation of FLC was carried out as described previously.Citation22 The flaxseed cake was defatted by n-hexane to remove residual oil. The defatted cake was then hydrolyzed with aqueous sodium hydroxide for 1 h at room temperature with intermittent shaking followed by extraction with 50% ethanol. The filtrate was acidified to pH 3 using 1 M hydrochloric acid. The filtrate was dried using rotavac apparatus at 50 °C. The dry powder of hydroalcoholic extract was labeled as FLC.
High-performance liquid chromatography
The SDG content in FLC was estimated using high-performance liquid chromatography (HPLC) analysis using previously reported modifications relating to the column specification.Citation31 The SDG peaks were identified and quantified by comparison with those of standard SDG.
Preparation of drug solution and selection of FLC dose
Captopril and FLC were dissolved in distilled water and DOCA-salt was dissolved in dimethyl formamide (DMF). This study was carried out using three doses of FLC (i.e. 200, 400 and 800 mg/kg, p.o.) and one dose of captopril (i.e. 30 mg/kg, p.o.).
Experimental induction of hypertension
The Wistar rats were anesthetized with intraperitoneal injection of 50 mg/kg thiopental sodium. The kidney of each rat was visualized by a left lateral abdominal incision and the left renal artery and ureter were ligated by silk thread. Then the left kidney was removed and weighed. The muscle and skin layers (incision site) were sutured with sterile suture needle. After 1 week, the rats were used for further experiment. The uninephrectomized (UNTZD) rats were allowed to drink water containing 1% sodium chloride (NaCl) and twice a week they were administered subcutaneous injection of DOCA (25 mg/kg body weight) in 0.4 ml DMF for 5 consecutive weeks.Citation32
Experiment design
After 1 week, the rats were randomly divided into following six groups each comprising six rats:
Group I: UNTZD: UNTZD control (vehicle dist. water p.o.)
Group II: UNTZD + DOCA: UNTZD + 1% NaCl + DOCA-salt control (25 mg/kg s.c. twice weekly)
Group III: UNTZD + C (30): UNTZD + 1% NaCl + DOCA-salt + std. drug captopril (30 mg/kg, p.o.)
Group IV: UNTZD + FLC (200): UNTZD + 1% NaCl + DOCA-salt + FLC (200 mg/kg, p.o.)
Group V: UNTZD + FLC (400): UNTZD + 1% NaCl + DOCA-salt + FLC (400 mg/kg, p.o.)
Group VI: UNTZD + FLC (800): UNTZD + 1% NaCl + DOCA-salt + FLC (800 mg/kg, p.o.)
Flax lignan concentrate (FLC) and captopril were dissolved in distilled water and administered to the rats orally using an oral feeding needle daily for a period of 5 consecutive weeks. The UNTZD control and DOCA-salt control rats received vehicle distilled water. At the end of the study period, blood was collected from each rat by retro orbital puncture for the measurement of biochemical parameters.
Assessment of hemodynamic changes
The rats were anesthetized with intraperitoneal injection of urethane (1.25 g/kg). The trachea was cannulated to assist respiration. Systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial blood pressure (MABP) were measured by invasive technique at the end of fifth week. Polyethylene cannula (PE 50) filled with heparinized saline (100 IU/mL) was inserted into the right carotid artery. The cannula was connected to a transducer and the signal was amplified. The left ventricular hemodynamic changes were measured by means of a Millar micro-tip transducer catheter (Model SRP-320, Millar instrument, INC 320–7051, Houston, TX) inserted into the left ventricle via the right carotid artery and connected to a bioamplifier. Maximum first derivative of ventricular pressure (dP/dt max), minimum first derivative of ventricular pressure (dP/dt min) and left ventricular end diastolic pressure (EDP) signals were obtained from primary signals (left ventricular systolic pressure and blood pressure) by means of Powerlab 8-channel data acquisition system (AD Instruments Pvt. Ltd., with Lab Chart 7.3 Prosoftware, Australia).Citation33–35
Estimation of biological serum markers
The electrolytes such as Na+, K+, and cardiac damage markers [creatine kinase (CK-MB), lactate dehydrogenase (LDH)], hepatic markers [aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total protein], renal markers [blood urea nitrogen (BUN), uric acid and creatinine] and lipid profile [triglycerides (TG), total cholesterol (TC), very low-density lipoproteins (VLDL), and high-density lipoprotein (HDL)] were estimated using commercially available measurement kits (Accurex Pvt. Ltd., Mumbai, India).
Estimation of endogenous antioxidant enzymes
At the end of experimental period, the rats were humanely euthanized. Heart and kidney were removed for further experiments. The portion of renal tissue was individually homogenized in 10% ice cold Tris-hydrochloride buffer (10 mmol/L, pH 7.4) in tissue homogenizer (Remi, India) and centrifuged at 7500 rpm for 15 min at 0 °C. The clear supernatant was collected after centrifugation and used for assay of endogenous antioxidant enzyme, namely superoxide dismutase (SOD), glutathione (GSH) and malondialdehyde (MDA) according to previously reported methods.Citation36–42
Histopathological examination
The excised heart and kidney samples were cleaned and immediately fixed in a neutral buffered solution of 10% formalin. Specimens were routinely processed and embedded in paraffin. Specimens were cut in section of 5 μm thickness by microtome and stained by hematoxylin and eosin for microscopic examination. The sections were observed under the microscope and photomicrographs of tissue section were taken using a microscope camera (Nikon Cool pix). The parameters of histopathological assessment of kidney sections were mainly atrophy of tubular cell, necrosis, and glomerulus congestion and vacuolization. The parameters of histopathological assessment of hearts sections were myocardial degeneration, hypertrophy, and infiltration of inflammatory cells.
Statistical analysis
The data were expressed as mean ± standard error mean (SEM). Analysis of the data was performed using GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA). The body weight data was analyzed by two-way analysis of variance (ANOVA) and Bonferroni test was applied for post-hoc analysis. However, hemodynamic and biochemical parameters were analyzed using one-way analysis of variance (ANOVA) and Dunett’s test was applied for post-hoc analysis. A value of p < 0.05 was considered to be statistically significant.
Results
Standardization of flax lignan concentrate
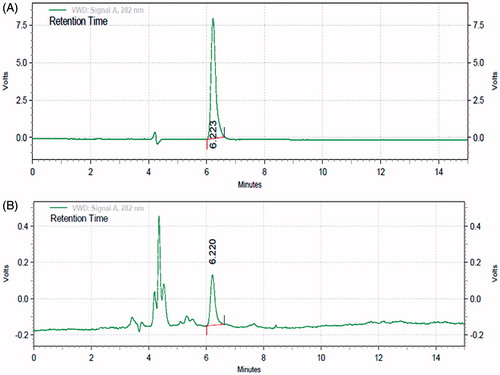
presents HPLC chromatogram of the standard SDG and flax lignan concentrate, respectively. The separation of SDG in FLC was achieved within 7 min. The peak eluting 6.22 min was assigned to be SDG after comparing with standard SDG chromatogram. The SDG content in FLC fraction was found to be 3.58%.
Effect of FLC on hemodynamic parameters and left ventricular contractile function
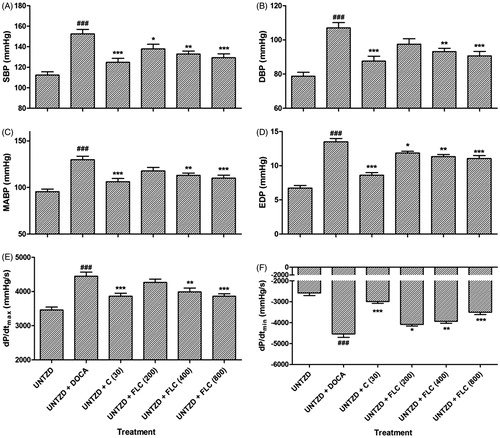
The effect of FLC at three different concentrations (200, 400, and 800 mg/kg) in all the I–VI groups after 5 weeks on hemodynamic parameters are shown in . In the UNTZD + DOCA-salt control group-II, after 5 weeks of dosing there was significant (p < 0.001 each) increase in SBP (152.5 ± 4.41 mmHg), DBP (107.1 ± 3.13 mmHg), MABP (129.8 ± 3.76 mmHg), EDP (13.49 ± 0.49 mmHg), dP/dt max (4450 ± 119.6 mmHg/s), and dP/dt min (−4547 ± 156 mmHg/s) compared to UNTZD control group-I. Captopril (30 mg/kg) and FLC (800 mg/kg) treatment respectively showed a significant (p < 0.001 each) decrease in the SBP (124.8 ± 3.9 and 129.3 ± 3.88 mmHg), DBP (87.55 ± 2.9 and 90.65 ± 2.6 mmHg), MABP (106.2 ± 3.4 and 110 ± 3.2 mmHg), EDP (8.6 ± 0.39 and 11.04 ± 0.44 mmHg), dP/dt max (3865 ± 84.2 and 3861 ± 72.55 mmHg/s), and dP/dt min (−2995 ± 85.97 and −3507 ± 108 mmHg/s). FLC (400 mg/kg) treatment also showed significant (p < 0.01each) decrease in SBP (132.9 ± 2.86 mmHg), DBP (93.16 ± 1.9 mmHg), MABP (113 ± 2.38 mmHg), EDP (11.32 ± 0.32 mmHg), dP/dt max (3989 ± 113.4 mmHg/s), and dP/dt min (−3942 ± 93.64 mmHg/s) compared to UNTZD DOCA-hypertensive group-II. Treatment with FLC (200 mg/kg) showed significant (p < 0.05) decrease in SBP (137.9 ± 4.51 mmHg), EDP (11.86 ± 0.25 mmHg), and dP/dt min (−4088 ± 74.32 mmHg/s); on the other hand, FLC (200 mg/kg) did not show any significant decrease in DBP (97.5 ± 3.20 mmHg), MABP (117.7 ± 3.85 mmHg), and dP/dt max (4268 ± 92.36 mmHg/s) values.
Figure 2. Effect of administration of captopril and FLC on various hemodynamic parameters viz. SBP (A), DBP (B), MABP (C), EDP (D), dP/dtmax (E), and dP/dtmin (F) in DOCA-salt hypertensive Wistar rats. Values are expressed as mean ± SEM (n = 6). Data were analyzed by one-way ANOVA followed by Dunnett's test. *p < 0.05, **p < 0.01, ***p < 0.001 as compared with UNTZD + DOCA control group and ###p < 0.001 compared to UNTZD control group.
Effect of FLC on organs weight, electrolyte and animal body weight
UNTZD DOCA-salt control group-II rats showed significant (p < 0.001) increase in organs weight (kidney and heart) and serum sodium ion level and significant (p < 0.001) decrease in serum potassium ion level. Treatment with captopril (30 mg/kg) and FLC (200, 400, and 800 mg/kg) brought back those values toward near to normal ().
Table 1. Effects of captopril and FLC on organs weight and serum electrolytes in DOCA-salt induced hypertensive Wistar rats.
The body weight of UNTZD DOCA-salt hypertensive animals started to decrease significantly (p < 0.01; p < 0.001) from the second week of DOCA-salt treatment compared to UNTZD control group-I. Treatments with captopril (30 mg/kg) and FLC (400 and 800 mg/kg) significantly (p < 0.01) prevented weight loss from the second week of the treatment. On the other hand, FLC (200 mg/kg) did not show significant inhibition of body weight loss in the second and third week but showed significant (p < 0.01; p < 0.001) inhibition after the fourth and fifth week of the treatment ().
Effect of FLC on serum cardiac, hepatic, and renal markers
The activities of CK-MB, LDH, AST, ALT, ALP, total protein, BUN, uric acid, and creatinine were significantly (p < 0.001 each) increased in DOCA salt hypertensive rats. The treatments with captopril (30 mg/kg) and FLC (200, 400, and 800 mg/kg) decreased these values toward near normal. Captopril (30 mg/kg) showed the highest activity by significant (p < 0.001) reduction in cardiac, hepatic, and renal markers. FLC (800 mg/kg) also showed significant (p < 0.001) reduction in serum level of LDH, AST, ALT, ALP, total protein, BUN, uric acid, and creatinine. Treatment with FLC (400 mg/kg) also significantly (p < 0.01) reduced the levels of AST, ALT, ALP, CK-MB, and LDH. On the other hand, FLC (200 mg/kg) did not show any significant reduction in serum AST, ALP, CK-MB, and LDH; but showed significant (p < 0.05) reduction in serum level of ALT, BUN, uric acid, and creatinine ().
Table 2. Effect of captopril and FLC on serum cardiac, hepatic and renal markers in DOCA-salt induced hypertensive Wistar rats.
Effect of FLC on lipid profile
There was significant (p < 0.001 each) increase in levels of TG, TC and VLDL and significant (p < 0.001) decrease in level of HDL in UNTZD DOCA-salt control group-II. After the treatments with captopril (30 mg/kg) and FLC (200, 400, and 800 mg/kg), there was decrease in serum TG, TC, and VLDL levels and increase in HDL level as compared to UNTZD DOCA-salt control group-II. Captopril (30 mg/kg) and FLC (800 mg/kg) showed significant (p < 0.001) reduction in TG, TC, and VLDL levels and also showed significant (p < 0.001) increase in serum HDL level. FLC (400 mg/kg) showed similar activity (i.e. p < 0.001) as compared to higher dose (800 mg/kg) of FLC in the case of TG, TC, and VLDL levels but showed relatively less activity (i.e. p < 0.01) in the case of HDL and total protein levels. Lower dose of FLC (200 mg/kg) also showed significant (p < 0.05) decrease in TG, TC, and VLDL levels and significant (p < 0.05) increase in HDL level ().
Table 3. Effect of captopril and FLC on lipid profile in DOCA-salt induced hypertensive Wistar rats.
Endogenous antioxidant enzymes
The effect of captopril (30 mg/kg) and FLC (200, 400, and 800 mg/kg) on endogenous antioxidant enzymes is as follows. After 5 weeks, there was significant (p < 0.001) decrease in activities of SOD and GSH in the tissues (kidney and heart) of the UNTZD DOCA-salt hypertensive rats, but the captopril (30 mg/kg) and FLC (800 mg/kg)-treated rats showed significant (p < 0.001) restoration of the activities of SOD and GSH. FLC (400 mg/kg) also showed significant (p < 0.01) restoration of SOD and GSH, whereas FLC (200 mg/kg) did not show any significant restoration. MDA level in the tissues (kidney and heart) of the UNTZD DOCA-salt hypertensive rats (group-II) increased significantly (p < 0.001), whereas the captopril (30 mg/kg) and FLC (800 mg/kg)-treated groups had significantly lower level (p < 0.001) of MDA than in the FLC (400 mg/kg)-treated group (p < 0.01). However, FLC (200 mg/kg)-treated group did not show any significant restoration ().
Table 4. Effect of captopril and FLC on endogenous antioxidant enzymes in DOCA-salt induced hypertensive Wistar rats.
Effect of FLC on histopathology of kidney
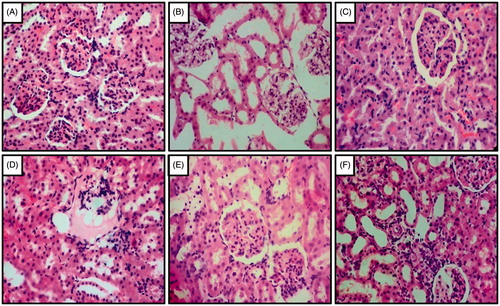
Histopathological examination of the kidney tissues in the UNTZD control group-I rats showed normal glomerulus and tubule structure without any damage. However, the tissues of the UNTZD DOCA-salt control group-II rats showed atrophy of tubules, necrosis, and congestion of glomerulus. Captopril (30 mg/kg) showed maximum protection against the hypertensive damage and FLC (400, 800 mg/kg) showed significant protection and reduced atrophy, necrosis, and glomerulus congestion. However, FLC (200 mg/kg) did not show significant protection from the hypertensive damage ().
Figure 3. Effect of captopril and FLC on DOCA-salt induced alteration in kidney histology of rats. Photomicrograph of sections of kidney of (A) UNTZD control rats showed normal glomerulus cell and tubuli with the absence of any congestion, (B) UNTZD + DOCA-salt control group showed atrophy of tubular cell, necrosis and glomerulus congestion, (C) UNTZD + DOCA-salt + captopril (30 mg/kg) showed decrease in glomerulus congestion, atrophy and necrosis, (D) UNTZD + DOCA-salt + FLC (200 mg/kg) showed no significant protection against hypertension, (E) UNTZD + DOCA-salt + FLC (400 mg/kg) showed decrease in glomerulus atrophy, congestion and necrosis, and (F) UNTZD + DOCA-salt + FLC (800 mg/kg) showed significantly reduced glomerulus congestion and necrosis. Hematoxylin and eosin staining (at 40×).
Effect of FLC on histopathology of heart
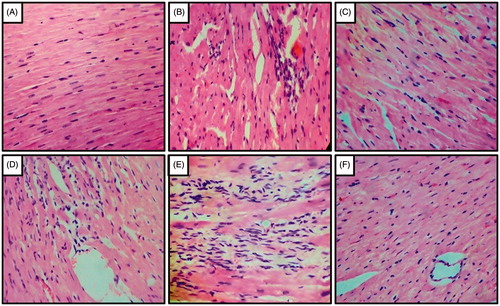
Histopathological examination of the heart tissues showed normal architecture in UNTZD control group-I but UNTZD DOCA-salt control group-II showed severe myocardial degeneration, hypertrophy, and infiltration of inflammatory cells. Captopril (30 mg/kg) showed maximum protection and minimum myocardial degeneration, hypertrophy, and infiltration of inflammatory cell. FLC (400 and 800 mg/kg) also showed significant protection against hypertension induced cardiac damage. However, FLC (200 mg/kg) did not show any significant protection ().
Figure 4. Effect of captopril and FLC on DOCA-salt induced alteration in heart histology of rats. Photomicrograph of sections of heart of (A) UNTZD rats showed normal cardiac muscle fibers, (B) UNTZD + DOCA-salt control showed severe myocardial degeneration, hypertrophy and infiltration of inflammatory cells, (C) UNTZD + DOCA-salt + captopril (30 mg/kg) showed minimal myocardial degeneration and infiltration of inflammatory cells, (D) UNTZD + DOCA-salt + FLC (200 mg/kg) showed no significant change in myocardial tissues, (E) UNTZD + DOCA-salt + FLC (400 mg/kg) showed decrease in myocardial degeneration and infiltration of inflammatory cells, and (F) UNTZD + DOCA-salt + FLC (800 mg/kg) showed significant reduction in myocardial degeneration and infiltration of inflammatory cells. Hematoxylin and eosin staining (at 40×).
Discussion
In this study, we observed that higher doses of FLC significantly decreased systolic, diastolic, and mean arterial blood pressures similar to those of standard ACE inhibitor drug captopril. The major lignan found in FLC is SDG, hypotensive, and ACE inhibitor-like activity of SDG from flaxseed have been reported in normotensive and angiotensin-I induced acute hypertension in rats.Citation19,Citation43 The blood pressure lowering effect of FLC in DOCA-salt induced hypertension in rat model explains its antihypertensive potential in the chronic hypertensive conditions. Comparison between captopril and FLC-treated group shall further clarify the mechanism of action of FLC. The current study also showed that FLC dose-dependently reduced EDP, dP/dt max, dP/dt min after 5 weeks of treatment. Our results suggest that FLC provides sufficient contractile reserve in hypertensive condition.
High blood pressure in DOCA salt-induced hypertension is responsible for loss of body weight in the laboratory animals.Citation44 In this study, similar type of weight loss was observed in the DOCA-salt control group; but in the captopril and FLC treatment groups, there were significant inhibition in the weight loss due to antihypertensive effects. Several studies reported that DOCA-salt hypertension is always associated with significant hypertrophy of kidney and heart.Citation45,Citation46 FLC and captopril significantly prevented the increase in kidney and heart weight associated with the hypertrophy and thus confirmed that the prevention of hypertrophy might be due to antihypertensive effects of FLC and captopril. According to pathophysiology of hypertension, intracellular sodium ion concentration increases, and potassium ion concentration decreases significantly in human.Citation47 It is also responsible for increase in sympathetic activity accompanying various vascular structural changes and altered ion exchanges.Citation48 In this study, we observed increase in serum sodium concentration and decrease in serum potassium concentration in DOCA-salt hypertensive rats. After the treatment with FLC and captopril, significant decrease in serum sodium and significant increase in serum potassium was observed. This may be due to antihypertensive effect of captopril and FLC.
The DOCA-salt hypertensive rat model is the model of cardiovascular oxidative and inflammatory stress.Citation49 The natural compounds that are able to lower the concentration of reactive free radical species can effectively restrain inflammation in the cardiovascular system in DOCA-salt hypertension model. The production of reactive oxygen species (oxidative stress) is the important etiological factor in the development of various diseases including hypertension.Citation50–62 GSH has been reported to have free radical scavenging activity.Citation63,Citation64 It is considered that GSH act as cofactor for several protective enzymes that are important in defense mechanism of body from oxidative stress.Citation33,Citation65–67 There is association between the increase in blood pressure and the oxidative stress. Several clinical investigations showed that serum concentrations of MDA are high in the hypertensive patients than in normal individuals.Citation68 In this study, serum MDA levels were significantly increased, while GSH and SOD levels were significantly decreased in the heart and kidney tissues of the DOCA-salt hypertensive group compared to the UNTZD control group. This indicates that the increase in blood pressure is responsible for the production of oxidative stress. Our results showed that FLC reduced the increase in blood pressure, which may be due to antioxidant activity of FLC. Recently, effect of different contents of flaxseed cake in diets and their administration period on the development of rat and selected parameters of their health status was reported by previous researcher.Citation69 The authors observed that administration of diet with 30% additions of flaxseed cake for 90 days improve the antioxidants status in rats. Our results are thus agreement with these previous findings.
Several clinical as well as experimental animal studies including DOCA-salt hypertension have been reported that in hypertension, there were increased plasma levels of LDL, VLDL, TG, TC, and decreased plasma level of HDL.Citation70–73 Irregular lipid and lipoprotein metabolism play important role in experimental hypertensive rats.Citation74 Oxidative stress, lipid peroxidation, and altered lipid metabolism may be the reason to decrease HDL and increase LDL, VLDL and TC level in DOCA-salt hypertensive rats. Antioxidants are known to inhibit lipid peroxidation by preventing accumulation of the reactive oxygen species.Citation75–79 In this study, antioxidant activity of FLC decreased lipid peroxidation, controlled abnormal lipid metabolism, and restored altered lipid profile values.
Oxidative stress induced by DOCA-salt induced hypertension is an important etiological factor for the liver damage or hepatotoxicity.Citation80,Citation81 The liver damage leads to increased levels of AST, ALT, ALP, and total protein in plasma mainly because of secretion of these enzymes in blood circulation.Citation82,Citation83 In this investigation, we have observed increase in serum level of this enzyme in the UNTZD DOCA-salt control hypertensive group confirming liver damage. After the administration of FLC and captopril, there was significant decrease in serum levels of AST, ALT, ALP, and total protein, which clearly showed that FLC had prevented oxidative damage in the DOCA-salt induced hypertension in rats. Kidney is the organ which regulates salt and water balance and plays an important role in maintaining blood pressure. Hypertensive disease always leads to develop volume and pressure loads on the kidneys, which leads to dysfunction and damage to renal tissues.Citation84 Several studies reported increase in the level of serum renal marker enzymes in the DOCA salt hypertension animal model.Citation85 Oxidative stress due to high blood pressure may be the reason for elevation of urea, creatinine, and uric acid levels in serum. In the present investigation, the increase in levels of serum urea, creatinine, and uric acid was observed in the UNTZD DOCA-salt hypertensive rats. FLC significantly reduced the levels of serum urea, creatinine, and uric acid suggested its protective effect in hypertension.
CK-MB and LDH are known to be the standard markers for identification of cardiac damage. Increase in blood pressure may be responsible for myocardial damage to leak CK-MB and LDH and detected in serum in the DOCA-salt hypertensive rats.Citation86 Decreased serum level of these enzymes in FLC-treated groups (400 and 800 mg/kg) showed protective effect of FLC in DOCA-salt hypertension.
Histopathological examination revealed that DOCA-salt induced hypertension damaged the heart and kidney tissues. However, the hearts and kidneys of the FLC-treated DOCA-salt hypertensive rats showed reduced cardiac and renal damage. This confirmed the antihypertensive activity of FLC in the DOCA-salt induced renal hypertension in rats.
Conclusion
It may be concluded that, FLC in high doses (400 and 800 mg/kg) significantly restored elevated blood pressure, altered left ventricular functions, biochemical changes, altered lipid profile, and oxidative injuries associated with DOCA-salt induced hypertension. Antihypertensive property of FLC (800 mg/kg) was similar to the standard ACE inhibitor drug captopril (30 mg/kg) in the DOCA-salt induced hypertensive rats. The protective effects of FLC were further revealed by histopathological examination of heart and kidney tissue of the rats. Based on these observations, we concluded that FLC possessed antihypertensive effect in DOCA-salt induced renal hypertension in rats. Antihypertensive effect of FLC may be due to its antioxidant nature which inhibited reactive oxygen species and possible antagonism of renin–angiotensin–aldosterone system.
Acknowledgements
Authors acknowledge Dr S. S. Kadam, Vice-Chancellor and Dr K. R. Mahadik, Principal; Poona College of Pharmacy, Bharati Vidyapeeth Deemed University, Pune, India, for providing necessary facility to carry out present research work.
Declaration of interest
The authors declare that there is no conflict of interest.
References
- Gosavi T, Kandhare A, Raygude K, Ghosh P, Bodhankar S. A comparative study on the efficacy, safety and cost effectiveness of Viscum album and Rauwolfia serpentina mother tincture in hypertensive patients. Deccan J Nat Prod. 2011;2:29–35.
- Ghosh P, Kandhare AD, Raygude KS, et al. Determination of the long term diabetes related complications and cardiovascular events using UKPDS risk engine and UKPDS outcomes model in a representative western Indian population. Asian Pac J Trop Dis. 2012;2:S642–S650.
- Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2224–2260.
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365:217–223.
- Patel V, Chatterji S, Chisholm D, et al. Chronic diseases and injuries in India. Lancet. 2011;377:413–428.
- Badole SL, Chaudhari SM, Jangam GB, Kandhare AD, Bodhankar SL. Cardioprotective activity of Pongamia pinnata in streptozotocin-nicotinamide induced diabetic rats. BioMed Res Int. 2015;2015:1–8.
- Visnagri A, Kandhare AD, Ghosh P, Bodhankar SL. Endothelin receptor blocker bosentan inhibits hypertensive cardiac fibrosis in pressure overload-induced cardiac hypertrophy in rats. Cardiovasc Endocrinol. 2013;2:85–97.
- Mohan S, Reddy KS, Prabhakaran D. Chronic Non-communicable Diseases in India – Reversing the Tide. New Delhi: Public Health Foundation of India; 2011.
- Lee DH, Kim JH, Park JS, Choi YJ, Lee JS. Isolation and characterization of a novel angiotensin I-converting enzyme inhibitory peptide derived from the edible mushroom Tricholoma giganteum. Peptides. 2004;25:621–627.
- de Champlain J, van Ameringen MR. Regulation of blood pressure by sympathetic nerve fibers and adrenal medulla in normotensive and hypertensive rats. Circ Res. 1972;31:617–628.
- Mulrow PJ, Forman BH. The tissue effects of mineralocorticoids. Am J Med. 1972;53:561–572.
- Brown L, Duce B, Miric G, Sernia C. Reversal of cardiac fibrosis in deoxycorticosterone acetate-salt hypertensive rats by inhibition of the renin-angiotensin system. J Am Soc Nephrol. 1999;10:S143–S148.
- Zhou M-S, Adam AG, Jaimes EA, Raij L. In salt-sensitive hypertension, increased superoxide production is linked to functional upregulation of angiotensin II. Hypertension. 2003;42:945–951.
- Callera GE, Touyz RM, Teixeira SA, et al. ETA receptor blockade decreases vascular superoxide generation in DOCA-salt hypertension. Hypertension. 2003;42:811–817.
- Iwashima F, Yoshimoto T, Minami I, Sakurada M, Hirono Y, Hirata Y. Aldosterone induces superoxide generation via Rac1 activation in endothelial cells. Endocrinology. 2008;149:1009–1014.
- Kandhare AD, Raygude KS, Ghosh P, Gosavi TP, Bodhankar SL. Patentability of Animal Models: India and the Globe. Int J Pharm Biol Arch. 2011;2:1024–1032.
- Shivakumar V, Kandhare A, Rajmane A, et al. Estimation of the long-term cardiovascular events using ukpds risk engine in metabolic syndrome patients. Indian J Pharm Sci. 2014;76:174–178.
- Westcott ND, Muir AD. Process for extracting lignans from flaxseed. Google Patents; 1998.
- Prasad K. Secoisolariciresinol diglucoside (SDG) isolated from flaxseed, an alternative to ACE inhibitors in the treatment of hypertension. Int J Angiol. 2013;22:235.
- Zanwar AA, Hegde M, Bodhankar S. In vitro antioxidant activity of ethanolic extract of Linum usitatissimum. Pharmacol Online. 2010;1:683–696.
- Zanwar A, Hegde M, Bodhankar S. Cardioprotective activity of flax lignan concentrate extracted from seeds of Linum usitatissimum in isoprenalin induced myocardial necrosis in rats. Interdiscip Toxicol. 2011;4:90–97.
- Zanwar AA, Hegde MV, Bodhankar SL. Protective role of concomitant administration of flax lignan concentrate and omega-3-fatty acid on myocardial damage in doxorubicin-induced cardiotoxicity. Food Sci Hum Wellness. 2013;2:29–38.
- Ghule AE, Jadhav SS, Bodhankar SL. Renoprotective effect of Linum usitatissimum seeds through hemodynamic changes and conservation of antioxidant enzymes in renal ischemia-reperfusion injury in rats. Arab J Urol. 2011;9:215–221.
- Lemay A, Dodin S, Kadri N, Jacques H, Forest J-C. Flaxseed dietary supplement versus hormone replacement therapy in hypercholesterolemic menopausal women. Obstet Gynecol. 2002;100:495–504.
- Prasad K. Dietary flax seed in prevention of hypercholesterolemic atherosclerosis. Atherosclerosis. 1997;132:69–76.
- Prasad K, Mantha S, Muir A, Westcott N. Reduction of hypercholesterolemic atherosclerosis by CDC-flaxseed with very low alpha-linolenic acid. Atherosclerosis. 1998;136:367–375.
- Chen J, Stavro PM, Thompson LU. Dietary flaxseed inhibits human breast cancer growth and metastasis and downregulates expression of insulin-like growth factor and epidermal growth factor receptor. Nutr Cancer. 2002;43:187–192.
- Collins TF, Sprando RL, Black TN, et al. Effects of flaxseed and defatted flaxseed meal on reproduction and development in rats. Food Chem Toxicol. 2003;41:819–834.
- Kinniry P, Amrani Y, Vachani A, et al. Dietary flaxseed supplementation ameliorates inflammation and oxidative tissue damage in experimental models of acute lung injury in mice. J Nutr. 2006;136:1545–1551.
- Rajesha J, Murthy KNC, Kumar MK, Madhusudhan B, Ravishankar GA. Antioxidant potentials of flaxseed by in vivo model. J Agric Food Chem. 2006;54:3794–3799.
- Ghule AE, Kandhare AD, Jadhav SS, Zanwar AA, Bodhankar SL. Omega-3-fatty acid adds to the protective effect of flax lignan concentrate in pressure overload-induced myocardial hypertrophy in rats via modulation of oxidative stress and apoptosis. Int Immunopharmacol. 2015;28:751–763.
- Fenning A, Harrison G, Rose’meyer R, Hoey A, Brown L. LArginine attenuates cardiovascular impairment in DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol. 2005;289:H1408–H1416.
- Adil M, Kandhare A, Visnagri A, Bodhankar S. Naringin ameliorates sodium arsenite-induced renal and hepatic toxicity in rats: decisive role of KIM-1, Caspase-3, TGF-β and TNF-α. Ren Fail. 2015;37:1396–1407.
- Kandhare AD, Patil MVK, Bodhankar SL. L-Arginine attenuates the ethylene glycol induced urolithiasis in ininephrectomized hypertensive rats: role of KIM-1, NGAL, and NOs. Ren Fail. 2015;37:709–721.
- Visnagri A, Kandhare AD, Bodhankar SL. Renoprotective effect of berberine via intonation on apoptosis and mitochondrial-dependent pathway in renal ischemia reperfusion-induced mutilation. Ren Fail. 2015;37:482–493.
- Kumar VS, Rajmane AR, Adil M, Kandhare AD, Ghosh P, Bodhankar SL. Naringin ameliorates acetic acid induced colitis through modulation of endogenous oxido-nitrosative balance and DNA damage in rats. J Biomed Res. 2014;28:132–145.
- Patil MVK, Kandhare AD, Ghosh P, Bhise SD. Determination of role of GABA and nitric oxide in anticonvulsant activity of Fragaria vesca L. ethanolic extract in chemically induced epilepsy in laboratory animals. Orient Pharm Exp Med. 2012;12:255–264.
- Raygude KS, Kandhare AD, Ghosh P, Ghule AE, Bodhankar SL. Evaluation of ameliorative effect of quercetin in experimental model of alcoholic neuropathy in rats. Inflammopharmacology. 2012;20:331–341.
- Saraswathi KY, Muthal A, Kandhare A, Rojatkar S, Bodhankar S. Study of methanolic extract of Artemisia pallens wall on endurance of laboratory animals. Pharmacologia. 2014;5:298–309.
- Sarkar A, Sengupta A, Mukhrjee A, et al. Antiulcer potential of morin in acetic acid-induced gastric ulcer via modulation of endogenous biomarkers in laboratory animals. Pharmacologia. 2015;6:273–281.
- Visnagri A, Kandhare AD, Chakravarty S, Ghosh P, Bodhankar SL. Hesperidin, a flavanoglycone attenuates experimental diabetic neuropathy via modulation of cellular and biochemical marker to improve nerve functions. Pharm Biol. 2014;52:814–828.
- Visnagri A, Kandhare AD, Shiva Kumar V, et al. Elucidation of ameliorative effect of Co-enzyme Q10 in streptozotocin-induced diabetic neuropathic perturbation by modulation of electrophysiological, biochemical and behavioral markers. Biomed Aging Pathol. 2012;2:157–172.
- Prasad K. Antihypertensive activity of secoisolariciresinol diglucoside (SDG) isolated from flaxseed: role of guanylate cyclase. Int J Angiol. 2004;13:7–14.
- Hayakawa H, Hirata Y, Suzuki E, et al. Long-term administration of L-arginine improves nitric oxide release from kidney in deoxycorticosterone acetate-salt hypertensive rats. Hypertension. 1994;23:752–756.
- Frohlich ED, Apstein C, Chobanian AV, et al. The heart in hypertension. N Engl J Med. 1992;327:998–1008.
- Chan V, Hoey A, Brown L. Improved cardiovascular function with aminoguanidine in DOCA-salt hypertensive rats . Br J Pharmacol. 2006;148:902–908.
- Leiba A, Vald A, Peleg E, Shamiss A, Grossman E. Does dietary recall adequately assess sodium, potassium, and calcium intake in hypertensive patients? Nutrition. 2005;21:462–466.
- Schenk J, McNeill JH. The pathogenesis of DOCA-salt hypertension. J Pharmacol Toxicol Methods. 1992;27:161–170.
- Iyer A, Chan V, Brown L. The DOCA-salt hypertensive rat as a model of cardiovascular oxidative and inflammatory stress. Curr Cardiol Rev. 2010;6:291.
- Kojda G, Harrison D. Interactions between NO and reactive oxygen species: Pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res. 1999;43:652–671.
- Goswami S, Kandhare A, Zanwar AA, et al. Oral l-glutamine administration attenuated cutaneous wound healing in Wistar rats. Int Wound J. 2016;13:116–124.
- Kamble H, Kandhare AD, Bodhankar S, Mohan V, Thakurdesai P. Effect of low molecular weight galactomannans from fenugreek seeds on animal models of diabetes mellitus. Biomed Aging Pathol. 2013;3:145–151.
- Kandhare AD, Bodhankar SL, Mohan V, Thakurdesai PA. Acute and repeated doses (28 days) oral toxicity study of glycosides based standardized fenugreek seed extract in laboratory mice. Regul Toxicol Pharmacol. 2015;72:323–334.
- Kandhare AD, Bodhankar SL, Singh V, Mohan V, Thakurdesai PA. Anti-asthmatic effects of type-A procyanidine polyphenols from cinnamon bark in ovalbumin-induced airway hyperresponsiveness in laboratory animals. Biomed Aging Pathol. 2013;3:23–30.
- Kandhare AD, Ghosh P, Bodhankar SL. Naringin, a flavanone glycoside, promotes angiogenesis and inhibits endothelial apoptosis through modulation of inflammatory and growth factor expression in diabetic foot ulcer in rats. Chem Biol Interact. 2014;219:101–112.
- Kandhare AD, Ghosh P, Ghule AE, Bodhankar SL. Elucidation of molecular mechanism involved in neuroprotective effect of Coenzyme Q10 in alcohol induced neuropathic pain. Fundam Clin Pharmacol. 2013;27:603–622.
- Kandhare AD, Ghosh P, Ghule AE, Zambare GN, Bodhankar SL. Protective effect of Phyllanthus amarus by modulation of endogenous biomarkers and DNA damage in acetic acid induced ulcerative colitis: Role of phyllanthin and hypophyllanthin. Apollo Med. 2013;10:87–97.
- Kandhare AD, Kumar VS, Adil M, Rajmane AR, Ghosh P, Bodhankar SL. Investigation of gastro protective activity of Xanthium strumarium L. by modulation of cellular and biochemical marker. Orient Pharm Exp Med. 2012;12:287–299.
- Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Bodhankar SL. Neuroprotective effect of naringin by modulation of endogenous biomarkers in streptozotocin induced painful diabetic neuropathy. Fitoterapia. 2012;83:650–659.
- Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Bodhankar SL. Therapeutic role of curcumin in prevention of biochemical and behavioral aberration induced by alcoholic neuropathy in laboratory animals. Neurosci Lett. 2012;511:18–22.
- Kandhare AD, Raygude KS, Shiva Kumar V, et al. Ameliorative effects quercetin against impaired motor nerve function, inflammatory mediators and apoptosis in neonatal streptozotocin-induced diabetic neuropathy in rats. Biomed Aging Pathol. 2012;2:173–186.
- Kandhare AD, Shivakumar V, Rajmane A, Ghosh P, Bodhankar SL. Evaluation of the neuroprotective effect of chrysin via modulation of endogenous biomarkers in a rat model of spinal cord injury. J Nat Med. 2014;68:586–603.
- Ketkar S, Rathore A, Kandhare A, et al. Alleviating exercise induced muscular stress using neat and processed bee pollen: Oxidative markers, mitochondrial enzymes and myostatin expression in rats. Integr Med Res. 2015;4:147–160
- Raygude KS, Kandhare AD, Ghosh P, Bodhankar SL. Anticonvulsant effect of fisetin by modulation of endogenous biomarkers. Biomed Prev Nutr. 2012;2:215–222.
- Kidd PM. Glutathione: Systemic protectant against oxidative and free radical damage. Altern Med Rev. 1997;2:155–176.
- Aswar UM, Kandhare AD, Mohan V, Thakurdesai PA. Anti-allergic effect of intranasal administration of type-A procyanidin polyphenols based standardized extract of cinnamon bark in ovalbumin sensitized BALB/c mice. Phytother Res. 2015;29:423–433.
- Gosavi TP, Ghosh P, Kandhare AD, et al. Therapeutic effect of H. pylori nosode, a homeopathic preparation in healing of chronic H. pylori infected ulcers in laboratory animals. Asian Pac J Trop Dis. 2012;2:S603–S611.
- Armas-Padilla MC, Armas-Hernández MJ, Sosa-Canache B, et al. Nitric oxide and malondialdehyde in human hypertension. Am J Ther. 2007;14:172–176.
- Matusiewicz M, Kosieradzka I, Zuk M, Szopa J. Effect of dose and administration period of seed cake of genetically modified and non-modified flax on selected antioxidative activities in rats. Int J Mol Sci. 2015;16:14259–14275.
- Sarkar D, Latif S, Uddin M, et al. Studies on serum lipid profile in hypertensive patient. Mymensingh Med J. 2007;16:70–76.
- Dimitrova A, Strashimirov D, Betova T, Russeva A, Alexandrova M. Zinc content in the diet affects the activity of Cu/ZnSOD, lipid peroxidation and lipid profile of spontaneously hypertensive rats. Acta Biol Hung. 2008;59:305–314.
- Ameijeiras AH, Paz JL, Seijo MP, et al. Lipid profile in hypertensive patients treated with lipid lowering therapy: PP.23.446. J Hypertens. 2010;28:e378–e379.
- Veeramani C, Al-Numair KS, Chandramohan G, Alsaif MA, Pugalendi KV. Antihyperlipidemic effect of Melothria maderaspatana leaf extracts on DOCA-salt induced hypertensive rats. Asian Pac J Trop Med. 2012;5:434–439.
- Girard A, Madani S, El Boustani ES, Belleville J, Prost J. Changes in lipid metabolism and antioxidant defense status in spontaneously hypertensive rats and Wistar rats fed a diet enriched with fructose and saturated fatty acids. Nutrition. 2005;21:240–248.
- Bernard L, Kathy K. Reactive oxygen species in hypertension. Am J Hypertens. 2004;17:852–860.
- Honmore V, Kandhare A, Zanwar AA, Rojatkar S, Bodhankar S, Natu A. Artemisia pallens alleviates acetaminophen induced toxicity via modulation of endogenous biomarkers. Pharm Biol. 2015;53:571–581.
- Kandhare A, Raygude K, Ghosh P, Bodhankar S. The ameliorative effect of fisetin, a bioflavonoid, on ethanol-induced and pylorus ligation-induced gastric ulcer in rats. Int J Green Pharm. 2011;5:236–243.
- Kandhare AD, Alam J, Patil MVK, Sinha A, Bodhankar SL. Wound healing potential of naringin ointment formulation via regulating the expression of inflammatory, apoptotic and growth mediators in experimental rats. Pharm Biol. 2015;54:419–432.
- Kandhare AD, Bodhankar SL, Mohan V, Thakurdesai PA. Effect of glycosides based standardized fenugreek seed extract in bleomycin-induced pulmonary fibrosis in rats: Decisive role of Bax, Nrf2, NF-κB, Muc5ac, TNF-α and IL-1β. Chem Biol Interact. 2015;237:151–165.
- Hoetzel A, Welle A, Schmidt R, et al. Nitric oxide-deficiency regulates hepatic heme oxygenase-1. Nitric Oxide. 2008;18:61–69.
- Hemalatha G, Pugalendi KV, Saravanan R. Modulatory effect of sesamol on DOCA-salt-induced oxidative stress in uninephrectomized hypertensive rats. Mol Cell Biochem. 2013;379:255–265.
- Navarro MC, Montialla M, Martín A, Jiménez J, Utrilla MP. Free radical scavenger and antihepatotoxic activity of Rosmarinus tomentosus. Planta Med. 1993;59:312–314.
- Bhattacharjee N, Pathak S, Khuda-Bukhsh AR. Amelioration of carcinogen-induced toxicity in mice by administration of a potentized homeopathic drug, Natrum Sulphuricum 200. Evid Based Complement Alternat Med. 2009;6:65–75.
- Mohring J, Mohring B, Naumann H, et al. Salt and water balance and renin activity in renal hypertension of rats. Am J Physiol Legacy Content. 1975;228:1847–1855.
- Prahalathan P, Kumar S, Raja B. Effect of morin, a flavonoid against DOCA-salt hypertensive rats: A dose dependent study. Asian Pac J Trop Biomed. 2012;2:443–448.
- Mair J, Wagner I, Jakob G, et al. Different time courses of cardiac contractile proteins after acute myocardial infarction. Clin Chim Acta. 1994;231:47–60.