Abstract
Background The roles of antioxidant therapy on non-thyroidal illness syndrome (NTIS) in uremic rats is still unclear. Materials and methods Twenty-four Sprague-Dawley (SD) rats were randomly divided into blank, 5/6 nephrectomy (Nx), pyrrolidine dithiocarbamate (PDTC, 10 mg/100 g), sodium bicarbonate (SB, 0.1 g/100 g), N-acetylcysteine (NAC, 80 mg/100 g) and thyroid hormones (TH, levothyroxine 2 μg/100 g) groups. The serum levels of malondialdehyde (MDA), superoxide dismutase (SOD), advanced oxidation protein products (AOPP), interleukin (IL)-1β, free triiodothyronine (FT3), and thyroid stimulating hormone (TSH) were detected in the sixth week. The expressions of IL-1β and deiodinase type 1 (DIO1) were assessed by western blotting. The nuclear factor kappa B (NF-κB) inflammatory signal pathway was confirmed by electrophoretic mobility shift assay (EMSA). Results Compared with 5/6 Nx group, PDTC and NAC significantly reduced the levels (p < 0.01, respectively) of serum MDA, AOPP, TSH, and elevated levels of serum SOD (p < 0.01, respectively) and FT3 (p = 0.016 and p < 0.01). Neither had significant effects on serum IL-1β content (p = 0.612 and p = 0.582). PDTC and NAC markedly decreased the protein expression of IL-1β (p < 0.01) and increased the protein expression of DIO1 (p < 0.01), respectively. Both had been considerably blunted NF-κB activity (p < 0.01). Conclusions In uremic rat model, PDTC and NAC can effectively improve oxidative stress level and NTIS. In terms of improving oxidative stress level, NAC is probably superior to PDTC.
Introduction
Non-thyroidal illness syndrome (NTIS), known as low triiodothyronine (T3) syndrome, refers to low serum T3 and raised reverse T3 (rT3) level. Moreover, serum thyroxine (T4) level and thyroid stimulating hormone (TSH) level are normal or reduced in NTIS. Low serum free T3 (FT3) is primarily due to impaired extra-thyroidal conversion of T4 to active T3. Deiodinase type 1 (DIO1) is the crucial enzyme, which convert T4 to T3.Citation1 NTIS is widely observed in end-stage renal disease (ESRD) patients. A recent study demonstrated that the accumulated uremic toxins inhibited the enzyme activity of DIO1.Citation2
The progression of chronic kidney disease (CKD) is closely linked to oxidative stressCitation3,Citation4 and chronic inflammation.Citation5 By activating nuclear factor kappa B (NF-κB) signaling pathway, stimulating the release of pro-inflammatory cytokines and inducing expression of monocyte chemoattractant protein,Citation6 oxidative stress promotes inflammation in ESRD. In addition, inflammatory cytokines could trigger the expression of the nicotinamide adenine dinucleotide phosphate oxidase, and intensify the oxidative stress subsequently,Citation7 thus setting up a vicious cycle. Carrero et al.Citation8 have found that a reduction in serum T3 was positively correlated with inflammation, malnutrition, and endothelial cell activation. Available data showed that T3 could reduce oxidative stress and weaken the damage mediated by reactive oxidative species (ROS).Citation9
Antioxidant treatment is regarded as a potential effective measure to attenuate the oxidative stress. Pyrrolidine dithiocarbamate (PDTC), a metal chelator and antioxidant, is a specific inhibitor of NF-κB. By inhibiting the activation of NF-κB signal pathways, PDTC thereby reduces the release of inflammatory cytokines.Citation10 In non-immune proteinuria rats, PDTC can markedly decrease inflammation and tubulointerstitial injury.Citation11 N-acetylcysteine (NAC) is a thiol-containing antioxidant, which increased intracellular glutathione (GSH) level. Added intracellular GSH level improves the ability of antioxidant. Araki et al.Citation12 have found that NAC inhibited NF-κB activation and decreased serum level of interleukin-6 (IL-6). In uremic rats, NAC attenuated the oxidative stress and the inflammatory reactions, finally reduced renal tissue damage.Citation13
Several studies showed that PDTC and NAC attenuated oxidative stress in 5/6 Nx rats.Citation13,Citation14 However, there have been no studies about the effects of antioxidant therapy on NTIS in ESRD rats. Therefore, to observe the effects of PDTC and NAC on rats with 5/6 Nx model in the present study, we detected the levels of serum malondialdehyde (MDA), superoxide dismutase (SOD), advanced oxidation protein products (AOPP), leukocytes interleukin-1β (IL-1β), FT3, and TSH. Furthermore, we investigated the effects of PDTC and NAC on the expression of IL-1β and DIO1, and their impacts on NF-κB signal pathways.
Materials and methods
Animals
Experiments were conducted on 7-week-old male and female Sprague-Dawley (SD) rats, weight ranging from 250 to 300 g. Animals were purchased from Shanghai Experimental Animal Center of Chinese Academy of Sciences (Shanghai, China). The rats were housed in a barrier facility at a temperature of 22–24 °C and a relative humidity of 45–65%. Artificial indoor lighting was provided by alternating 12 h light and 12 h dark with a luminance of 200 to 300 lx. Indoor ventilation frequency was 20 times/h, the ammonia concentration was below 14 mg/m3. The rats were fed with 60Co-irradiated pellet feeds and sterile drinking water. The study was approved by the ethics committee of the Second Affiliated Hospital to Nanchang University.
Study groups
These SD rats were equally randomly divided into six groups with equal number of males and females. Blood was collected from the tail vein for detecting preoperative serum creatinine level. 5/6 nephrectomy (Nx) were conducted on rats as previously described.Citation15 Five groups were randomly selected for 5/6 Nx with surgical resections by performing a right Nx and two-thirds of the left kidney, and observed for four weeks under stable feeding. Blood was collected from the tail vein at the end of the fourth week after operation. The uremic rat models were successfully established if postoperative serum creatinine level was two times higher than the preoperative.Citation16 The remaining group did not undergo 5/6 Nx was settled as Blank group. The 5/6 Nx rats were grouped and intervened respectively as follows: 5/6 Nx group, PDTC group [PDTC (Sigma-Aldrich, St. Louis, MO) were intraperitoneally injected at 10 mg/100 g, once daily], sodium bicarbonate (SB) group (SB was intragastric administrated at 0.1 g/100 g, once daily), NAC group [NAC effervescent tablet (Sigma-Aldrich, St. Louis, MO) was intragastrically administrated at 80 mg/100g, once daily], thyroxin (TH) group (levothyroxine was intragastrically administrated at 2 μg/100 g, once daily). Animals were sacrificed after successful intervention, blood and liver were sampled. Serum samples were stored at −80 °C, liver samples were frozen in liquid nitrogen and transferred to dry ice for shipping. The level of MDA, SOD, AOPP, IL-1β, FT3, and TSH were detected. The protein expression of IL-1β and DIO1 was assessed by western blotting. NF-κB inflammatory signal pathways were confirmed by electrophoretic mobility shift assay (EMSA).
Blood sampling
Routine biochemical parameters were assayed in automated analyzer using commercial kits. MDA and SOD were determined according to the instructions on the kit (Sigma-Aldrich, St. Louis, MO). AOPP, IL-1β, FT3, and TSH production was measured in serum by using rat AOPP ELISA kit (CUSABIO, Barksdale, LA), rat IL-1β ELISA kit (R&D Systems, Wiesbaden, Germany), rat FT3 ELISA kit (CUSABIO, Barksdale, LA), and rat TSH ELISA kit (CUSABIO, Barksdale, LA), respectively. All samples were measured in triplicate.
Western blotting
In order to assess the effects of PDTC and NAC on uremic rats, the protein expressions of IL-1β and DIO1 were determined by western blot. Nuclear and cytosolic extracts were prepared using a Nuclear and Cytoplasmic Protein Extraction Kit (Pierce Biotechnology, Rockford, IL) according to the manufacturer’s instructions. Protein concentrations were determined by using a bicinchoninic acid protein assay kit (Pierce Biotechnology, Rockford, IL). Protein extractions of 50 μg were loaded onto 12% sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to polyvinylidene difluoride membranes. In short, the membrane was blocked with 5% skimmed milk and incubated with primary rabbit antibody against IL-1β (Santa Cruz Biotechnology, Dallas, TX), DIO1 (Santa Cruz Biotechnology, Dallas, TX) overnight at 4 °C. After washing with Tris-buffered saline with 0.1% Tween (TBS-T), the blots were incubated with horseradish peroxidase-labeled secondary antibody for 1 h. The signals were visualized by using enhanced chemical luminescence. Western analysis of quantification was performed with Image J (National Institutes of Health, Baltimore, MD).
EMSA
Nuclear protein was extracted and EMSA for the transcription factor NF-κB were carried out according to the manufacturer’s instructions. Protein concentrations were determined by the BioRad protein reagents (Hercules, CA). The NF-κB double-stranded consensus oligonucleotide sequence used was 5'-AGTTGAGGGGACTTTCCCAGGC-3'. The NF-κB oligonucleotide probe was end-labeled with Cy5.5-lectin. Unincorporated nucleotides were removed by passing the reaction mixture through a Sephadex G-25 spin column (Amersham-Pharmacia, Uppsala, Sweden). Briefly, binding reactions were added to 1 μl of binding buffer, 2 μl of labeled probe, 1 μl of poly-dIdC, 1 μl of poly-L-Lysine, and 5 μg nuclear extracts for 15 min. Then, 5 μl of loading buffer was added to each sample. DNA protein complexes were separated by electrophoresis through a 6% native polyacrylamide gel in a running buffer containing 1.5 M Tris, pH 8.8, 1 M Glycine and 0.5 M EDTA for 50 min at 90 V. Acquire image using normal image scanning methods for colorimetric detection. Quantification was performed with Image J (National Institutes of Health, Baltimore, MD).
Statistical analysis
Data were presented as the mean ± standard deviation (SD) of triplicate experiments, unless otherwise specified. Statistically significant differences in mean values were analyzed by one-way ANOVA followed by the Least Significant Difference (LSD) test. The data were analyzed with SPSS 19.0 software (SPSS Inc., Chicago, IL). A p-value below 0.05 was considered statistically significant.
Results
The serum levels of MDA, SOD, IL-1β, AOPP, T3, and TSH
No rat died in each group. The levels of MDA, SOD, IL-1β, AOPP, T3, and TSH of each group are summarized in . PDTC showed benefit effects that reduced the levels (p < 0.01) of MDA, AOPP, and TSH (), increased the levels of SOD (p < 0.01) and T3 (p = 0.016) (), but had no significant influence on the levels of IL-1β (p = 0.612) compared with the 5/6 Nx group (). NAC administration significantly reduced the contents (p < 0.01) of MDA, AOPP, and TSH, increased the contents (p < 0.01) of SOD and T3, but had no significant effect on the contents of IL-1β (p = 0.582) versus 5/6 Nx group. SB treatment notably decreased the levels of MDA (p = 0.018), AOPP (p < 0.01), and TSH (p = 0.038), ascended the contents of SOD (p = 0.042) and T3 (p = 0.012) compared with the 5/6 Nx group, but that of IL-1β level was no statistically different (p = 0.127). TH treatment notably reduced the levels of MDA (p < 0.01), AOPP (p = 0.021), and TSH (p < 0.01), added levels of SOD (p < 0.01) and T3 (p = 0.026) compared with the 5/6 Nx group. There was no statistical difference in IL-1β content between TH group and 5/6 Nx group (p = 0.168).
Figure 1. The levels of serum MDA (A), SOD (B), AOPP (C), IL-1β (D), FT3 (E), and TSH (F) in each group. Data are given as mean ± SD of four animals per group. #p < 0.05 compared with Blank group. *p < 0.05 compared with 5/6 Nx group.
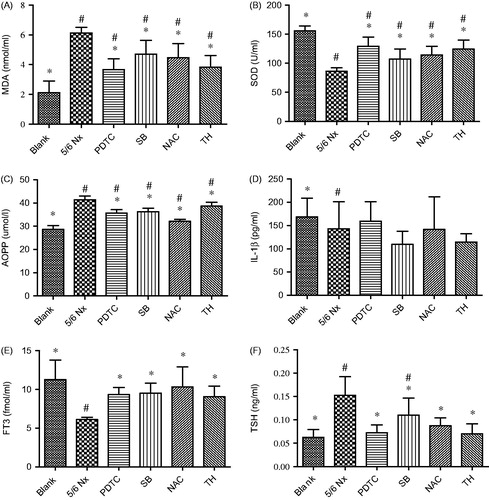
Table 1. The levels of serum MDA, SOD, AOPP, IL-1β, FT3, and TSH in each group.
From the results, we learned that PDTC, SB, NAC, and TH significantly reduced the levels of serum MDA, AOPP, and TSH, elevated the levels of serum SOD and FT3 compared with 5/6 Nx group. However, the level of IL-1β had no significant difference between all drug intervention groups and 5/6 Nx group.
Although both PDTC group and SB group had the trend of increased level of SOD, there was statistically significant difference between the two groups (p = 0.033). This result showed that in terms of elevated serum SOD level, the effect of PDTC is superior to SB. At the level of AOPP, there was a significant statistical difference between NAC and PDTC (p < 0.01), NAC and SB (p < 0.01), NAC and TH (p < 0.01), PDTC and TH (p < 0.01), respectively. PDTC group and SB group had no statistical difference (p = 0.543). All of them could decrease the contents of AOPP effectively. This result illustrated that regarding to reduce the contents of AOPP, NAC was the best, followed by PDTC and SB, with TH the worst.
Western blotting analysis of IL-1β and DIO1 expressions
Western blot demonstrated that the IL-1β protein expression was significantly inhibited by approximately 39% (p < 0.01) under the NAC treatment, approximately 43% (p < 0.01) under the SB treatment, approximately 10% (p < 0.01) under PDTC treatment, and approximately 41% (p < 0.01) under the TH treatment, compared with 5/6 Nx group (). However, the expression of IL-1β under NAC, SB, and TH treatment made a great statistical difference (p < 0.01) versus PDTC treatment.
Figure 2. Effects of PDTC, SB, NAC, and TH treatments on uremic rats by western blot. Normalized densitometric data of IL-1β (A) and DIO1 (B) bands obtained from the protein extractions. RR stands for relative ratio. Values are expressed as fold changes relative to the appropriate controls. Values are given as mean ± SD of four animals per group. #p < 0.05 compared with Blank group. *p < 0.05 compared with 5/6 Nx group.
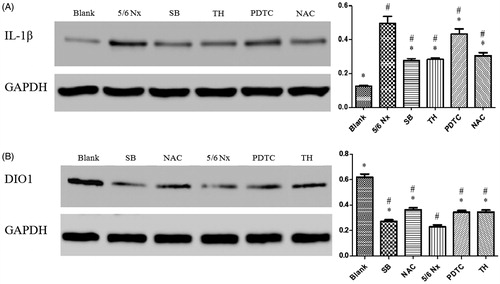
The DIO1 protein expression in 5/6 Nx group was reduced significantly (p < 0.01) compared with Blank group (). SB, NAC, PDTC, and TH treatment was significantly up-regulated DIO1 expression, approximately 8, 47, 37, and 39% (p < 0.01) compared with 5/6 Nx group, respectively. The western blot of DIO1 expressions suggested that the SB group had a dramatical difference between the NAC, PDTC, and TH group (p < 0.01). As indicated in , PDTC, SB, NAC, and TH had the prominent effects on ascending the serum FT3 levels. Owing to the elevated DIO1 protein expression induced by PDTC, SB, NAC, and TH treatments, it was demonstrated that the DIO1 function could be improved by antioxidant and TH therapy.
NF-κB signal pathway by EMSA
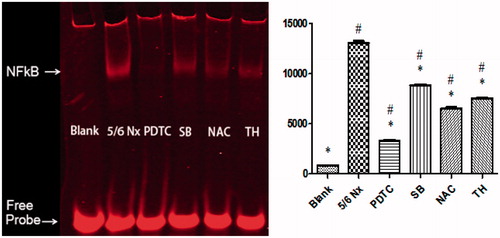
Accumulated uremic toxins in 5/6 Nx group activated the NF-κB signal pathway (p < 0.01) in contrast with Blank group (). The NF-κB signal was prominently reduced by approximate 75, 33, 50, and 42% after PDTC, SB, NAC, and TH treatments, compared with 5/6 Nx group (p < 0.01). There was an obvious inactivation of NF-κB while improving oxidative stress and/or serum T3 level was elevated.
Figure 3. Effects of PDTC, SB, NAC, and TH treatments on uremic rats by EMSA. Normalized densitometric data of NF-κB band obtained from the extracts of proteins with unclear interaction. Data are given as mean ± SD of 4 animals per group. #p < 0.05 compared with Blank group. *p < 0.05 compared with 5/6 Nx group.
Discussion
Several studies showed that PDTC and NAC attenuated oxidative stress in 5/6 Nx rats.Citation13,Citation14,Citation17–19 At present, there is no study that explores the effects of PDTC and NAC on NTIS in uremic rats. Firstly, the present study showed the impact of PDTC and NAC on NTIS in 5/6 Nx rats.
Our study showed that in terms of increasing the serum SOD level, PDTC was superior to SB (). We speculated that via inhibit NF-κB activity, PDTC ameliorated inflammation and oxidative stress, scavenged oxygen-free radicals. SOD is the antioxidant enzyme and prime substance to remove free radicals. PDTC could directly reduce the consumption of SOD. SB probably through correcting metabolic acidosis, then indirectly decreased the oxidative stress level.Citation17 The present study found in the field of lowering AOPP content, NAC was superior to PDTC and SB, PDTC and SB was superior to TH (). AOPP is the product of plasma albumin and chlorine oxide, and is regarded as a novel marker of protein oxidative damage. The antioxidant NAC properties, bases on its ability to prevent GSH depletion exposed to uremic serumCitation18 and inhibit the pro-inflammatory transcription factor activators protein-1 (AP-1) and NF-κB.Citation19 So, in terms of reducing the concentration of AOPP, the effect of NAC was the best among the four drugs. Several studies showed that TH could reduce the levels of ROS and attenuate the systemic oxidative stress.Citation20–22 TH therapy appears to protect against lipid peroxidation.Citation23 and enhance mitochondrial aerobic capacity.Citation24 Thus, indirectly improve oxidative stress level in uremic rats.
Inflammatory cytokines, such as IL-1β, IL-6, and tumor necrosis factor (TNF)-α were higher with lower levels of glomerular filtration rate.Citation25 The results showed IL-1β protein expression was markedly reduced under SB, TH, PTDC, and NAC administration, separately (). And the expression of IL-1β under NAC, SB, and TH treatment made a great difference compared with PDTC treatment. ROS may serve as signal transduction messengers for several important transcription factors, such as NF-κB and AP-1.Citation26 PDTC is a specific NF-κB depressor, but that it has no influence on AP-1, cAMP response element-binding protein (CREB) or specificity protein (Sp-1).Citation27,Citation28 However, IL-1 was not only regulated by NF-κB but also by both activator protein AP-1Citation29 and CREB. Therefore, the specific NF-κB inhibitor, PDTC had little influence on IL-1β protein expression. Moreover, as indicated in , each of the four interventions had very little effect on the serum level of IL-1β. The possible reason is that these treatments had a relatively prominent effect on the transcription level, but had little effect on the post-translational level of cytokine production.
SB, NAC, PDTC, and TH treatments were significantly up-regulated DIO1 expressions (). SB had been demonstrated to improve nutritional status and slow the rate of progression of renal failure to ESRD.Citation30 A prospective randomized study found that SB could improve thyroid function by correcting metabolic acidosis.Citation31 However, further study regarding the mechanism will be required. All of them exert direct or indirect anti-oxidative effects on promoting the expression of DIO1 protein in the ESRD.
NF-κB DNA binding activity was measured by EMSA (). The results presented that PDTC and NAC significantly inhibited NF-κB signal pathways, nearly 75 and 50%, respectively. Combined with the effects of PDTC and NAC on oxidative stress parameters, which further verified NF-κB plays a crucial role in the process of oxidative stress.
According to the effects of the four drugs intervened in uremic rats, the present study showed that PDTC and NAC prominently improved oxidative stress level and NTIS. There are very limited studies about clinical application of PDTC. A high intravenous dose of PDTC was found to induce acute toxicity in mice and rats, which were mainly affected autonomous and central nervous system.Citation32 NAC is a safe drug without any significant adverse effect.Citation33 NAC was demonstrated safe and well-tolerated in hemodialysis patients.Citation34 In the present study, PDTC and NAC could availably improve oxidative stress level and NTIS. NAC is probably better than PDTC regarding the improved oxidative stress level and relatively safe. Based on the above reasons, prefer to recommend clinical application of NAC.
There are some limitations of the present study. Firstly, the number of animal models is relatively small. Secondly, there are three types of deiodinases. The protein expression of DIO2 and/or DIO3 could be investigated. Thirdly, pathological changes in rat kidney under the four drugs administration should be further investigated.
Conclusions
In CRF model, PDTC and NAC can effectively improve oxidative stress level and non-thyroidal illness syndrome (NTIS). In terms of improving oxidative stress level, NAC is probably superior to PDTC.
Funding information
This research was supported by the National Natural Science Foundation of China (No. 81360122/H0518).
Disclosure statement
No conflict of interests is declared.
References
- Chopra IJ. Clinical review 86: Euthyroid sick syndrome: Is it a misnomer? J Clin Endocrinol Metab. 1997;82:329–334.
- Xu G, Tu W, Qin S. The relationship between deiodinase activity and inflammatory responses under the stimulation of uremic toxins. J Transl Med. 2014;12:239.
- Dounousi E, Papavasiliou E, Makedou A, et al. Oxidative stress is progressively enhanced with advancing stages of CKD. Am J Kidney Dis. 2006;48:752–760.
- Xu G, Yan W, Li J. An update for the controversies and hypotheses of regulating nonthyroidal illness syndrome in chronic kidney diseases. Clin Exp Nephrol. 2014;18:837–843.
- Stenvinkel P. New insights on inflammation in chronic kidney disease-genetic and non-genetic factors. Nephrol Ther. 2006;2:111–119.
- Stenvinkel P, Ketteler M, Johnson RJ, et al. IL-10, IL-6, and TNF-alpha: Central factors in the altered cytokine network of uremia - the good, the bad, and the ugly. Kidney Int. 2005;67:1216–1233.
- Sela S, Shurtz-Swirski R, Cohen-Mazor M, et al. Primed peripheral polymorphonuclear leukocyte: A culprit underlying chronic low-grade inflammation and systemic oxidative stress in chronic kidney disease. J Am Soc Nephrol. 2005;16:2431–2438.
- Carrero JJ, Qureshi AR, Axelsson J, et al. Clinical and biochemical implications of low thyroid hormone levels (total and free forms) in euthyroid patients with chronic kidney disease. J Intern Med. 2007;262:690–701.
- Menzies KJ, Robinson BH, Hood DA. Effect of thyroid hormone on mitochondrial properties and oxidative stress in cells from patients with mtDNA defects. Am J Physiol Cell Physiol. 2009;296:C355–C362.
- Yao YM, Xu CL, Yao FH, Yu Y, Sheng ZY. [The pattern of nuclear factor-kappa B activation in rats with endotoxin shock and its role in biopterin-mediated nitric oxide induction]. Zhonghua Shao Shang Za Zhi. 2006;22:405–410.
- Nobel CI, Kimland M, Lind B, Orrenius S, Slater AF. Dithiocarbamates induce apoptosis in thymocytes by raising the intracellular level of redox-active copper. J Biol Chem. 1995;270:26202–26208.
- Araki S, Dobashi K, Kubo K, Kawagoe R, Yamamoto Y, Shirahata A. N-acetylcysteine inhibits induction of nitric oxide synthase in 3T3-L1 adipocytes. J UOEH. 2007;29:417–429.
- Shimizu MH, Coimbra TM, de Araujo M, Menezes LF, Seguro AC. N-acetylcysteine attenuates the progression of chronic renal failure. Kidney Int. 2005;68:2208–2217.
- Fujihara CK, Antunes GR, Mattar AL, Malheiros DM, Vieira JJ, Zatz R. Chronic inhibition of nuclear factor-kappa B attenuates renal injury in the 5/6 renal ablation model. Am J Physiol Renal Physiol. 2007;292:F92–F99.
- Fujihara CK, De Nucci G, Zatz R. Chronic nitric oxide synthase inhibition aggravates glomerular injury in rats with subtotal nephrectomy. J Am Soc Nephrol. 1995;5:1498–1507.
- Zareie M, De Vriese AS, Hekking LH, et al. Immunopathological changes in a uraemic rat model for peritoneal dialysis. Nephrol Dial Transplant. 2005;20:1350–1361.
- Souma T, Abe M, Moriguchi T, et al. Luminal alkalinization attenuates proteinuria-induced oxidative damage in proximal tubular cells. J Am Soc Nephrol. 2011;22:635–648.
- Rushworth GF, Megson IL. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol Ther. 2014;141:150–159.
- Dodd S, Dean O, Copolov DL, Malhi GS, Berk M. N-acetylcysteine for antioxidant therapy: Pharmacology and clinical utility. Expert Opin Biol Ther. 2008;8:1955–1962.
- Forini F, Lionetti V, Ardehali H, et al. Early long-term L-T3 replacement rescues mitochondria and prevents ischemic cardiac remodelling in rats. J Cell Mol Med. 2011;15:514–524.
- Pantos C, Mourouzis I, Markakis K, et al. Thyroid hormone attenuates cardiac remodeling and improves hemodynamics early after acute myocardial infarction in rats. Eur J Cardiothorac Surg. 2007;32:333–339.
- Pantos C, Mourouzis I, Markakis K, Tsagoulis N, Panagiotou M, Cokkinos DV. Long-term thyroid hormone administration reshapes left ventricular chamber and improves cardiac function after myocardial infarction in rats. Basic Res Cardiol. 2008;103:308–318.
- De Castro AL, Tavares AV, Campos C, et al. Cardioprotective effects of thyroid hormones in a rat model of myocardial infarction are associated with oxidative stress reduction. Mol Cell Endocrinol. 2014;391:22–29.
- Venditti P, Bari A, Di Stefano L, Di Meo S. Tri-iodothyronine treatment differently affects liver metabolic response and oxidative stress in sedentary and trained rats. J Endocrinol. 2008;197:65–74.
- Gupta J, Mitra N, Kanetsky PA, et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol. 2012;7:1938–1946.
- Scheidegger KJ, Du J, Delafontaine P. Distinct and common pathways in the regulation of insulin-like growth factor-1 receptor gene expression by angiotensin II and basic fibroblast growth factor. J Biol Chem. 1999;274:3522–3530.
- Liu SF, Ye X, Malik AB. Inhibition of NF-kappaB activation by pyrrolidine dithiocarbamate prevents in vivo expression of proinflammatory genes. Circulation. 1999;100:1330–1337.
- Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992;175:1181–1194.
- Su YW, Chiou WF, Chao SH, Lee MH, Chen CC, Tsai YC. Ligustilide prevents LPS-induced iNOS expression in RAW 264.7 macrophages by preventing ROS production and down-regulating the MAPK, NF-kappaB and AP-1 signaling pathways. Int Immunopharmacol. 2011;11:1166–1172.
- De Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009;20:2075–2084.
- Disthabanchong S, Treeruttanawanich A. Oral sodium bicarbonate improves thyroid function in predialysis chronic kidney disease. Am J Nephrol. 2010;32:549–556.
- Chabicovsky M, Prieschl-Grassauer E, Seipelt J, et al. Pre-clinical safety evaluation of pyrrolidine dithiocarbamate. Basic Clin Pharmacol Toxicol. 2010;107:758–767.
- Sahraei Z, Salamzadeh J, Nafar M. Effect of N-acetyl cysteine and vitamin C on kidney allograft function biomarkers interleukin-18 and neutrophil gelatinase-associated lipocalin. Iran J Kidney Dis. 2015;9:56–62.
- Friedman AN, Bostom AG, Laliberty P, Selhub J, Shemin D. The effect of N-acetylcysteine on plasma total homocysteine levels in hemodialysis: A randomized, controlled study. Am J Kidney Dis. 2003;41:442–446.

