Abstract
This randomized, placebo-controlled, crossover trial assessed the lipid-altering efficacy of a dietary supplement (tablet form) providing 1.8 g/day free (non-esterified) plant sterols and stanols versus placebo for 6 weeks as part of a therapeutic lifestyle changes (TLC) diet in 32 men and women with primary hypercholesterolaemia. Mean ± SE baseline (end of a 5-week TLC diet lead-in) lipid concentrations (mmol/l) were total cholesterol (TC), 5.88 ± 0.08; non-high-density lipoprotein cholesterol (non-HDL-C), 4.71 ± 0.09; low-density lipoprotein cholesterol (LDL-C), 4.02 ± 0.08; HDL-C, 1.17 ± 0.06 and triglycerides (TGs), 1.51 ± 0.12. Differences from control in responses (plant sterol/stanol − control) were significant (p < 0.05) for LDL-C ( − 4.9%), non-HDL-C ( − 3.6%) and TC ( − 2.8%). HDL-C and TG responses were not significantly different between treatment conditions. These results indicate that 1.8 g/day free plant sterols/stanols administered in a tablet produced favourable lipoprotein lipid changes in men and women with hypercholesterolaemia.
Introduction
The guidelines of the National Cholesterol Education Program (NCEP) recommend incorporation of 2 g/day of plant sterols or stanols (phytosterols or phytostanols) into the therapeutic lifestyle changes (TLC) diet as a dietary adjunct for individuals who do not achieve their low-density lipoprotein cholesterol (LDL-C) treatment targets with diet alone (NCEP Expert Panel Citation2002). A large body of evidence supports the efficacy of plant sterol/stanol-enriched products providing 1.0–3.0 g/day plant sterols/stanols for lowering LDL-C, non-high-density lipoprotein (HDL)-C and total cholesterol (TC) concentrations (Food and Drug Administration Citation2000; NCEP Expert Panel Citation2002; Maki et al. Citation2003; Demonty et al. Citation2009; Sánchez-Muniz et al. Citation2009). Results from studies in subjects with hypercholesterolaemia have generally indicated that HDL-C and triglyceride (TG) concentrations are unchanged by consumption of plant sterols or stanols, although some evidence suggests that plant stanols (and presumably sterols) may lower the TG concentration in subjects with hypertriglyceridemia (Plat et al. Citation2009).
Because of their structural similarity to cholesterol, plant sterols and stanols compete with cholesterol for incorporation into micelles, as well as for transport across the brush border by the Nieman Pick C1–Like 1 transporter (von Bergmann et al. Citation2005; Hui et al. Citation2008; Jones Citation2008). In addition, the accumulation of plant sterols or stanols in the enterocyte appears to trigger the production of adenosine triphosphate binding cassette transporters G5 and G8, which function to transport sterols, including cholesterol, out of the enterocyte, into the intestinal lumen (von Bergmann et al. Citation2005; Hui et al. Citation2008; Jones Citation2008). The net result of these mechanisms is to reduce intestinal cholesterol absorption (Ikeda and Sugano Citation1998; Jones Citation2008). Consequently, hepatic cholesterol content is reduced, inducing an up-regulation of LDL receptor expression, thereby enhancing the removal of LDL and other apolipoprotein B-containing lipoprotein particles from the circulation (Jones Citation2008).
Various commercially available products contain plant sterols and/or stanols in free and/or esterified forms including margarine-type spreads, yogurt and yogurt-based drinks, orange juice and dietary supplements in capsule or tablet form. Most clinical trials examining the effects of plant sterols/stanols on lipid concentrations have administered food forms (Ostlund et al. Citation1999; Maki et al. Citation2001, Citation2003; Nestel et al. Citation2001; Volpe et al. Citation2001; de Graaf et al. Citation2002; Gremaud et al. Citation2002; Pouteau et al. Citation2003; Berger et al. Citation2004; Thomsen et al. Citation2004; Doornbos et al. Citation2006; Abumweis et al. Citation2008), while fewer have investigated the effects of tablet and capsule forms (McPherson et al. Citation2005; Goldberg et al. Citation2006; Acuff et al. Citation2007; Carr et al. Citation2009). The use of plant sterol/stanol capsules or tablets offers a practical option compared with traditional food applications because it provides a vehicle that can be easily incorporated into a cholesterol-lowering regimen without impacting dietary macronutrient distribution.
Recently, the US Food and Drug Administration reviewed the published data on phytosterols and risk for coronary heart disease (CHD; Food and Drug Administration Citation2010). In their review they concluded that ‘the available scientific evidence for the cholesterol-lowering effects of phytosterols in dietary supplements shows that formulation of the supplement product is an important factor in the effectiveness of the product’. They further concluded that ‘the totality of available scientific evidence for the cholesterol-lowering effects of nonesterified phytosterols in dietary supplements is inconsistent’. The agency requested submission of additional data to demonstrate the cholesterol-lowering efficacy of non-esterified phytosterols consumed as ingredients in dietary supplements. This trial was undertaken to assess the efficacy of a dietary supplement in a tablet providing a total of 1.8 g/day plant sterol/stanol in a non-esterified form, as part of a NCEP TLC diet, for improving the lipoprotein lipid profile in men and women with primary hypercholesterolaemia. Similar doses of non-esterified plant sterol/stanol administered in food products have been shown to significantly lower LDL-C levels (Jones et al. Citation1999; de Graaf et al. Citation2002). Daily plant sterol intake in the US diet is ∼200 mg (range 150–450 mg in various Western populations; Ostlund Citation2002), which is insufficient to materially alter cholesterol levels. Accordingly, the dosage of 1.8 g was chosen with the expectation that total plant sterol/stanol intake in study subjects would be ∼2.0 g/day from a combination of diet and the supplement provided. A dose of 1.7 g/day non-esterified sitostanol-containing phytosterols administered in margarine for 30 days resulted in a 15.5% LDL-C reduction (Jones et al. Citation1999), and 1.8 g/d administered in chocolate for 4 weeks decreased LDL-C by 10.3% (de Graaf et al. Citation2002).
Methods
Study design
This was a randomized crossover study consisting of a 5-week diet plus single-blind placebo lead-in, followed by two double-blind 6-week treatment periods during which subjects received either dietary supplement tablets (four tablets daily, two tablets with each of two meals) providing 1.8 g/day non-esterified plant sterols/stanols or the same number of matching placebo tablets. The study was conducted at two clinical research centres (Provident Clinical Research in Addison, IL, USA, and Bloomington, IN, USA) according to Good Clinical Practice Guidelines, the Declaration of Helsinki and the US 21 Code of Federal Regulations. Informed consent for the study was obtained from all subjects before protocol-specific procedures were carried out and subjects were informed of their rights to withdraw from the study at any time.
Subjects
Men and women of age 21–79 years, inclusive, each with a fasting LDL-C level ≥ 3.4 and < 5.7 mmol/l, and in good general health on the basis of medical history and routine laboratory tests were eligible for the study. Individuals were excluded from participation if they had a body mass index of >42.0 kg/m2, fasting blood glucose ≥ 7.0 mmol/l or diabetes mellitus, resting blood pressure ≥ 160 mm Hg systolic and/or ≥ 100 mm Hg diastolic, or CHD or a CHD risk equivalent as defined by the NCEP Adult Treatment Panel III (NCEP Expert Panel Citation2002). Additional exclusion criteria included a history of extreme dietary habits, eating disorders, alcoholism, cancer or any clinically important cardiovascular disorders. Use of any medications, dietary supplements or fortified foods with lipid-altering effects, including sterol and stanol products, were excluded for at least 4 weeks prior to study entry as was the use of weight-loss drugs or programmes and recent body weight change >4.5 kg.
Study products and diet instruction
Following the diet and the single-blind placebo lead-in, subjects were randomly assigned to receive, in a double-blind manner, either active (non-esterified sterol/stanol tablets, 0.45 g per tablet) or control (matched placebo tablets, the main ingredient of which was lactose) study product for 6 weeks (treatment period I) and then cross over to receive the opposite product for 6 weeks (treatment period II). Subjects were instructed to swallow, with water or another beverage, four active or control tablets daily (two with each of two meals) at consistent times each day. Compliance with active and control tablets was assessed by counting unused study product returned to the clinic. Subjects were instructed to maintain their usual physical activity patterns throughout the trial.
Beginning at week − 5 and throughout the study, subjects were counselled to follow the weight maintenance version of the TLC diet recommended by the NCEP, and handouts were provided to reinforce the diet instructions (NCEP Expert Panel Citation2002). To evaluate compliance with the TLC diet, diet records were completed on 3 consecutive days (2 weekdays and 1 weekend day) at baseline and the end of each treatment period using standardized forms that included instructions for obtaining complete information such as the use of condiments, brand names and cooking methods. Daily intakes of energy and key nutrients were calculated from these records utilizing Food Processor SQL Software (version 10.4, EHSA Research, Salem, OR, USA). Body weight was measured at each clinic visit throughout the trial.
Laboratory measurements
Fasting (9–15 h) plasma lipid profile measurements from blood samples collected in duplicate at baseline (weeks − 1 and 0) and at the end of each treatment period (weeks 5 and 6, 11 and 12) were conducted by the EMH Reference Laboratory (Elmhurst, IL, USA) according to the Standardization Program of the Centers for Disease Control and Prevention and the National Heart, Lung, and Blood Institute. Lipoprotein lipid assessments included TC, non-HDL-C (calculated as TC–HDL-C), LDL-C, HDL-C and TG in mg/dl and converted to mmol/l with conversion factors of 0.02586 for cholesterol and 0.01129 for TG. LDL-C concentration was calculated using Friedewald's (Citation1972) equation as follows: LDL-C = TC − HDL-C − TG/5. Because this equation is not valid when the TG concentration is above 4.51 mmol/l, LDL-C values were not calculated in the few instances when subjects had values in this range.
Statistical analyses
Statistical analyses were completed in SAS version 9.2 (SAS Institute, Cary, NC, USA). Efficacy analyses were performed on the sample that included all subjects who were randomized and provided at least one post-randomization fasting lipid profile during each treatment condition. The sample size for this study was selected for 90% power (5% α-level) to detect a 7% difference between treatments in the change from baseline LDL-C concentration. Baseline characteristics for subjects in the two treatment sequences were compared using analysis of variance (continuous variables) or Fisher's exact test (categorical variables). Repeated measures analysis of covariance (using SAS PROC MIXED) was used to compare lipid, dietary intake and vital signs response variables (changes or percent changes from baseline) for the two treatment conditions (active and control) using the baseline value as a covariate. The initial model included subject as a random effect and terms for treatment condition, sequence and treatment by sequence interaction. If the treatment by sequence interaction term for a variable was not statistically significant (p>0.05), it was dropped from the final model.
Examination of responses by sequence suggested that no material differences were present that would bring into question the appropriateness of pooling data from the two sequence groups. Residuals from the final model were examined to assess normality, and if clear evidence of non-normality was present, rank transformations were employed in the final models. An additional analysis of covariance was conducted using the results of Kris-Etherton and Yu's (Citation1997) predictive equation for LDL-C changes with response to dietary alteration with and without an adjustment for dietary cholesterol intake based on Hegsted et al.'s (Citation1993) equation according to diet record results. This was done in order to assess possible confounding by dietary changes during the treatment periods. Safety was assessed by the evaluation of treatment emergent adverse events and changes in vital signs measurements. McNemar's test was used for statistical comparisons between treatment conditions for categorical variables.
Results
Subjects and demographics
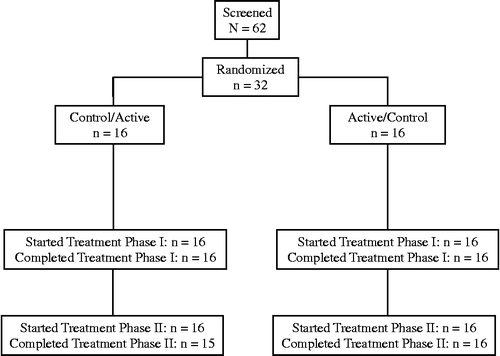
Sixty-two subjects were screened, 32 of whom entered and completed the first treatment period (). One subject withdrew consent after completing part of the second treatment period while receiving the active study product, but was retained in the efficacy analyses. Demographic and baseline characteristics of the subjects are presented in . Subjects were predominantly female (59%), of non-Hispanic White race/ethnicity (91%) and non-smokers (91%). Participants had a mean age of 57.6 years and a mean body mass index of 27.4 kg/m2. Compliance with active and control tablets was 98.0% and 98.4% of the expected tablets, respectively (p = 0.586).
Table I. Subject characteristics at baseline.*
Lipids
Fasting plasma lipids at baseline, percent changes from baseline for control and active treatments and differences in responses between conditions are shown in . Differences from control in responses (plant sterol/stanol − control) were significant (p < 0.05) for LDL-C ( − 4.9%), non-HDL-C ( − 3.6%) and TC ( − 2.8%). HDL-C and TG responses were not significantly different between treatment conditions.
Table II. Fasting lipids at baseline, percent changes from baseline and differences in responses between treatments.*,†
Diet
Total reported energy intake at baseline was 1634 kcal/day and increased by 142.5 kcal/day during the active condition compared with a decrease of 8.4 kcal/day during the control condition, a difference which approached statistical significance (p = 0.056). There were no significant differences in changes from baseline between active and control conditions, respectively, in percentages of intake from carbohydrate ( − 1.1% and 1.5%, p = 0.100), protein ( − 0.3% and 0.3%, p = 0.513), total fat (0.3% and − 1.7%, p = 0.128) and saturated fat (1.1% and 0.2%, p = 0.199); and intakes of dietary fibre (0.4 and 0.9 g/day, p = 0.645), soluble fibre (0.05 and − 0.01 g/d, p = 0.376) or cholesterol (30.8 and 29.5 mg/day, p = 0.762). Analysis of covariance to adjust for differences in energy intake or predicted LDL-C response using prediction equations (as described in ‘Statistical analysis’ section) did not materially alter the estimates of the treatment effects (data not shown).
Vital signs, body weight and safety
There were no statistically significant or clinically relevant changes in vital signs, body weight or clinical laboratory values. Mean body weight changed < 0.5 kg and mean systolic and diastolic blood pressures changed ≤ 1 mm Hg throughout the study. Adverse events assessed by non-leading questions at each clinic visit were reported by eight (12.5%) of the subjects during the active period and seven (10.9%) of the subjects during the control period. The most common adverse events were related to the respiratory system (rhinitis and sinusitis). None of the adverse events were serious and all but one adverse event (moderate endometrial hyperplasia experienced by one subject when receiving the placebo) were reported to be mild. Increased appetite reported by one subject during the control period was considered by the study physician to be possibly related to study product. All other adverse events were classified by the study physicians as not related or unlikely to be related to the study products.
Discussion
The results of the present study indicate that 1.8 g/day unesterified plant sterols/stanols administered orally in tablets resulted in significant reductions in atherogenic lipoprotein lipids (LDL-C, non-HDL-C and TC) in individuals with hypercholesterolaemia. The LDL-C lowering of 4.9% was within the expected range, albeit at the lower end, based on meta-analyses (Katan et al. Citation2003; Abumweis et al. Citation2008; Demonty et al. Citation2009). According to calculations from a meta-analysis, weighted mean percentage reductions in LDL-C range from ∼5% at 1.0 g/day phytosterol to ∼11% at 3.0 g/day phytosterol with no appreciable increase in response at higher dosages up to 9.0 g/day (Demonty et al. Citation2009).
Although LDL-C has long been considered the principal lipoprotein determinant of atherosclerosis, results from observational studies and clinical trials of lipid-altering therapies have consistently shown that the non-HDL-C level is a better predictor of CHD event risk than LDL-C alone, irrespective of whether the TG concentration is elevated (Cui et al. Citation2001; Bittner Citation2003; Pischon et al. Citation2005; Ridker et al. Citation2005; Liu et al. Citation2006; Miller et al. Citation2008). Non-HDL-C was reduced by 3.6% in the present trial.
Most prior clinical trials have been conducted using plant sterols and stanols provided in foods (Ostlund et al. Citation1999; Maki et al. Citation2001, Citation2003; Nestel et al. Citation2001; Volpe et al. Citation2001; de Graaf et al. Citation2002; Gremaud et al. Citation2002; Pouteau et al. Citation2003; Berger et al. Citation2004; Thomsen et al. Citation2004; Doornbos et al. Citation2006; Abumweis et al. Citation2008). Relatively few studies have investigated plant sterols/stanols in tablet or capsule forms, but the available results support the ability of plant sterols and stanols to reduce LDL-C concentrations in some supplement forms (McPherson et al. Citation2005; Goldberg et al. Citation2006; Acuff et al. Citation2007; Carr et al. Citation2009). Goldberg et al. (Citation2006) demonstrated that tablets providing 1.8 g/day soy stanols in addition to the subjects' usual statin therapy, reduced LDL-C by 9.1%. McPherson et al. (Citation2005) reported a reduction in LDL-C of 10.4% after administration of stanol lecithin tablets providing 1.26 g stanols/day. The use of plant sterols and stanols in capsules or tablets offers a practical option compared to traditional phytosterol-containing food vehicles by virtue of being easily incorporated into a cholesterol-lowering regimen without altering the macronutrient distribution. This feature might increase compliance during long-term use compared with the types of dietary changes needed to incorporate phytosterol-containing foods (Berger et al. Citation2004).
Because of the hypothesized mechanisms of action: (1) competing with cholesterol for incorporation into micelles and (2) up-regulation of the production of sterol transport proteins from the enterocyte into the intestinal lumen, it is recommended that sterol or stanol products be consumed with meals to ensure adequate availability of bile acids for micelle formation (Berger et al. Citation2004). In the present study, all subjects were advised to take the tablets twice daily with meals. It was believed at one time that plant sterols would be more effective when consumed with diets containing higher levels of cholesterol or fat (Mussner et al. Citation2002; Berger et al. Citation2004). Since only 10–20% of cholesterol passing through the intestine daily is of dietary origin, sterols and stanols appear to be equally effective when consumed with low-fat diets such as the weight maintenance version of the TLC diet used in the current study (Hallikainen and Uusitupa Citation1999; Jones et al. Citation1999; Hallikainen et al. Citation2000; Katan et al. Citation2003; Abumweis et al. Citation2008; Demonty et al. Citation2009).
Additional research is needed to further evaluate the effects of this and other plant sterol/stanol products on lipid levels in other types of dyslipidemia, particularly Fredrickson Type IIb (mixed) dyslipidemia. Also of interest would be an evaluation of the effect on cholesterol biosynthesis of the intake of plant sterol/stanol tablets during the day (only with lunch) versus in the evening (only with dinner).
Intervention trials have shown that each 1% reduction in LDL-C (or non-HDL-C) lowers the risk of a major cardiovascular event by ∼1% over a period of 5 years (Grundy et al. Citation2004; Robinson et al. Citation2005, Citation2009). However, the cardiovascular benefit of maintaining low levels of atherogenic lipoprotein cholesterol levels over decades may be larger than would be predicted on the basis of results from short-term cholesterol-lowering intervention trials. Each 1% reduction in LDL-C or non-HDL-C may be associated with as much as a 3% reduction in CHD event risk if maintained over an extended period (Cohen et al. Citation2006). Thus, the changes in atherogenic lipoprotein cholesterol observed in the present study are clinically relevant.
Conclusions
In conclusion, incorporation of a dietary supplement tablet containing 1.8 g/day unesterified plant sterols/stanols into the NCEP TLC diet produced favourable changes in apolipoprotein B-containing lipoprotein lipids in individuals with hypercholesterolaemia and would be expected to reduce the risk for cardiovascular disease if consumed over an extended period of time (Miettinen and Gylling Citation2004).
Acknowledgements
The authors wish to thank Arianne Schild of Provident Clinical Research/Biofortis North America for assistance with statistical analysis and Rachel Hubacher and Nicholas Shera, both formerly with Provident Clinical Research/Biofortis North America, for technical assistance.
Declaration of interest: This trial was funded by Pharmavite, LLC, Northridge, CA, USA. KCM, ALL, MSR and MRD were, at the time this study was conducted, employees of Provident Clinical Research, which has received research grant support and consulting fees from Pharmavite, LLC, the manufacturer of the product studied. BHJ, ES and JRB are employees of Pharmavite, LLC, the manufacturer of the product studied.
References
- Abumweis SS, Barake R, Jones PJ. 2008. Plant sterols/stanols as cholesterol lowering agents: a meta-analysis of randomized controlled trials. Food Nutr Res. 52 e-publication.
- Acuff RV, Cai DJ, Dong ZP, Bell D. 2007. The lipid lowering effect of plant sterol ester capsules in hypercholesterolemic subjects. Lipids Health Dis. 6:11.
- Berger A, Jones PJ, Abumweis SS. 2004. Plant sterols: factors affecting their efficacy and safety as functional food ingredients. Lipids Health Dis. 3:5.
- Bittner V. 2003. Non-high-density lipoprotein cholesterol and cardiovascular disease. Curr Opin Lipidol. 14:367–371.
- Carr TP, Krogstrand KL, Schlegel VL, Fernandez ML. 2009. Stearate-enriched plant sterol esters lower serum LDL cholesterol concentration in normo- and hypercholesterolemic adults. J Nutr. 139:1445–1450.
- Cohen JC, Boerwinkle E, Mosley THJr, Hobbs HH. 2006. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 354:1264–1272.
- Cui Y, Blumenthal RS, Flaws JA, Whitman MK, Langenberg P, Bachorik PS, Bush TL. 2001. Non-high-density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. Arch Intern Med. 161:1413–1419.
- de Graaf J, de Sauvage Nolting PR, Van Dam M, Belsey EM, Kastelein JJ, Haydn Pritchard P, Stalenhoef AF. 2002. Consumption of tall oil-derived phytosterols in a chocolate matrix significantly decreases plasma total and low-density lipoprotein-cholesterol levels. Br J Nutr. 88:479–488.
- Demonty I, Ras RT, van der Knaap HC, Duchateau GS, Meijer L, Zock PL, Geleijnse JM, Trautwein EA. 2009. Continuous dose-response relationship of the LDL-cholesterol-lowering effect of phytosterol intake. J Nutr. 139:271–284.
- Doornbos AM, Meynen EM, Duchateau GS, van der Knaap HC, Trautwein EA. 2006. Intake occasion affects the serum cholesterol lowering of a plant sterol-enriched single-dose yoghurt drink in mildly hypercholesterolaemic subjects. Eur J Clin Nutr. 60:325–333.
- Food and Drug Administration. 2000. Food labeling: health claims; plant sterol/stanol esters and coronary heart disease. Food and Drug Administration, HHS. Interim final rule. Fed Reg. 65:54686–54739.
- Food and Drug Administration. 2010. Food labeling: health claim; phytosterols and risk of coronary heart disease; proposed rule. Fed Reg. 75:76526–76571.
- Friedewald WT, Levy RI, Fredrickson DS. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 18:499–502.
- Goldberg AC, Ostlund REJr, Bateman JH, Schimmoeller L, McPherson TB, Spilburg CA. 2006. Effect of plant stanol tablets on low-density lipoprotein cholesterol lowering in patients on statin drugs. Am J Cardiol. 97:376–379.
- Gremaud G, Dalan E, Piguet C, Baumgartner M, Ballabeni P, Decarli B, Leser ME, Berger A, Fay LB. 2002. Effects of non-esterified stanols in a liquid emulsion on cholesterol absorption and synthesis in hypercholesterolemic men. Eur J Nutr. 41:54–60.
- Grundy SM, Cleeman JI, Merz CN, Brewer HBJr, Clark LT, Hunninghake DB, Pasternak RC, Smith SCJr, Stone NJ, Coordinating Committee of the National Cholesterol Education Program. 2004. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 44:720–732.
- Hallikainen MA, Uusitupa MI. 1999. Effects of 2 low-fat stanol ester-containing margarines on serum cholesterol concentrations as part of a low-fat diet in hypercholesterolemic subjects. Am J Clin Nutr. 69:403–410.
- Hallikainen MA, Sarkkinen ES, Gylling H, Erikkilä AT, Uusitupa MI. 2000. Comparison of the effects of plant sterol ester and plant stanol ester-enriched margarines in lowering serum cholesterol concentrations in hypercholesterolaemic subjects on a low-fat diet. Eur J Clin Nutr. 54:715–725.
- Hegsted DM, Ausman LM, Johnson JA, Dallal GE. 1993. Dietary fat and serum lipids: an evaluation of the experimental data. Am J Clin Nutr. 57:875–883.
- Hui DY, Labonté ED, Howles PN. 2008. Development and physiological regulation of intestinal lipid absorption. III. Intestinal transporters and cholesterol absorption. Am J Physiol Gastrointest Liver Physiol. 294:G839–G843.
- Ikeda I, Sugano M. 1998. Inhibition of cholesterol absorption by plant sterols for mass intervention. Curr Opin Lipidol. 9:527–531.
- Jones PJ. 2008. Dietary agents that target gastrointestinal and hepatic handling of bile acids and cholesterol. J Clin Lipidol. 2:S4–S10.
- Jones PJ, Ntanios FY, Raeini-Sarjaz M, Vanstone CA. 1999. Cholesterol-lowering efficacy of a sitostanol-containing phytosterol mixture with a prudent diet in hyperlipidemic men. Am J Clin Nutr. 69:1144–1150.
- Katan MB, Grundy SM, Jones P, Law M, Miettinen T, Paoletti R, Stresa Workshop Participants. 2003. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc. 78:965–978.
- Kris-Etherton PM, Yu S. 1997. Individual fatty acid effects on plasma lipids and lipoproteins: human studies. Am J Clin Nutr. 65 5 Suppl.: 1628S–1644S.
- Liu J, Sempos CT, Donahue RP, Dorn J, Trevisan M, Grundy SM. 2006. Non-high-density lipoprotein and very-low-density lipoprotein cholesterol and their risk predictive values in coronary heart disease. Am J Cardiol. 98:1363–1368.
- Maki KC, Davidson MH, Umporowicz DM, Schaefer EJ, Dicklin MR, Ingram KA, Chen S, McNamara JR, Gebhart BW, Ribaya-Mercado JD, Perrone G, Robins SJ, Franke WC. 2001. Lipid responses to plant-sterol-enriched reduced-fat spreads incorporated into a National Cholesterol Education Program Step I diet. Am J Clin Nutr. 74:33–43.
- Maki KC, Shinnick F, Seeley MA, Veith PE, Quinn LC, Hallissey PJ, Temer A, Davidson MH. 2003. Food products containing free tall oil-based phytosterols and oat beta-glucan lower serum total and LDL cholesterol in hypercholesterolemic adults. J Nutr. 133:808–813.
- McPherson TB, Ostlund RE, Goldberg AC, Bateman JH, Schimmoeller L, Spilburg CA. 2005. Phytostanol tablets reduce human LDL-cholesterol. J Pharm Pharmacol. 57:889–896.
- Miettinen TA, Gylling H. 2004. Plant stanol and sterol esters in prevention of cardiovascular diseases. Ann Med. 36:126–134.
- Miller M, Ginsberg HN, Schaefer EJ. 2008. Relative atherogenicity and predictive value of non-high-density lipoprotein cholesterol for coronary heart disease. Am J Cardiol. 101:1003–1008.
- Mussner MJ, Parhofer KG, Von Bergmann K, Schwandt P, Broedl U, Otto C. 2002. Effects of phytosterol ester-enriched margarine on plasma lipoproteins in mild to moderate hypercholesterolemia are related to basal cholesterol and fat intake. Metabolism. 51:189–194.
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). 2002. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 106:3143–3421.
- Nestel P, Cehun M, Pomeroy S, Abbey M, Weldon G. 2001. Cholesterol-lowering effects of plant sterol esters and non-esterified stanols in margarine, butter and low-fat foods. Eur J Clin Nutr. 55:1084–1090.
- Ostlund REJr. 2002. Phytosterols in human nutrition. Ann Rev Nutr. 22:533–549.
- Ostlund REJr, Spilburg CA, Stenson WF. 1999. Sitostanol administered in lecithin micelles potently reduces cholesterol absorption in humans. Am J Clin Nutr. 70:826–831.
- Pischon T, Girman CJ, Sacks FM, Rifai N, Stampfer MJ, Rimm EB. 2005. Non-high-density lipoprotein cholesterol and apolipoprotein B in the prediction of coronary heart disease in men. Circulation. 29:3375–3383.
- Plat J, Brufau G, Dallinga-Thie GM, Dasselaar M, Mensink RP. 2009. A plant stanol yogurt drink alone or combined with a low-dose statin lowers serum triacylglycerol and non-HDL cholesterol in metabolic syndrome patients. J Nutr. 139:1143–1149.
- Pouteau E, Monnard IE, Piguet-Welsch C, Groux MJ, Sagalowicz L, Berger A. 2003. Non-esterified plant sterols solubilized in low fat milks inhibit cholesterol absorption – a stable isotope double-blind crossover study. Eur J Nutr. 42:154–164.
- Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. 2005. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 294:326–333.
- Robinson JG, Smith B, Maheshwari N, Schrott H. 2005. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta-regression analysis. J Am Coll Cardiol. 46:1855–1862.
- Robinson JG, Wang S, Smith BJ, Jacobson TA. 2009. Meta-analysis of the relationship between non-high-density lipoprotein cholesterol reduction and coronary heart disease risk. J Am Coll Cardiol. 53:316–322.
- Sánchez-Muniz FJ, Maki KC, Schaefer EJ, Ordovas JM. 2009. Serum lipid and antioxidant responses in hypercholesterolemic men and women receiving plant sterol esters vary by apolipoprotein E genotype. J Nutr. 139:13–19.
- Thomsen AB, Hansen HB, Christiansen C, Green H, Berger A. 2004. Effect of free plant sterols in low-fat milk on serum lipid profile in hypercholesterolemic subjects. Eur J Clin Nutr. 58:860–870.
- Volpe R, Nittynen L, Korpela R, Sirtori C, Bucci A, Fraone N, Pazzucconi F. 2001. Effects of yoghurt enriched with plant sterols on serum lipids in patients with moderate hypercholesterolaemia. Br J Nutr. 86:233–239.
- von Bergmann K, Sudhop T, Lütjohann D. 2005. Cholesterol and plant sterol absorption: recent insights. Am J Cardiol. 96:10D–14D.