Abstract
This review summarizes some of the milestones of the research on the biological functions(s) of midbrain urocortin 1 (Ucn1) since its discovery 15 years ago. Detailed characterization of Ucn1 in the midbrain revealed its overall significance in food intake and regulation of homeostatic equilibrium and mood under stress. In addition, we have recently found a conspicuous alteration in midbrain Ucn1 levels in brains of depressed suicide victims. Furthermore, from the results from the genetically modified animals, a picture is emerging where corticotrophin-releasing factor promotes the initial reactions to stress, whereas Ucn1 seems to be crucial for management of the later adaptive phase. In the case of imbalance in action of these principle stress mediators, vulnerability to stress-related brain diseases is enhanced.
Introduction
Fifteen years ago, the second member of the corticotrophin-releasing factor (CRF) neuropeptide family, urocortin 1 (Ucn1) was discovered (Vaughan et al. Citation1995). It has a sequence identity of 45% with CRF (Vaughan et al. Citation1995). Interestingly, Vaughan et al. (Citation1995) localized Ucn1 to a discrete nucleus in the rostroventral midbrain, and to the Edinger–Westphal (EW) nucleus. This has been later confirmed by showing Ucn1 immunoreactive neurons in the EW nucleus in various mammalian as well as non-mammalian species (Bittencourt et al. Citation1999; Iino et al. Citation1999; CitationKozicz et al. 1998, Citation2002; Ryabinin et al. Citation2005). The presence of a stress-related neuropeptide in a nucleus classically associated with parasympathetic oculomotor functions (pupil construction, lens accommodation, and trophic functions of the eyes) not only has resulted in the extension of the classical biological significance of EW nucleus but also has become inevitable to revisit the nomenclature of this midbrain area. To distinguish preganglionic cholinergic neurons directly controlling oculomotor functions, neurons that express Ucn1 and do not project to the ciliary ganglion, but rather possess central projections that were renamed as EW central projecting (EWcp) neurons (Kozicz et al. 2011). Despite the ongoing debate on the nomenclature of this brain area, a significant advance has been made in putting together the puzzle on the biological role(s) of EWcp-Ucn1 in the last 15 years. These efforts have brought us closer to answer the questions that we raised at the end of the 1990s. Does midbrain Ucn1 matter? We do believe it does, but to underpin this statement, this review aims to summarize data showing that midbrain Ucn1 is regulated by basic physiological processes, that Ucn1 itself regulates/modulates various physiological processes, and that midbrain Ucn1 may also contribute to the pathophysiology of human diseases.
Does midbrain UCN 1 change under physiological conditions?
Daily rhythms
Period 2 (Per2) is an important clock gene involved in the regulation of the major circadian clock in the mammalian central nervous system, the suprachiasmatic nucleus. In a recent study, we have found that Per2 is expressed in the EWcp (Gaszner et al. Citation2009). Both the protein and the mRNA expression of Per2 in EWcp neurons showed a diurnal rhythm, with a minimum at light-off, and a maximum at light-on (Gaszner et al. Citation2009). Interestingly, a majority of Ucn1 neurons co-expressed Per2, suggesting that the Ucn1 is produced in a diurnal fashion. Indeed, we have found a diurnal rhythm in the number of Ucn1-immunopositive neurons (most probably reflecting change in the abundance of Ucn1 peptide in neurons) and in their Ucn1 peptide content, with a minimum at the beginning of the light period and a maximum at light-off, while the UCN1 expression paralleled that of Per2 rhythm (Gaszner et al. Citation2009). This apparent phase shift in the abundance of Ucn1 peptide and mRNA could represent a diurnal rhythm in the biosynthetic and secretory activity of EWcp-Ucn1 neurons; at light-on, Ucn1 mRNA is produced at its maximum rate, as well as Ucn1 peptide is made, transported, and released from neurons (lowest number of Ucn1 positive neurons), whereas at light-off, Ucn1 mRNA production is at its lowest, and Ucn1 peptide is stored/accumulated in neurons (highest number of Ucn1 positive neurons). These rhythms in the rat EWcp are accompanied by a diurnal rhythm in plasma corticosterone concentration. Diurnal production of Per2 and Ucn1 in the EWcp may be relevant for the physiological role(s) of Ucn1 (see later).
Sex steroids
The presence of estrogen receptor β (ERβ) in the EW nucleus has been previously demonstrated (Mitra et al. Citation2003). We confirmed this finding and extended it by demonstrating the absence of ERα in EWcp neurons (Derks et al. Citation2007, Citation2009). We also found that the majority of EWcp-Ucn1 neurons co-express ERβ in both rat and mouse (Derks et al. Citation2007, Citation2009). Furthermore, Ucn1 mRNA in males was nearly 10 times higher than in females in diestrus and 1.6 times higher than in females in proestrus. This clearly indicates a sex-dependent difference in Ucn1 biosynthetic activity. In line with this notion, it has been demonstrated that estrogen decreases the transcriptional activity of the UCN1 promoter via ERβ (Haeger et al. Citation2006). As an additional support for a sex-dependent expression of EWcp-Ucn1 is the fact that female rats possess significantly higher levels of Ucn1 immunoreactivity than male rats (Fonareva et al. Citation2009) and that Ucn1 immunoreactivity decreases during the course of pregnancy in rats (Fatima et al. Citation2007).
Peripheral metabolic signals
In a recent study, using a novel LepRb-IRES-Cre-EYFP-reporter mouse, substantial LepRb expressions were found in EWcp (Scott et al. Citation2009), and LepRb is expressed in about 50–60% of EWcp-Ucn1 neurons in both rat and mouse (Xu et al. Citation2009). Furthermore, Ucn1 is also co-expressed with ghrelin receptor mRNA (unpublished observation by the authors). These observations strongly support the view that EWcp-Ucn1 neurons are under the control of peripheral metabolism-related signals and, thus, would play an important role in controlling/modulating food intake and/or energy balance. In support of this notion, we have recently shown that fasting rats for 2 days causes a marked body weight loss, reduces leptin plasma level in both sexes, and results in a 3.3 times upregulation of Ucn1 mRNA in males but not in females (Xu et al. Citation2009). We have also shown that EWcp-Ucn1 neurons respond to leptin with the activation of JAK2–STAT3 pathway, in which leptin inhibits the electrical activity of EWcp neurons and systemic leptin administration significantly increases Ucn1 content in EWcp (Xu et al. Citation2010b). Furthermore, electrical lesioning of the EWcp results in inhibition of food intake (Weitemier and Ryabinin Citation2005) and that high fat diet decreases Ucn1 mRNA in the EWcp too, concomitant with increased plasma leptin level (Legendre et al. Citation2007). Collectively, these data show that a negative correlation exists between plasma leptin and Ucn1 specifically in the EWcp. Based on the anorexigenic action of centrally administered Ucn1 (Spina et al. Citation1996), one would expect the opposite. However, one should consider that Ucn1 administered via an intracerebroventricular route has access to both CRF receptor subtypes, which could lead to differential (even opposing) behavioral, physiological, and neuroendocrine responses.
Stress response
Stress is a physiological response to any threatening demand in order to restore homeostatic equilibrium (Selye Citation1951). Soon after the discovery of Ucn1 in EWcp, it was shown that Ucn1 neurons were recruited by acute pain stress (Kozicz et al. Citation2001), and that Ucn1 mRNA is significantly upregulated by acute (pain and restraint) stress (Weninger et al. Citation2000; Kozicz et al. Citation2001). Later it has become clear that EWcp-Ucn1 neurons are recruited by various acute stressors (Gaszner et al. Citation2004; Kozicz Citation2007, 2009), and their activation pattern suggest that they preferentially respond (as revealed by expression of Fos protein) to psychological/processive stressors (e.g. restraint and pain), but not to systemic stressors (hyperosmotic or hemorrhage stress). In addition, in peritoneal inflammation as well as surgical operation (bilateral nephrectomy) both forms of an acute stress, induce strong Fos and Fra-2 expression in EWcp (Lanteri-Minet et al. Citation1993; Palkovits et al. Citation2009).
Interestingly, the activation of EWcp neurons and the upregulation of Ucn1 mRNA after acute pain stress last more than 16 h (Kozicz et al. Citation2001), in contrast to the relative short-lasting (up to 2–4 h) activation of neurons in the hypothalamic paraventricular nucleus (PVN) and the upregulation of PVN-CRF mRNA (Viau and Sawchenko Citation2002). A similar difference in the dynamics of activation of EWcp and PVN has been found after acute renal failure. EWcp neurons were recruited within 30 min, however, unlike the Fos/Fra-2 expression in the PVN, and EWcp activation lasted at least 72 h during acute renal failure (Palkovits et al. Citation2009). This not only further supports the view that EWcp neurons are sensitive of an altered body homeostasis but also supports the notion that EWcp is rather involved in the later, adaptive phase of the stress response (Kozicz Citation2007).
Similarly to rats, restraint stress also increases the expression of egr-1 (another inducible transcription factor) in bird EWcp-Ucn1 neurons (Cunha et al. Citation2007). In mice, however, no induction of fos or egr-1-ir has been reported following various acute stressors (Turek and Ryabinin Citation2005; Spangler et al. Citation2009), suggesting that the stress responsiveness of EWcp neurons is species specific too. In agreement with this idea, electrolytic lesions of this brain area in the mouse, which produce several other robust behavioral effects, do not affect anxiety-like behavior in the plus maze, locomotor behavior in the open field, or levels of plasma corticosterone (Weitemier and Ryabinin Citation2005).
Chronic stress also recruits Ucn1 neurons in the rodent EWcp. Both chronic predictable/homotypic (chronic ether stress for 21 days in the mouse) and chronic unpredictable/heterotypic (chronic variable mild stress for 14 days in the rat) activated Ucn1 neurons as demonstrated by Fos immunoreactivity (Korosi et al. Citation2005; Xu et al. Citation2010a). These data show that EWcp neurons do not habituate to chronic stressors, and this is in clear contrast to the habituating response of PVN neurons upon chronic stress (Viau and Sawchenko Citation2002).
With respect to Ucn1 peptide and mRNA expression in EWcp neurons, it has to be noted that chronic homotypic stressor induces different dynamics as compared to chronic heterotypic stressor. Chronic ether stress (3 weeks) did not result in any change in Ucn1 mRNA expression in the mouse (Korosi et al. Citation2005). This response is very similar to that of CRF neurons in PVN, which also did not show any change in CRF mRNA expression (e.g. Romeo et al. Citation2007). In contrast, chronic unpredictable/heterotypic stress in mouse results in a significant increase in both Ucn1 peptide and mRNA expression (Derks Citation2010), similarly to the increased CRF mRNA expression after chronic variable stress (e.g. Herman et al. Citation1995, Citation2003).
Taken together, the differences in the dynamics of activation of PVN and EWcp neurons both in response to acute and chronic challenges, the hypothesis has been put forward that the complementary action of these stress-sensitive brain areas would serve to terminate the central stress response, thereby promoting successful adaptation to challenges (Weninger et al. Citation1999; Kozicz Citation2007, 2009). Consequently, this would imply that besides the CRF neurons in the PVN, EWcp-Ucn1 neurons have an important role in the stress adaptation response and in stress-related brain diseases such as anxiety and depression (Kozicz Citation2007, 2009).
Does midbrain UCN1 contribute to disease pathology?
Anxiety and depression
Ucn1, similarly to CRF, administered intracerebroventricularly is capable of accessing its cognate receptors (Bittencourt and Sawchenko Citation2000) and elicits strong anxiogenic properties (Skelton et al. Citation2000; Gysling et al. Citation2004; Kozicz Citation2007). In addition, a large body of pharmacological evidence indicates specific roles for EWcp-Ucn1 neurons in anxiety-like behaviors. Specifically, the recruitment of EWcp neurons after intracerebroventricular administration of benzodiazepines and selective agonists of the metabotropic glutamate receptors strongly suggests that their action may manifest, at least in part, via regulation of EWcp neuron activity (Linden et al. Citation2004, Citation2006; Skelton et al. Citation2004).
To further dissect the possible role of Ucn1 in disease pathology, with special emphasis on stress-related anxiety and depression, various mice models have been created with a null mutation in the UCN1 gene. One of these models showed significantly heightened anxiety-like behavior, but a normal HPA-axis response to stress, and no change in CRF mRNA expression (Vetter et al. Citation2002). Another Ucn1-null mice generated by Wang et al. (Citation2002) exhibit normal stress-induced responses, including anxiety-like behavior, autonomic control, and endocrine secretion. These clearly contrasting results make it difficult to conclude about the significance of Ucn1 in regulating anxiety-like behavior. Although some of these contrasting results on Ucn1's role in anxiety-like behaviors can be explained by developmental compensatory mechanisms activated in these mutants (e.g. altered expression in CRF receptors), further and more detailed dissection of the behavioral phenotype of UCN1-null mice and the use of site-specific, lentiviral modification of Ucn1 expression in EWcp are warranted to clarify its biological significance in stress-related behaviors and are the focus of future research in our group. However, it is also possible that in contrast to what was found for mice deficient for receptors CRF-R1 and CRF-R2 (Bale and Vale Citation2004; Bale Citation2006), neither CRF nor Ucn1 alone plays an essential role in acute stress-related behavioral and/or autonomic responses. This suggests that CRF and Ucn1 are redundant with respect to their functions in regulating/controlling acute stress response. Instead, Ucn1 may play an important role in regulating the HPA-axis' response to chronic challenges. In line with this notion, another model with a null mutation in the UCN1 gene reveals impaired adaptation to repeated restraint stress (Zalutskaya et al. Citation2007). Furthermore, in a recent study, Neufeld-Cohen et al. (2010) demonstrated decreased anxiety and increased forebrain serotonergic functioning in UCN1/UCN2 double knockout mice. This together with the fact that Ucn1 neurons project to the dorsal raphe nucleus (DRN; Bittencourt et al. Citation1999; Weitemier and Ryabinin Citation2005) clearly puts the Ucn1 neurons in EWcp in critical position to control dorsal raphe serotonergic activity and thereby modulating the stress response and stress-related behavior(s) (Kozicz Citation2010). Thus, if the lack of innervations by Ucn1/Ucn2 to the DRN results in increased serotonin releases in forebrain anxiety circuitries, it is reasonable to assume that overexpression of UCN1 in EWcp would increase anxiety and depression due to decreased serotonergic activity in the forebrain. And indeed, male depressed suicide victims show an approximately 10 times higher UCN1 expression in EWcp compared to controls (Kozicz et al. Citation2008).
Although the above data strongly implicate EWcp-Ucn1 DRN–serotonin interaction in controlling/modulating anxiety and depression-like behaviors, the projections of EWcp-Ucn1 neurons to the lateral septum (Bittencourt et al. Citation1999), which has also been postulated in stress-related anxiety (Koolhaas et al. Citation1998; Eckart et al. Citation1999), may also play a specific role.
Mechanistically, dysregulation of the CRF receptor signaling may play a role in stress-related depression. It is well documented that CRF is hypersecreted in forebrain centers and has been suggested to contribute to depression pathology (Holsboer et al. Citation1984; Nemeroff Citation1988; Reul and Holsboer Citation2002; de Kloet et al. Citation2005; Bale Citation2006; Joels and Baram Citation2009). However, current concepts concerning G protein-coupled receptor regulation (Lefkowitz and Shenoy Citation2005) state that agonist-activated CRF1 receptors undergo rapid desensitization and internalization in response to high agonist concentrations (for review, see Hauger et al. Citation2006). Therefore, it is unlikely that CRF hypersecretion alone is sufficient to account for the neuropathology of major depression (Hauger et al. Citation2006). Thus, hypothetically, the concomitant hypersecretion of CRF and Ucn1 could produce exaggerated CRF receptor signaling in brain regions mediating the symptoms of depression (Kozicz Citation2009).
Gender difference in Ucn1 expression in stress-related mood
Although an increased susceptibility of females to depression has been well documented (Kornstein 1997; Frackiewicz et al. 2000; Kudielka and Kirschbaum 2005), the underlying mechanism(s) is/are not well known. Possibly, gender-specific actions of EWcp-Ucn1 neurons account for this difference. This idea is in line with the finding that chronic variable mild stress does increase Ucn1 mRNA in male but not in female rats and mouse (Derks Citation2010). The same phenomenon was found in humans, where UCN1 expression appeared to be strongly upregulated (more than 10 times) in male suicide victims with major depression but no such upregulation was observed in female depressed suicides (Kozicz et al. Citation2008).
Obesity
Peripheral injection of Ucn1 in nanomolar concentration (0.3–3 nmol) produces high and prolonged inhibitory effects on food intake (Asakawa et al. Citation1999; Wang et al. Citation2001), an effect that can also be seen in leptin receptor (ob/ob)-deficient mice (Asakawa et al. Citation2001). The ability of Ucn1 to reduce food intake was interestingly accompanied by a reduced motivation to eat (Kinney et al. Citation2001), a phenomenon that will need attention in future studies. Intracerebroventricular administration of Ucn1 also potently reduces food intake in food-deprived and free-feeding rats (Spina et al. Citation1996). This action of Ucn1 is most probably mediated via endogenous Ucn1 in the ventromedial hypothalamic nucleus (VMH), because injection of Ucn1 into the VMH significantly inhibited food intake over 3 h, but no significant effect was observed following injection into other hypothalamic nuclei. This effect of Ucn1 was completely reversed by injecting an antibody against rat Ucn1 into the VMH (Ohata et al. Citation2000). Interestingly, the VMH, which expresses predominantly CRF-R2 (Van Pett et al. Citation2000), is densely innervated by Ucn1 fibers (Kozicz et al. Citation1998; Bittencourt et al. Citation1999) most probably originating from EWcp. In addition, intracerebroventricular injection of Ucn1 results in strong Fos labeling in VMH (Bittencourt and Sawchenko Citation2000). Taken together, the function of Ucn1 controlling food intake might be mediated by a EWcp-VMH neuronal circuitry.
To conclude, these data strongly suggest a role for central Ucn1 in regulating food intake and possibly obesity. To underpin this notion, Delplanque et al. (Citation2002) analyzed the association between single nucleotide polymorphisms in the UCN1 gene and obesity in French Caucasians. No strong associations have been found (at least not in French Caucasians), suggesting that the UCN1 gene has a major role in obesity (Delplanque et al. Citation2002). This clearly contrasts the evidence of linkage of obesity-related phenotypes (i.e. leptin resistance, body mass index) to human chromosome 2 at a position of 2p21–23, a locus where two potentially interesting genes lie, i.e. UCN1 and proopiomelanocortin (Comuzzie et al. Citation1997; Rotimi et al. Citation1999; Delplanque et al. Citation2002). It is, however, possible that Ucn1 indirectly affects the development of obesity. Synergistic effects of leptin and Ucn1 on food intake have been shown in several studies. More specifically, Kotz et al. (Citation2002) showed that the satiety effect of Ucn1 is enhanced by its induction of plasma leptin. Also, co-treatment of rats with doses of leptin and Ucn1 that are ineffective when given alone effectively suppresses appetite (Pan and Kastin Citation2008). Furthermore, leptin shows a facilitatory effect on Ucn1 transport across the blood–brain barrier, and there is evidence that Ucn1 potentiates leptin signaling by increasing leptin receptor-induced STAT3 phosphorylation (Pan et al. Citation2007). Such an action of Ucn1 may amplify the cellular response of leptin and suggests that Ucn1 can play an important compensatory role during leptin resistance in obesity (Pan and Kastin Citation2008).
Beside Ucn1's involvement in regulating food intake, Ucn1 has also been implicated in another consumptive behavior, i.e. intake of alcohol and addictive drugs. This interesting aspect of the biology of midbrain Ucn1 is, however, beyond the scope of this review and has recently been reviewed (e.g. Ryabinin and Weitemier Citation2006; Vilpoux et al. Citation2009; Ciccocioppo et al. Citation2009; CitationKozicz et al. 2010; Lowery and Thiele Citation2010). Collectively, data on addictive drugs and eating behavior suggest that EWcp-Ucn1 could have a unique role in consumption of rewarding substances.
A model
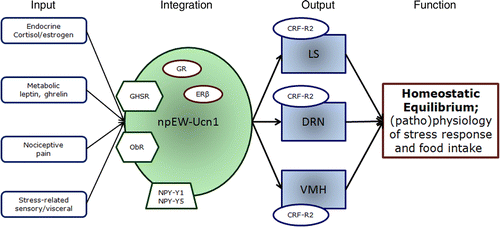
On the basis of the data mentioned above, we have constructed a model of the possible biological significance of midbrain Ucn1 neurons in the EWcp (). These neurons receive multiple inputs transmitting various sorts of information ranging from endocrine (e.g. estrogen, cortisol), metabolic (e.g. leptin, ghrelin), nociceptive, and visceral signals (Morimoto et al. Citation1996; Derks et al. Citation2007, Citation2009; Gaszner et al. Citation2007; Xu et al. Citation2009). These will be received by receptors for estrogen (ERβ; Derks et al. Citation2009), cortisol (GR; data under publication), ghrelin (GHSR; Kaur and Ryabinin Citation2010), leptin LepRB (Xu et al. Citation2009), and neuropeptide Y (NPY-Y1 and Y5; Gaszner et al. Citation2007). EWcp-Ucn1 neurons integrate these diverse input signals, which will consequently alter their secretory activity. This will result in changes in the synaptic release of Ucn1 in brain areas targeted by Ucn1 afferents, including the lateral septum (LS), DRN, and VMH (Bittencourt et al. Citation1999; Weitemier and Ryabinin Citation2005). All these brain nuclei express CRF-R2, a receptor to which Ucn1 binds with a very high affinity (Vaughan et al. Citation1995), and thus Ucn1 released in these brain areas is in state to influence the functioning of these brain centers. Clearly, these centers have also been implicated in regulating the (patho)physiology of the stress response and food intake, as well as in controlling mood. According to our model, dysfunctioning of EWcp neurons would contribute to dysregulation of neuronal activity in LS, DRN, and VMH. This consequently will result in alterations in homeostatic equilibrium and in maladaptive regulation of stress and food intake.
Figure 1. The proposed model. CRF-R2, corticotrophin-releasing factor receptor type 2; DRN, dorsal raphe nucleus; GHSR, ghrelin receptor; GR, glucocorticoid receptor; ERβ, estrogen receptor β; LS, lateral septal nucleus; NPY-Y1 and Y5, neuropeptide Y receptor Y1 and Y5; ObR, leptin receptor; VMH, ventromedial hypothalamic nucleus.
Does midbrain UCN1 matter?
We do believe it does! However, there are still several pieces of the puzzle that are missing, despite the large body of (mostly correlative) evidence that has been collected in the past 15 years. In the future, we will need comprehensive neuroanatomical studies mapping all afferent and efferent connections to and from EWcp-Ucn1 neurons, identifying the phenotype of neurons targeted by Ucn1 efferents. In addition, further and detailed physiological and behavioral characterization of existing Ucn1-null mice will be inevitable under control and challenge conditions (including yet untested hypotheses, such as reward processing and cognition) to gain better insights into the (patho)physiological significance of Ucn1. Ultimately, viral-mediated down- or upregulation of UCN1, specifically in the EWcp would serve us with direct evidence on the roles of EWcp neurons, so we could confidently state that MIDBRAIN UCN1 DOES MATTER!
Acknowledgements
We apologize to those colleagues whose work could not be cited here owing to space limitations. L.S. and X.L are on a 4-year PhD program sponsored by MSD, Oss, The Netherlands (L.S.), and by Radboud University Nijmegen, Faculty of Science, Mathematics and Informatics (X.L.). This work was also supported by the Netherlands Organization for Scientific Research Grant 864.05.008 (to TK).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Asakawa A, Inui A, Ueno N, Makino S, Fujino MA, Kasuga M. 1999. Urocortin reduces food intake and gastric emptying in lean and ob/ob obese mice. Gastroenterology. 116 6: 1287–1292.
- Asakawa A, Inui A, Ueno N, Makino S, Fujimiya M, Fujino MA, Kasuga M. 2001. Urocortin reduces oxygen consumption in lean and ob/ob mice. Int J Mol Med. 7 5: 539–541.
- Bale TL. 2006. Stress sensitivity and the development of affective disorders. Horm Behav. 50 4: 529–533.
- Bale TL, Vale WW. 2004. CRF and CRF receptors: Role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 44:525–557.
- Bittencourt JC, Sawchenko PE. 2000. Do centrally administered neuropeptides access cognate receptors?: An analysis in the central corticotropin-releasing factor system. J Neurosci. 20 3: 1142–1156.
- Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. 1999. Urocortin expression in rat brain: Evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol. 415 3: 285–312.
- Ciccocioppo R, Gehlert DR, Ryabinin A, Kaur S, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Economidou D, Stopponi S, Cannella N, Braconi S, Kallupi M, de GG, Massi M, George DT, Gilman J, Hersh J, Tauscher JT, Hunt SP, Hommer D, Heilig M. 2009. Stress-related neuropeptides and alcoholism: CRH, NPY, and beyond. Alcohol. 43 7: 491–498.
- Comuzzie AG, Hixson JE, Almasy L, Mitchell BD, Mahaney MC, Dyer TD, Stern MP, MacCluer JW, Blangero J. 1997. A major quantitative trait locus determining serum leptin levels and fat mass is located on human chromosome 2. Nat Genet. 15 3: 273–276.
- Cunha RP, Reiner A, Toledo CA. 2007. Involvement of urocortinergic neurons below the midbrain central gray in the physiological response to restraint stress in pigeons. Brain Res. 1147:175–183.
- de Kloet ER, Joels M, Holsboer F. 2005. Stress and the brain: From adaptation to disease. Nat Rev Neurosci. 6 6: 463–475.
- Delplanque J, Vasseur F, Durand E, Abderrahmani A, Dina C, Waeber G, Guy-Grand B, Clement K, Weill J, Boutin P, Froguel P. 2002. Mutation screening of the urocortin gene: Identification of new single nucleotide polymorphisms and association studies with obesity in French Caucasians. J Clin Endocrinol Metab. 87 2: 867–869.
- Derks NM. 2010. The role of the non-preganglionic Edinger–Westphal nucleus in sex-dependent stress adaptation in rodents Donders Series. Nijmegen: PrintPartners Ipskamp.
- Derks NM, Roubos EW, Kozicz T. 2007. Presence of estrogen receptor beta in urocortin 1-neurons in the mouse non-preganglionic Edinger–Westphal nucleus. Gen Comp Endocrinol. 153 1–3: 228–234.
- Derks NM, Gaszner B, Roubos EW, Kozicz LT. 2009. Sex differences in urocortin 1 dynamics in the non-preganglionic Edinger–Westphal nucleus of the rat. Neurosci Res. 66 1: 117–123.
- Eckart K, Radulovic J, Radulovic M, Jahn O, Blank T, Stiedl O, Spiess J. 1999. Actions of CRF and its analogs. Curr Med Chem. 6 11: 1035–1053.
- Fatima A, Haroon MF, Wolf G, Engelmann M, Spina MG. 2007. Reduced urocortin 1 immunoreactivity in the non-preganglionic Edinger–Westphal nucleus during late pregnancy in rats. Regul Pept. 143 1–3: 34–38.
- Fonareva I, Spangler E, Cannella N, Sabino V, Cottone P, Ciccocioppo R, Zorrilla EP, Ryabinin AE. 2009. Increased perioculomotor urocortin 1 immunoreactivity in genetically selected alcohol preferring rats. Alcohol Clin Exp Res. 33 11: 1956–1965.
- Frackiewicz EJ, Sramek JJ, Cutler NR. 2000. Gender differences in depression and antidepressant pharmacokinetics and adverse events. Ann Pharmacother. 34 1: 80–88.
- Gaszner B, Csernus V, Kozicz T. 2004. Urocortinergic neurons respond in a differentiated manner to various acute stressors in the Edinger–Westphal nucleus in the rat. J Comp Neurol. 480 2: 170–179.
- Gaszner B, Korosi A, Palkovits M, Roubos EW, Kozicz T. 2007. Neuropeptide Y activates urocortin 1 neurons in the nonpreganglionic Edinger–Westphal nucleus. J Comp Neurol. 500 4: 708–719.
- Gaszner B, Van Wijk DC, Korosi A, Jozsa R, Roubos EW, Kozicz T. 2009. Diurnal expression of period 2 and urocortin 1 in neurones of the non-preganglionic Edinger–Westphal nucleus in the rat. Stress. 12 2: 115–124.
- Gysling K, Forray MI, Haeger P, Daza C, Rojas R. 2004. Corticotropin-releasing hormone and urocortin: Redundant or distinctive functions?. Brain Res Brain Res Rev. 47 1–3: 116–125.
- Haeger P, Andres ME, Forray MI, Daza C, Araneda S, Gysling K. 2006. Estrogen receptors alpha and beta differentially regulate the transcriptional activity of the Urocortin gene. J Neurosci. 26 18: 4908–4916.
- Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. 2006. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: New molecular targets. CNS Neurol Disord Drug Targets. 5 4: 453–479.
- Herman JP, Adams D, Prewitt C. 1995. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 61 2: 180–190.
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. 2003. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 24 3: 151–180.
- Holsboer F, Gerken A, Steiger A, Benkert O, Muller OA, Stalla GK. 1984. Corticotropin-releasing-factor induced pituitary-adrenal response in depression. Lancet. 1 8367: 55.
- Iino K, Sasano H, Oki Y, Andoh N, Shin RW, Kitamoto T, Takahashi K, Suzuki H, Tezuka F, Yoshimi T, Nagura H. 1999. Urocortin expression in the human central nervous system. Clin Endocrinol (Oxf). 50 1: 107–114.
- Joels M, Baram TZ. 2009. The neuro-symphony of stress. Nat Rev Neurosci. 10 6: 459–466.
- Kaur S, Ryabinin AE. 2010. Ghrelin receptor antagonism decreases alcohol consumption and activation of perioculomotor urocortin-containing neurons. Alcohol Clin Exp Res. 34 9: 1525–1534.
- Kinney JW, Scruggs B, Avery DD. 2001. Peripheral administration of urocortin suppresses operant responding for food reward. Peptides. 22 4: 583–587.
- Koolhaas JM, Everts H, de Ruiter AJ, De Boer SF, Bohus B. 1998. Coping with stress in rats and mice: Differential peptidergic modulation of the amygdala-lateral septum complex. Prog Brain Res. 119:437–448.
- Korosi A, Schotanus S, Olivier B, Roubos EW, Kozicz T. 2005. Chronic ether stress-induced response of urocortin 1 neurons in the Edinger–Westphal nucleus in the mouse. Brain Res. 1046 1–2: 172–179.
- Kornstein SG. 1997. Gender differences in depression: implications for treatment. J Clin Psychiatry 58 Suppl. 15:12–8.
- Kotz CM, Wang C, Levine AS, Billington CJ. 2002. Urocortin in the hypothalamic PVN increases leptin and affects uncoupling proteins-1 and -3 in rats. Am J Physiol Regul Integr Comp Physiol. 282 2: R546–R551.
- Kozicz T. 2007. On the role of urocortin 1 in the non-preganglionic Edinger–Westphal nucleus in stress adaptation. Gen Comp Endocrinol. 153 1–3: 235–240.
- Kozicz T. 2009. Neurobiology of corticotropin releasing factor system components in stress; from adaptation to maladaptation. In: Kovacs M, Merchenthaler I. editors. Neuropeptides and peptide analogs. Kerala, India: Research Signpost. p 59–90.
- Kozicz T. 2010. The missing link; the significance of urocortin 1/urocortin 2 in the modulation of the dorsal raphe serotoninergic system. Mol Psychiatry. 15 4: 340–341.
- Kozicz T, Yanaihara H, Arimura A. 1998. Distribution of urocortin-like immunoreactivity in the central nervous system of the rat. J Comp Neurol. 391 1: 1–10.
- Kozicz T, Min L, Arimura A. 2001. The activation of urocortin immunoreactive neurons in the Edinger–Westphal nucelus following acute pain stress in rats. Stress. 4:85–90.
- Kozicz T, Arimura A, Maderdrut JL, Lazar G. 2002. Distribution of urocortin-like immunoreactivity in the central nervous system of the frog Rana esculenta. J Comp Neurol. 453 2: 185–198.
- Kozicz T, Tilburg-Ouwens D, Faludi G, Palkovits M, Roubos E. 2008. Gender-related urocortin 1 and brain-derived neurotrophic factor expression in the adult human midbrain of suicide victims with major depression. Neuroscience. 152 4: 1015–1023.
- Kozicz T, Bittencourt JC, May PJ, Reiner A, Gamlin PDR, Palkovits M, Horn AKE, Toledo CA, Ryabinin AE. The Edinger–Westphal nucleus: A historical, structural and functional perspective on a dichotomous terminology. J Comp Neurol. 2011 Accepted manuscript online: 23 December 2010 11:00AM EST DOI: 10.1002/cne.22580.
- Kudielka BM, Kirschbaum C. 2005. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 69 1: 113–32.
- Lanteri-Minet M, Isnardon P, de PJ, Menetrey D. 1993. Spinal and hindbrain structures involved in visceroception and visceronociception as revealed by the expression of Fos, Jun and Krox-24 proteins. Neuroscience. 55 3: 737–753.
- Lefkowitz RJ, Shenoy SK. 2005. Transduction of receptor signals by beta-arrestins. Science. 308 5721: 512–517.
- Legendre A, Papakonstantinou E, Roy MC, Richard D, Harris RB. 2007. Differences in response to corticotropin-releasing factor after short- and long-term consumption of a high-fat diet. Am J Physiol Regul Integr Comp Physiol. 293 3: R1076–R1085.
- Linden AM, Greene SJ, Bergeron M, Schoepp DD. 2004. Anxiolytic activity of the MGLU2/3 receptor agonist LY354740 on the elevated plus maze is associated with the suppression of stress-induced c-Fos in the hippocampus and increases in c-Fos induction in several other stress-sensitive brain regions. Neuropsychopharmacology. 29 3: 502–513.
- Linden AM, Baez M, Bergeron M, Schoepp DD. 2006. Effects of mGlu2 or mGlu3 receptor deletions on mGlu2/3 receptor agonist (LY354740)-induced brain c-Fos expression: Specific roles for mGlu2 in the amygdala and subcortical nuclei, and mGlu3 in the hippocampus. Neuropharmacology. 51 2: 213–228.
- Lowery EG, Thiele TE. 2010. Pre-clinical evidence that corticotropin-releasing factor (CRF) receptor antagonists are promising targets for pharmacological treatment of alcoholism. CNS Neurol Disord Drug Targets. 9 1: 77–86.
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. 2003. Immunolocalization of estrogen receptor beta in the mouse brain: Comparison with estrogen receptor alpha. Endocrinology. 144 5: 2055–2067.
- Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M. 1996. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: An immunohistochemical and in situ hybridization study. Neurosci Res. 26 3: 235–269.
- Nemeroff CB. 1988. The role of corticotropin-releasing factor in the pathogenesis of major depression. Pharmacopsychiatry. 21 2: 76–82.
- Neufeld-Cohen A, Evans AK, Getselter D, Spyroglou A, Hill A, Gil S, Tsoory M, Beuschlein F, Lowry CA, Vale W, Chen A. 2009. Urocortin-1 and -2 double-deficient mice show robust anxiolytic phenotype and modified serotonergic activity in anxiety circuits. Mol Psychiatry. 15 4: 426–41.
- Ohata H, Suzuki K, Oki Y, Shibasaki T. 2000. Urocortin in the ventromedial hypothalamic nucleus acts as an inhibitor of feeding behavior in rats. Brain Res. 861 1: 1–7.
- Palkovits M, Sebekova K, Gallatz K, Boor P, Sebekova KJr, Klassen A, Bahner U, Heidland A. 2009. Neuronal activation in the CNS during different forms of acute renal failure in rats. Neuroscience. 159 2: 862–882.
- Pan W, Kastin AJ. 2008. Urocortin and the brain. Prog Neurobiol. 84 2: 148–156.
- Pan W, Tu H, Hsuchou H, Daniel J, Kastin AJ. 2007. Unexpected amplification of leptin-induced Stat3 signaling by urocortin: Implications for obesity. J Mol Neurosci. 33 3: 232–238.
- Reul JM, Holsboer F. 2002. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol. 2 1: 23–33.
- Romeo RD, Karatsoreos IN, Jasnow AM, McEwen BS. 2007. Age- and stress-induced changes in corticotropin-releasing hormone mRNA expression in the paraventricular nucleus of the hypothalamus. Neuroendocrinology. 85 4: 199–206.
- Rotimi CN, Comuzzie AG, Lowe WL, Luke A, Blangero J, Cooper RS. 1999. The quantitative trait locus on chromosome 2 for serum leptin levels is confirmed in African–Americans. Diabetes. 48 3: 643–644.
- Ryabinin AE, Weitemier AZ. 2006. The urocortin 1 neurocircuit: Ethanol-sensitivity and potential involvement in alcohol consumption. Brain Res Brain Res Rev. 52 2: 368–380.
- Ryabinin AE, Tsivkovskaia NO, Ryabinin SA. 2005. Urocortin 1-containing neurons in the human Edinger–Westphal nucleus. Neuroscience. 134 4: 1317–1323.
- Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. 2009. Leptin targets in the mouse brain. J Comp Neurol. 514 5: 518–532.
- Selye H. 1951. The general-adaptation-syndrome. Annu Rev Med. 2:327–342.
- Skelton KH, Owens MJ, Nemeroff CB. 2000. The neurobiology of urocortin. Regul Pept. 93 1–3: 85–92.
- Skelton KH, Nemeroff CB, Owens MJ. 2004. Spontaneous withdrawal from the triazolobenzodiazepine alprazolam increases cortical corticotropin-releasing factor mRNA expression. J Neurosci. 24 42: 9303–9312.
- Spangler E, Cote DM, Anacker AM, Mark GP, Ryabinin AE. 2009. Differential sensitivity of the perioculomotor urocortin-containing neurons to ethanol, psychostimulants and stress in mice and rats. Neuroscience. 160 1: 115–125.
- Spina M, Merlo-Pich E, Chan RK, Basso AM, Rivier J, Vale W, Koob GF. 1996. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science. 273 5281: 1561–1564.
- Turek VF, Ryabinin AE. 2005. Expression of c-Fos in the mouse Edinger–Westphal nucleus following ethanol administration is not secondary to hypothermia or stress. Brain Res. 1063 2: 132–139.
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. 2000. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 428 2: 191–212.
- Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C. 1995. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 378 6554: 287–292.
- Vetter DE, Li C, Zhao L, Contarino A, Liberman MC, Smith GW, Marchuk Y, Koob GF, Heinemann SF, Vale W, Lee KF. 2002. Urocortin-deficient mice show hearing impairment and increased anxiety-like behavior. Nat Genet. 31 4: 363–369.
- Viau V, Sawchenko PE. 2002. Hypophysiotropic neurons of the paraventricular nucleus respond in spatially, temporally, and phenotypically differentiated manners to acute vs. repeated restraint stress: Rapid publication. J Comp Neurol. 445 4: 293–307.
- Vilpoux C, Warnault V, Pierrefiche O, Daoust M, Naassila M. 2009. Ethanol-sensitive brain regions in rat and mouse: A cartographic review, using immediate early gene expression. Alcohol Clin Exp Res. 33 6: 945–969.
- Wang L, Martinez V, Rivier JE, Tache Y. 2001. Peripheral urocortin inhibits gastric emptying and food intake in mice: Differential role of CRF receptor 2. Am J Physiol Regul Integr Comp Physiol. 281 5: R1401–R1410.
- Wang X, Su H, Copenhagen LD, Vaishnav S, Pieri F, Shope CD, Brownell WE, De BM, Paylor R, Bradley A. 2002. Urocortin-deficient mice display normal stress-induced anxiety behavior and autonomic control but an impaired acoustic startle response. Mol Cell Biol. 22 18: 6605–6610.
- Weitemier AZ, Ryabinin AE. 2005. Lesions of the Edinger–Westphal nucleus alter food and water consumption. Behav Neurosci. 119 5: 1235–1243.
- Weninger SC, Dunn AJ, Muglia LJ, Dikkes P, Miczek KA, Swiergiel AH, Berridge CW, Majzoub JA. 1999. Stress-induced behaviors require the corticotropin-releasing hormone (CRH) receptor, but not CRH. Proc Natl Acad Sci USA. 96 14: 8283–8288.
- Weninger SC, Peters LL, Majzoub JA. 2000. Urocortin expression in the Edinger–Westphal nucleus is up-regulated by stress and corticotropin-releasing hormone deficiency. Endocrinology. 141 1: 256–263.
- Xu L, Bloem B, Gaszner B, Roubos EW, Kozicz T. 2009. Sex-specific effects of fasting on urocortin 1, cocaine- and amphetamine-regulated transcript peptide and nesfatin-1 expression in the rat Edinger–Westphal nucleus. Neuroscience. 162 4: 1141–1149.
- Xu L, Bloem B, Gaszner B, Roubos E, Kozicz T. Stress-related changes in the activity of cocaine- and amphetamine-regulated transcript and nesfatin neurons in the midbrain non-preganglionic Edinger–Westphal nucleus in the rat. Neuroscience. 2010a; 170 2: 478–488.
- Xu L, Scheenen WJJM, Leshan RL, Patterson CM, Elias CF, Bouwhuis S, Roubos EW, Myers MJJ, Kozicz T. Evidence that leptin signaling modulates the activity of stress-sensitive urocortin 1 neurons in the non-preganglionic Edinger–Westphal nucleus in the mouse. Endocrinology. 2010b; 152 3: 979–988.
- Zalutskaya AA, Arai M, Bounoutas GS, Abou-Samra AB. 2007. Impaired adaptation to repeated restraint and decreased response to cold in urocortin knockout mice. Am J Physiol Endocrinol Metab. 293:E259–E263.
