Abstract
A suboptimal prenatal environment may induce permanent changes in cells, organs and physiology that alter social, emotional and cognitive functioning, and increase the risk of cardiometabolic and mental disorders in subsequent life (“developmental programming”). Although animal studies have provided a wealth of data on programming and its mechanisms, including on the role of stress and its glucocorticoid mediators, empirical evidence of these mechanisms in humans is still scanty. We review the existing human evidence on the effects of prenatal maternal stress, anxiety and depression, glucocorticoids and intake of liquorice (which inhibits the placental barrier to maternal glucocorticoids) on offspring developmental outcomes including, for instance, alterations in psychophysiological and neurocognitive functioning and mental health. This work lays the foundations for biomarker discovery and affords opportunities for prevention and interventions to ameliorate adverse outcomes in humans.
Introduction
Mounting epidemiological evidence suggests that smaller body size at birth and/or a shorter length of gestation increase the risk of poorer physical health later in life (Barker et al. Citation1989; Eriksson et al. Citation2006, Citation2007). The brain is particularly affected, manifesting as altered cognitive and affective functioning and as an increased risk of mental health disorders. These associations may not be linear, as children born at the upper end of the birth weight distribution may also be at a higher risk for poorer health later in life (Gunnell et al. Citation2003). These findings can be best understood within the framework of prenatal “programming” or developmental origins of health and disease (DOHaD; Barker Citation1998). According to the DOHaD paradigm, adverse environmental experiences during critical periods of early development can permanently alter or programme the structure and function of cells, organs and physiological systems. Recent research has shown that the influences of programming may not be limited to the prenatal period, but may extend across the entire human growth period from preconception to adulthood (Barker et al. Citation2005). The effects of programming are not limited to development in childhood, but are influential across the life span (Barker et al. Citation1989; Eriksson et al. Citation2006, Citation2007; Räikkönen et al. Citation2007, Citation2008a, Citation2009a).
Recently, much interest has been focused on whether developmental programming—as reflected in alterations in body size at birth and length of gestation—plays a role in determining individual differences in cognitive, social and emotional functioning, including characteristic traits of temperament and personality, in neuroendocrine and autonomic nervous system (ANS) functioning and in mental health. A series of studies have shown that smaller body size at birth and/or shorter length of gestation associate, for instance, with alterations in hypothalamic–pituitary–adrenocortical (HPA) axis and ANS functioning during daily living and under psychosocial stress (Reynolds et al. Citation2001; IJzerman et al. Citation2003; Wust et al. Citation2005; Jones et al. Citation2006, Citation2007; Phillips and Jones Citation2006; Feldt et al. Citation2007; Kajantie et al. Citation2007) and with increased risks of depression (Räikkönen et al. Citation2007, Citation2008b), suicide (Barker et al. Citation1995), schizophrenia (Wahlbeck et al. Citation2001), personality disorders (Lahti et al. Citation2010b), schizotypal personality traits (Lahti et al. Citation2009), hostility (Räikkönen et al. Citation2008a), behavioural symptoms of attention deficit/hyperactivity disorder (Lahti et al. Citation2006; Strang-Karlsson et al. Citation2008b; Heinonen et al. Citation2010), poorer cognitive functioning (Gale et al. Citation2004; Heinonen et al. Citation2008; Broekman et al. Citation2009; Räikkönen et al. Citation2009a) internalizing and externalizing behaviour problems (Schlotz et al. Citation2008), poorer sleep (Strang-Karlsson et al. Citation2008a; Pesonen et al. Citation2009) and negative and positive affectivity characteristics of temperament and personality (Pesonen et al. Citation2006, Citation2008a, Schlotz et al. Citation2008, Schmidt et al. Citation2008; Lahti et al. Citation2010a). These associations may not be linear as children born with higher birth weights may display higher levels of anxiety (Lahti et al. Citation2010a) and distress (Cheung Citation2002), and be at an increased risk for psychosis later in life (Gunnell et al. Citation2003). The extent to which the associations with higher birth weight reflect maternal obesity, poorer health and pregnancy disorders, such as maternal diabetes/glucose intolerance, is not clear. In addition to the original empirical studies, several papers exist that have reviewed the evidence linking smaller body size at birth and/or shorter length of gestation with psychological and psychophysiological functioning and mental health later in life (Shenkin et al. Citation2004; Gluckman et al. Citation2005, Citation2008, Citation2009; Seckl and Meaney Citation2006; Seckl and Holmes Citation2007; Hanson and Gluckman Citation2008; Kajantie Citation2008; Seckl Citation2008; Räikkönen and Pesonen Citation2009; Kajantie and Räikkönen Citation2010).
Despite the increasing evidence pointing to the importance of early life programming in inducing alterations in psychological and psychophysiological functioning and mental health, the mechanisms through which these prenatal influences operate remain largely unknown. Two major hypotheses have been advanced to explain the link between events in utero and the later risk of neuropsychiatric and cardiometabolic disorders: maternal malnutrition and foetal overexposure to glucocorticoid stress hormones (Barker Citation1991; Edwards et al. Citation1993). These notions have been extensively explored in preclinical studies. In a range of experimental species, maternal malnutrition (global undernutrition or selective protein deficiency) reduces birth weight and reliably leads to higher blood pressure, glucose levels, altered behaviour and HPA axis function in adult offspring (Warner and Ozanne Citation2010). Similarly, maternal stress or administration of glucocorticoids, such as dexamethasone or betamethasone that freely crosses the placenta, also reduces birth weight and produces a similar or identical phenotype in the adult offspring (Seckl and Holmes Citation2007). Similar effects occur in non-human primates exposed to glucocorticoids in the last half of gestation (de Vries et al. Citation2007). The two hypotheses may be linked. Thus, circulating levels of physiological glucocorticoids (cortisol and corticosterone) are much higher in the maternal than in the foetal blood. This gradient is ensured by a placental enzyme, 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), that catalyzes the rapid inactivation of glucocorticoids to their inert 11-keto forms (cortisone, 11-dehydrocorticosterone), thus forming a physiological “barrier” to maternal glucocorticoids. Liquorice contains compounds, such as glycyrrhizic acid and glycyrrhetinic acid, that potently inhibit 11β-HSDs. Maternal administration of these agents or the derived drug carbenoxolone (Lindsay et al. Citation1996a,Citationb) or genetic knock out of the enzyme (Holmes et al. Citation2006) reduces birth weight and generates similar programmed CNS and peripheral outcomes in the adult offspring. Moreover, maternal protein malnutrition selectively lowers placental 11β-HSD2 (Langley-Evans et al. Citation1996), affording a possible link between the proposed mechanisms. Maternal stress in rodents also downregulates placental 11β-HSD2 (Mairesse et al. Citation2007), perhaps delimited by genotype (Lucassen et al. Citation2009), suggesting a double hit of elevated maternal glucocorticoids and reduced placental barrier function.
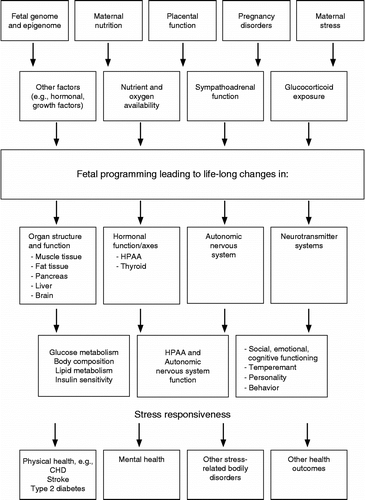
Despite this increasingly coherent body of evidence in model organisms, understanding the key mechanisms of programming in humans has been much less explored. outlines the mechanisms that are potentially involved. A series of human studies have demonstrated that prenatal environmental adversities, such as exposure to malnutrition (Roseboom et al. Citation2001), maternal pre-pregnancy overweight (Rodriguez Citation2010), pregnancy disorders, such as hypertension (Tuovinen et al. Citation2010, Citation2011), tobacco (Cornelius and Day Citation2009; Espy et al. Citation2011; Heinonen et al. Citation2011), cannabis (El Marroun et al. Citation2009) and alcohol exposure (Hellemans et al. Citation2010), have the potential to induce alterations in the offspring growth, birth anthropometry, development and adult functions. In this paper, we concentrate on reviewing the existing human evidence of programming induced by prenatal maternal stress, depression and anxiety, maternal glucocorticoid therapy and intake of liquorice during pregnancy.
Figure 1. Mechanisms involved in the offspring programming. (Modified from Räikkönen et al. Citation2008b).
Exposure to prenatal maternal stress, depression and anxiety: Overview of results of studies, examining offspring behaviour with questionnaires, standardized observations, neurocognitive tasks, EEG, MRI and fMRI
Prenatal maternal stress, depression and anxiety may exert effects on offspring development through multiple pathways. These pathways, which include alterations, for instance, in maternal lifestyle, physiological stress-regulatory mechanisms and placental function, are outlined in and described in more detail in previous reviews (O'Regan et al. Citation2001; Seckl Citation2001, Citation2008; Seckl and Meaney Citation2004b; Van den Bergh et al. Citation2005b; Seckl and Holmes Citation2007).
In humans, prospective follow-up studies have provided accumulating evidence for an association between prenatal exposure to high levels of maternal stress, anxiety and depression and altered neurodevelopment in the offspring at stages ranging from birth up to 20 years of age. Importantly, these associations persisted after controlling for postnatal maternal mood and/or other potentially important pre- and postnatal confounders, such as alcohol consumption and smoking, during pregnancy, birth weight and socio-economic status, and in some studies also use of medication, such as antidepressants, have been taken into account. Results of the studies reviewed here are described in . The studies are organized by the age of the offspring at the final follow-up assessment. In newborn babies, maternal prenatal stress was associated with neurodevelopmental alterations manifesting as less optimal scores on the Brazelton Neonatal Assessment Scale (Brouwers et al. Citation2001; Rieger et al. Citation2004; Hernandez-Reif et al. Citation2006) and on quantitative neurological examination (Lou et al. Citation1994). The newborn babies also displayed higher cardiac vagal tone, lower Apgar score (Ponirakis et al. Citation1998), less optimal behavioural states (Van den Bergh Citation1990, Citation1992) and higher cortisol levels 1 week postpartum (Diego et al. Citation2004). In the study of Harvison et al. (Citation2009), neonates of mothers scoring lower on perinatal anxiety displayed more negative frontal slow wave amplitudes in response to their mother's voice compared to a female stranger's voice, while neonates of mothers scoring higher on perinatal anxiety showed the opposite pattern. The latter results indicate that maternal anxiety may induce neurophysiologically based differences in attentional allocation, processes known to be crucial to an infant's ability to maintain homoeostasis.
Table I. Studies testing associations between prenatal maternal stress, anxiety and depression with offspring development.
Infants of mothers scoring higher on prenatal stress were rated by an observer as having poorer interactions with their mother (Field et al. Citation1985), being more irritable (DiPietro et al. Citation2008) and reactive (Davis et al. Citation2004, Citation2007), having problems with regulation of attention (Huizink et al. Citation2002, Citation2003) and having poorer language abilities and a lower IQ (Laplante et al. Citation2004, Citation2008). Their mothers rated them as having more sleeping, feeding and activity problems (Van den Bergh Citation1990, Citation1992), and being more irritable, difficult and showing more negative affectivity characteristics of temperament (Vaughn et al. Citation1987; Van den Bergh Citation1990, Citation1992; Huizink et al. Citation2002, Citation2003; Austin et al. Citation2005; Pesonen et al. Citation2005; McGrath et al. Citation2008; Henrichs et al. Citation2009) and crying more excessively (van der Wal et al. Citation2007). Scores on the Bayley Scales of Infant Development were worse at 8 and 24 months (Brouwers et al. Citation2001; Huizink et al. Citation2002, Citation2003; Laplante et al. Citation2004), but not in another study of 7-month-old infants (Van den Bergh Citation1990, Citation1992). Methodological differences, such as differences in sample size and variables that were used as covariates, may explain the conflicting findings between the studies using the Bayley Scales. Dawson and colleagues found that during mother–infant interaction, children of depressed mothers showed higher than normal heart rates and higher levels of cortisol, and reduced activity in brain regions that mediate positive approach behaviour. The authors indicate that there is suggestive evidence from their follow-up study that the postnatal experience with the mother may have had more effect on infant frontal EEG than on prenatal factors (Dawson and Ashman Citation2000; Dawson et al. Citation2001).
Pre-school children and children were rated by their mothers (Martin et al. Citation1999; O'Connor et al. Citation2002, Citation2003; Niederhofer and Reiter Citation2004; Van den Bergh and Marcoen Citation2004), teachers (Niederhofer and Reiter Citation2004; Rodriguez and Bohlin Citation2005), an external observer (Van den Bergh and Marcoen Citation2004) or themselves (Van den Bergh and Marcoen Citation2004) as showing poorer attention, hyperactivity, behavioural and emotional problems, and they were rated by their teacher as having lower school grades and problem behaviour (Niederhofer and Reiter Citation2004). O'Connor et al. (Citation2002) found that the effects of antenatal anxiety were stronger than the effects of antenatal depression. Finally, the results of Obel et al. (Citation2003)indicated that stressful life events in the mother during pregnancy increased the risk for ADHD problems in pre-adolescence between ages, 9–11 years.
Adolescents showed problems with cognitive control when performing computerized cognitive tasks measuring prefrontal cortex functioning and scored lower on intelligence subtests at the age of 14–15 and 17 years (Mennes et al. Citation2006; Van den Bergh et al. Citation2005a, Citation2006). Girls displayed higher levels of depressive symptoms (Van den Bergh et al. Citation2008). Mennes et al. (Citation2009) showed that 17-year-old children of mothers who were more anxious between 12 and 22 weeks of gestation displayed changes in event-related potentials (ERP) measured with EEG, when performing a gambling task, requiring endogenous control. Functional magnetic resonance (fMRI) measures revealed that in the 20-year-old offspring, differences related to the level of antenatal maternal anxiety were found in the activation patterns of several important prefrontal regions (Mennes Citation2008). Buss et al. (Citation2009) showed in a MRI study that high pregnancy-specific anxiety in mid gestation, but not later, is associated with decreased grey matter density in specific brain areas, in 8-year-old children.
Some evidence also suggests that the major life events inducing severe prenatal stress, such as death of a close relative during pregnancy, may be associated with stillbirth (Wisborg et al. Citation2008), low birth weight and smallness for gestational age status (Khashan et al. Citation2008b), cerebral palsy (Li et al. Citation2009b) and schizophrenia (Khashan et al. Citation2008a) in the offspring. One study has linked such events also with the risk of autism in the offspring (Kinney et al. Citation2008), but another study failed to replicate this association (Li et al. Citation2009a).
In humans, birth anthropometry associates with subsequent HPA axis function. Higher plasma and urinary glucocorticoid levels are found in children and adults who were of lower birth weight (Clark et al. Citation1996; Phillips et al. Citation1998). Cortisol responses to ACTH stimulation are exaggerated in those of low birth weight (Levitt et al. Citation2000; Reynolds et al. Citation2001, Citation2007). This occurs in myriad populations (Phillips et al. Citation2000; Kajantie and Räikkönen Citation2010) and precedes overt adult disease (Levitt et al. Citation2000). It is well known that psychological stress causes activation of the HPA axis with a consequent elevation in the levels of cortisol. Elevated levels of cortisol are also among the most consistently demonstrated biological abnormalities found in depression (Knorr et al. Citation2010), and in some contexts in anxiety (Roelofs et al. Citation2009). As maternal and foetal levels of cortisol are correlated (Gitau et al. Citation1998; O'Keane et al. Citation2011), the experience of stress and higher levels of depression and anxiety during pregnancy may directly result in higher foetal exposure to maternal cortisol. In a recent study, Sarkar et al. (Citation2008) demonstrated that the correlation between amniotic fluid cortisol (reflecting foetal exposure) and maternal plasma cortisol becomes stronger the higher with levels of maternal prenatal anxiety, while maternal prenatal anxiety correlated with higher plasma cortisol levels of the mother, it was not directly related to amniotic fluid cortisol (Glover et al. Citation2009).
As a consequence of this biology, a number of studies have explored the effects of maternal psychopathology on offspring HPA axis function, mainly observing the effects by comparing the offspring of high vs. low anxiety, stressed or depressed pregnant women (Glover et al. Citation2010). Prenatal maternal anxiety and depression are in general associated with raised cortisol in the offspring, but infants of mothers exposed to the trauma of 9/11 who themselves developed symptoms of posttraumatic stress disorder (PTSD) had lower cortisol levels, though this is a common finding in PTSD (Yehuda et al. Citation2005). One study has provided evidence that an altered cortisol diurnal profile in the offspring associated with prenatal maternal anxiety was also associated with an altered behavioural phenotype, i.e. with depressed mood (Van den Bergh et al. Citation2008). Differences have been reported throughout the lifespan from infancy, ages 1 week to 9 months (Diego et al. Citation2004; Yehuda et al. Citation2005; Brennan et al. Citation2008; Oberlander et al. Citation2008; Grant et al. Citation2009), 4–15 years of age (Gutteling et al. Citation2004, Citation2005; O'Connor et al. Citation2005; Huizink et al. Citation2008; Van den Bergh et al. Citation2008) and in adults (Entringer et al. Citation2009). These data suggest that, as in animal models, the HPA axis is a prime target for prenatal influences and programming.
The studies on prenatal maternal stress, anxiety and depression, even when controlling for postnatal maternal stress, anxiety and depression, cannot overrule a possibility that a shared genetic basis may underlie the associations with offspring neurodevelopmental outcomes. Most evidence pointing to a hereditary component comes from the studies of depression (Belmaker and Agam Citation2008). It is, however, unlikely that individual differences in offspring neurodevelopmental outcomes could be attributed to a single genetic, epigenetic or pre- or postnatal environmental factor. Multiple mechanisms do exist (see ), and these may act in concert. Further studies on the exact underlying mechanisms through which maternal stress, depression and anxiety induce programming of the offspring are clearly warranted.
Maternal glucocorticoid therapy
In humans, therapeutic treatment with synthetic glucocorticoids is common in women at risk of preterm delivery, serving to accelerate foetal lung maturation and thus reduce neonatal morbidity and mortality (Seckl and Meaney Citation2004a). The long-term effects of this treatment are not fully determined, although there are suggestions that foetal growth and subsequent development may be impaired (for a review see Bolt et al. Citation2001). Glucocorticoid treatment during pregnancy typically reduces birth weight (for a review see Sloboda et al. Citation2005), but long-term follow up studies are few. Antenatal glucocorticoid administration has been linked with higher blood pressure in adolescence (Doyle et al. Citation2000) and higher insulin levels in adulthood (Dalziel et al. Citation2005). Studies aimed at establishing the long-term neurological and developmental effects of antenatal glucocorticoid therapy are complicated by the frequency of neurological sequelae common in such children anyway. However, in a group of 6-year-old children, antenatal glucocorticoid exposure is associated with subtle effects on neurological function, including reduced visual closure and visual memory (MacArthur et al. Citation1982). Multiple doses of antenatal glucocorticoids given to women at risk of preterm delivery reduced birth weight and head circumference in the offspring (French et al. Citation1999). There are also effects on behaviour: three or more courses of glucocorticoids associate with an increased risk of externalizing behaviour problems, distractibility and inattention (Yeh et al. Citation2004), and children exposed to a longer (>24 h to delivery) relative to a shorter duration ( < 24 h to delivery) of a single, repeat dose of betamethasone were rated by their mothers as more impulsive at the age of 3 years (Pesonen et al. Citation2009a). Children exposed to dexamethasone in early pregnancy because of risk of congenital adrenal hyperplasia and born at term showed increased emotionality, unsociability, avoidance and behavioural problems (Trautman et al. Citation1995). Postnatally, dexamethasone in premature babies lowers later IQ and other higher brain functions (Yeh et al. Citation2004). Overall, whilst far from complete, evidence indicates that exposure of the foetus (or premature neonate) to excess glucocorticoids that readily cross the placental and foetal tissue barriers to physiological cortisol (because they are poor substrates for 11β-HSD2) may induce alterations in the developing CNS.
Maternal intake of liquorice
If glucocorticoid exposure underpins some developmental effects on the foetus, what does 11β-HSD2 do? Rare children homozygous for deleterious mutations of the HSD11B2 gene encoding 11β-HSD2 are of substantially lower birth weight than their siblings, many of whom will be heterozygous for the mutations (Dave-Sharma et al. Citation1998). In humans, as in rodents (Benediktsson et al. Citation1993), placental 11β-HSD2 activity correlates with birth weight in some (Stewart et al. Citation1995), but not all studies (Rogerson et al. Citation1997). However, such correlations are weak and mechanisms can only be inferred.
A handful of recent studies have focused on prenatal maternal intake of liquorice confectionery as a natural experimental platform to address mechanisms of programming. These have been conducted in Finland where consumption of large quantities of liquorice confectionery is common among young women: nearly half of the pregnant women consume at least some liquorice (Räikkönen et al. Citation2009b; Strandberg et al. Citation2001). Glycyrrhizin (3β-d-diglucuronyl-18β-glycyrrhetinic or -glycyrrhizic acid) is a natural constituent of liquorice. Its hemisuccinate synthetic analogue, carbenoxolone, was a clinical drug formerly used to treat peptic ulcers. Its water solubility makes it attractive for use in preclinical and human studies (Welberg et al. Citation2000). These agents are potent (low nanomolar Ki) inhibitors of 11β-HSD2. Though they also potently inhibit 11β-HSD type 1, this is little expressed in the foetus at least in rodents (Speirs et al. Citation2004). In normal circumstances, this placental enzyme metabolizes up to 80–90% of maternal active cortisol to inactive cortisone, a function inhibited by carbenoxolone, at least in intact placenta ex vivo (Benediktsson et al. Citation1997).
In 1998, a project was initiated to test whether varying levels of maternal consumption of glycyrrhizin in liquorice during pregnancy was associated with body size at birth and length of gestation. In over 1000 pregnant women, prenatal exposure to high (>500 mg/wk) compared to zero-low (0–249 mg/wk) or moderate (250–499 mg/wk) glycyrrhizin in liquorice was associated with a slightly shorter duration of gestation (Strandberg et al. Citation2001). Glycyrrhizin intake during pregnancy was not significantly associated with birth anthropometry. The findings on shorter length of gestation were replicated in another Finnish cohort: high intake of glycyrrhizin during pregnancy was associated with over a twofold increased risk in the rate of preterm delivery (Strandberg et al. Citation2002).
In a follow-up study, the long-term effects of maternal intake of liquorice during gestation on 8-year-old offspring psychological development, mental health and psychophysiological functioning were determined. In comparison to the group whose mothers had zero-low glycyrrhizin exposure, those with high exposure scored significantly lower on verbal and visuo-spatial abilities and in narrative memory. In addition, children in the high-exposure group had significant 2.4–3.0-fold increased risk of externalizing symptoms, attention, rule breaking, aggression problems and DSM IV-based symptoms of obsessive defiant disorder (Räikkönen et al. Citation2009b). The effects on cognitive performance appeared dose-related. None of the associations were affected by birth weight or duration of gestation and other pre- and perinatal, maternal and child characteristics implicated as risks for pregnancy and/or cognitive and psychiatric outcomes.
The HPA axis of these children was also affected. Children of mothers consuming high levels of glycyrrhizin had a higher salivary cortisol peak and area under the curve upon awakening and higher overall salivary cortisol throughout a Trier Social Stress Test for Children (TSST-C; Räikkönen et al. Citation2010c). The associations with salivary cortisol peak and salivary cortisol baseline during the TSST-C were dose-related. As far as could be ascertained, the results were not due to confounding factors such as maternal health during pregnancy, including blood pressure levels, obesity and pregnancy disorders, birth anthropometry and length of gestation, maternal social class at birth and in a follow-up at the child's age of 8 years, maternal smoking and alcohol consumption during pregnancy, and child characteristics, such as sex, growth in height, head circumference and difficulties in cognitive functioning that might interfere with cognitive performance, associate with psychiatric symptomatology, and alter performance in the TSST-C. We have previously reported, in this same sample of 8-year-old children, that shorter sleep duration and lower sleep efficiency (percent time spent asleep whilst in bed) are associated with alterations in the diurnal salivary cortisol pattern and in salivary cortisol responses during the TSST-C (Räikkönen et al. Citation2010b). Poorer sleep may thus introduce another factor that may confound the associations between liquorice consumption and HPA axis activity. Maternal intake of liquorice during pregnancy is, however, not associated with the children's sleep patterns (Räikkönen, unpublished data). Thus, when we further controlled the associations of maternal liquorice intake during pregnancy and their children's HPA axis function for the children's sleep duration and sleep efficiency, the associations remained identical.
These findings suggest that high maternal liquorice consumption during pregnancy may exert deleterious effects upon cognitive and psychiatric outcomes and HPAA functioning in children paralleling the effects seen in preclinical models. Whilst it remains to be ascertained whether the dose of liquorice consumed affects placental 11β-HSD2 function appreciably in vivo (mice lacking one copy of the gene encoding 11β-HSD2 have intermediate reductions in birth weight), it is plausible to suggest that this may occur and that placental glucocorticoid “leakiness” may have an impact on foetal brain development during periods critical for affective and cognitive development with persisting impacts. If humans resemble rodents, then maternal stress-related disorders may further lower placental glucocorticoid barrier function.
Conclusion
This review shows, first, that accumulating evidence exists suggesting that maternal negative emotions during pregnancy are linked with the long-term behaviour and physiology of her child, even after controlling for relevant covariates. The range of maternal “stressors” that predict the child outcomes is quite wide. Moreover, a wide range of different outcomes have been found to be affected by these maternal stressors (Glover et al. Citation2010). This should come as no surprise. In terms of mechanisms, we are far from understanding how and when the early hormonal environment may affect the refinement of neural circuits in specific brain layers and areas which will later determine the way in which sensory-cognitive, motor, arousal and emotional structure–function relationships are affected (Van den Bergh et al. Citation2005a; Fox et al. Citation2010).
Second, this review shows that there are links between prenatal maternal intake of glucocorticoids and of liquorice confectionery and long-term behaviour and physiology of her child, even after controlling for relevant covariates. These findings may not generalize merely to intake of liquorice confectionery.
Because of its sweetening (50–200 times sweeter than refined sugar) and flavouring capacity, glycyrrhizin is also used as a natural sweetener and is also found in other foodstuffs, including some candies and chewing gum, herbal teas, alcoholic and non-alcoholic drinks, tobacco and some traditional (e.g. cough medicine) as well as some herbal medicine (to treat stomach ulcers, sore throat and viral infections). Hence, not surprisingly, according to some estimates the daily consumption levels of glycyrrhizin range from 1.6 to 215.2 mg (Isbrucker and Burdock Citation2006). This estimate was derived from the USA where consumption of liquorice confectionery is not popular. Glycyrrhizin is generally recognized as safe for use in foods, though the European Community's Scientific Committee on Food and the FAO/WHO Expert Committee (http://ec.europa.eu/food/fs/sc/scf/out186_en.pdf) have considered that a consumption of 100 mg/day may be a reasonable upper limit for the majority of the population, and the FDA recommends that if glycyrrhizin-containing foods are not consumed in excess or by sensitive individuals, these foods do not pose a health hazard (http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr = 184.1408). The findings reviewed here suggest that consumption of large quantities of glycyrrhizin in foodstuffs may alter offspring's brain development and subsequent function, and therefore, it may be prudent to inform pregnant women of the potential hazards of eating large quantities of liquorice.
Future studies should try to increase knowledge about mechanisms underlying the effect of prenatal influences on social-emotional, cognitive and behavioural problems, particularly about whether prenatal stress and gene-environment interactions affecting the HPA-axis play a role in early developmental processes. It has become increasingly clear that epigenetic changes, including DNA methylation and histone modifications, are causally involved in a range of developmental processes by affecting transcriptional activity. For the future, it is important that these epigenetic processes, that take place also in the placenta, are integrated in the studies of prenatal stress. They provide “a physical basis for the influence of the perinatal environmental signals over the life of the individual” (Meaney Citation2010). Moreover, the eventual moderating influence of other environmental factors, such as the postnatal caregiving environment, should also be studied. There is evidence that severe early life stress may carry consequences on a wide range of developmental outcomes, including physical and mental health, psychophysiological functioning and cognitive abilities and attained social class in adulthood (Pesonen et al. Citation2007, Citation2008b, Citation2010, Citation2011; Alastalo et al. Citation2009; Räikkönen et al. Citation2011). Whether the postnatal environmental influences buffer or add to the prenatal influences is little studied. Results of these research efforts would open the way for the development of targeted pre- and perinatal prevention and intervention strategies that could reduce the risk that prenatal environmental adversities carry for early functioning and later mental health of children, and have significant consequences for the long-term health and well-being of the children.
Declaration of interest: This work was sponsored by the grants from the European Science Foundation, Stress and Mental Health programme (EuroSTRESS), the Finnish Academy, the Medical Research Council (UK), The Netherlands Organisation for Scientific Research (NWO). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alastalo H, Räikkönen K, Pesonen A-K, Osmond C, Barker DJ, Kajantie E, Heinonen K, Forsen TJ, Eriksson JG. 2009. Cardiovascular health of Finnish war evacuees 60 years later. Ann Med. 41:66–72.
- Austin MP, Hadzi-Pavlovic D, Leader L, Saint K, Parker G. 2005. Maternal trait anxiety, depression and life event stress in pregnancy: Relationships with infant temperament. Early Hum Dev. 81:183–190.
- Barker DJ. 1991. The intrauterine environment and adult cardiovascular disease. Ciba Found Symp. 156:3–10 discussion 10–6.
- Barker DJ. 1998. In utero programming of chronic disease. Clin Sci (Lond). 95:115–128.
- Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. 1989. Weight in infancy and death from ischaemic heart disease. Lancet. 2:577–580.
- Barker DJ, Osmond C, Rodin I, Fall CH, Winter PD. 1995. Low weight gain in infancy and suicide in adult life. Br Med J. 311:1203.
- Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. 2005. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 353:1802–1809.
- Belmaker RH, Agam G. 2008. Major depressive disorder. N Engl J Med. 358:55–68.
- Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CR. 1993. Glucocorticoid exposure in utero: New model for adult hypertension. Lancet. 341:339–341.
- Benediktsson R, Calder AA, Edwards CR, Seckl JR. 1997. Placental 11 beta-hydroxysteroid dehydrogenase: A key regulator of fetal glucocorticoid exposure. Clin Endocrinol (Oxf). 46:161–166.
- Bergman K, Sarkar P, O'Connor TG, Modi N, Glover V. 2007. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. J Am Acad Child Adolesc Psychiatry. 46:1454–1463.
- Bolt RJ, van Weissenbruch MM, Lafeber HN, Delemarre-van de Waal HA. 2001. Glucocorticoids and lung development in the fetus and preterm infant. Pediatr Pulmonol. 32:76–91.
- Brand SR, Engel SM, Canfield RL, Yehuda R. 2006. The effect of maternal PTSD following in utero trauma exposure on behavior and temperament in the 9-month-old infant. Ann N Y Acad Sci. 1071:454–458.
- Brennan PA, Pargas R, Walker EF, Green P, Newport DJ, Stowe Z. 2008. Maternal depression and infant cortisol: Influences of timing, comorbidity and treatment. J Child Psychol Psychiatry. 49:1099–1107.
- Broekman BF, Chan YH, Chong YS, Quek SC, Fung D, Low YL, Ooi YP, Gluckman PD, Meaney MJ, Wong TY, Saw SM. 2009. The influence of birth size on intelligence in healthy children. Pediatrics. 123:e1011–e1016.
- Brouwers EPM, van Baar AL, Pop VJM. 2001. Maternal anxiety during pregnancy and subsequent infant development. Inf Behav Dev. 24:95–106.
- Buss C, Davis EP, Muftuler LT, Head K, Sandman CA. 2009. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9-year-old children. Psychoneuroendocrinology. 35:141–153.
- Cheung YB. 2002. Early origins and adult correlates of psychosomatic distress. Soc Sci Med. 55:937–948.
- Clark PM, Hindmarsh PC, Shiell AW, Law CM, Honour JW, Barker DJP. 1996. Size at birth and adrenocortical function in childhood. Clin Endocrinol. 45:721–726.
- Cornelius MD, Day NL. 2009. Developmental consequences of prenatal tobacco exposure. Curr Opin Neurol. 22:121–125.
- Dalziel SR, Walker NK, Parag V, Mantell C, Rea HH, Rodgers A, Harding JE. 2005. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 365:1856–1862.
- Dave-Sharma S, Wilson RC, Harbison MD, Newfield R, Azar MR, Krozowski ZS, Funder JW, Shackleton CH, Bradlow HL, Wei JQ, Hertecant J, Moran A, Neiberger RE, Balfe JW, Fattah A, Daneman D, Akkurt HI, De Santis C, New MI. 1998. Examination of genotype and phenotype relationships in 14 patients with apparent mineralocorticoid excess. J Clin Endocrinol Metab. 83:2244–2254.
- Davis EP, Snidman N, Wadhwa PD, Glynn LM, Schetter CD, Sandman CA. 2004. Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy. 6:319–331.
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA. 2007. Prenatal exposure to maternal depression and cortisol influences infant temperament. J Am Acad Child Adolesc Psychiatry. 46:737–746.
- Dawson G, Ashman SB. 2000. On the origins of a vulnerability to depression: The influence of the early social environment on the development of psychobiological systems related to risk for affective disorder. Mahwah, NJ: Erlbaum.
- Dawson G, Ashman SB, Hessl D, Spieker S, Frey K, Panagiotides H, Embry L. 2001. Autonomic and brain electrical activity in securely- and insecurely-attached infants of depressed mothers. Inf Behav Dev. 24:135–149.
- de Bruijn ATCE, van Bakel HJA, van Baar AL. Sex differences in the relation between prenatal maternal emotional complaints and child outcome. Early Hum Dev. 2009a; 85:319–324.
- de Bruijn ATCE, van Bakel HJA, Wijnen H, Pop VJM, van Baar AL. Prenatal maternal emotional complaints are associated with cortisol responses in toddler and preschool aged girls. Dev Psychobiol. 2009b; 51:553–563.
- de Vries A, Holmes MC, Heijnis A, Seier JV, Heerden J, Louw J, Wolfe-Coote S, Meaney MJ, Levitt NS, Seckl JR. 2007. Prenatal dexamethasone exposure induces changes in nonhuman primate offspring cardiometabolic and hypothalamic–pituitary–adrenal axis function. J Clin Invest. 117:1058–1067.
- Diego MA, Field T, Hernandez-Reif M, Cullen C, Schanberg S, Kuhn C. 2004. Prepartum, postpartum, and chronic depression effects on newborns. Psychiatry. 67:63–80.
- DiPietro JA, Ghera MM, Costigan KA. 2008. Prenatal origins of temperamental reactivity in early infancy. Early Hum Dev. 84:569–575.
- Doyle LW, Ford GW, Davis NM, Callanan C. 2000. Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin Sci. 98:137–142.
- Edwards CR, Benediktsson R, Lindsay RS, Seckl JR. 1993. Dysfunction of placental glucocorticoid barrier: Link between fetal environment and adult hypertension?. Lancet. 341:355–357.
- El Marroun H, Tiemeier H, Steegers EA, Jaddoe VW, Hofman A, Verhulst FC, van den Brink W, Huizink AC. 2009. Intrauterine cannabis exposure affects fetal growth trajectories: The generation R study. J Am Acad Child Adolesc Psychiatry. 48 12: 1173–1181.
- Entringer S, Kumsta R, Hellhammer DH, Wadhwa PD, Wust S. 2009. Prenatal exposure to maternal psychosocial stress and HPA axis regulation in young adults. Horm Behav. 55:292–298.
- Eriksson JG, Osmond C, Kajantie E, Forsen TJ, Barker DJ. 2006. Patterns of growth among children who later develop type 2 diabetes or its risk factors. Diabetologia. 49:2853–2858.
- Eriksson JG, Forsén TJ, Kajantie E, Osmond C, Barker DJ. 2007. Childhood growth and hypertension in later life. Hypertension. 49:1415–1421.
- Espy KA, Fang H, Johnson C, Stopp C, Wiebe SA, Respass J. 2011. Prenatal tobacco exposure: Developmental outcomes in the neonatal period. Dev Psychol. 47:153–156.
- Feldt K, Räikkönen K, Eriksson JG, Andersson S, Osmond C, Barker DJ, Phillips DI, Kajantie E. 2007. Cardiovascular reactivity to psychological stressors in late adulthood is predicted by gestational age at birth. J Hum Hypertens. 21:401–410.
- Field T, Sandberg D, Garcia R, Vega-Lahr N, Goldstein S, Guy L. 1985. Pregnancy problems, postpartum depression, and early mother–infant interactions. Dev Psychol. 21:1152–1156.
- Fox SE, Levitt P, Nelson CA3rd. 2010. How the timing and quality of early experiences influence the development of brain architecture. Child Dev. 81:28–40.
- French NP, Hagan R, Evans SF, Godfrey M, Newnham JP. 1999. Repeated antenatal corticosteroids: Size at birth and subsequent development. Am J Obstet Gynecol. 180:114–121.
- Gale CR, O'Callaghan FJ, Godfrey KM, Law CM, Martyn CN. 2004. Critical periods of brain growth and cognitive function in children. Brain. 127:321–329.
- Gitau R, Cameron A, Fisk NM, Glover V. 1998. Fetal exposure to maternal cortisol. Lancet. 352:707–708.
- Glover V, Bergman K, Sarkar P, O'Connor TG. 2009. Association between maternal and amniotic fluid cortisol is moderated by maternal anxiety. Psychoneuroendocrinology. 34:430–435.
- Glover V, O'Connor TG, O'Donnell K. 2010. Prenatal stress and the programming of the HPA axis. Neurosci Biobehav Rev. 35:17–22.
- Gluckman PD, Hanson MA, Pinal C. 2005. The developmental origins of adult disease. Matern Child Nutr. 1:130–141.
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. 2008. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 359:61–73.
- Gluckman PD, Hanson MA, Bateson P, Beedle AS, Law CM, Bhutta ZA, Bhutta ZA, Anokhin KV, Bougnères P, Chandak GR, Dasgupta P, Smith GD, Ellison PT, Forrester TE, Gilbert SF, Jablonka E, Kaplan H, Prentice AM, Simpson SJ, Uauy R, West-Eberhard MJ. 2009. Towards a new developmental synthesis: Adaptive developmental plasticity and human disease. Lancet. 373:1654–1657.
- Grant KA, McMahon C, Austin MP, Reilly N, Leader L, Ali S. 2009. Maternal prenatal anxiety, postnatal caregiving and infants' cortisol responses to the still-face procedure. Dev Psychobiol. 51:625–637.
- Gunnell D, Rasmussen F, Fouskakis D, Tynelius P, Harrison G. 2003. Patterns of fetal and childhood growth and the development of psychosis in young males: A cohort study. Am J Epidemiol. 158:291–300.
- Gutteling BM, de Weerth C, Buitelaar JK. 2004. Maternal prenatal stress and 4–6 year old children's salivary cortisol concentrations pre- and post-vaccination. Stress. 7:257–260.
- Gutteling BM, de Weerth C, Buitelaar JK. 2005. Prenatal stress and children's cortisol reaction to the first day of school. Psychoneuroendocrinology. 30:541–549.
- Gutteling BM, de Weerth C, Willemsen-Swinkels SH, Huizink AC, Mulder EJ, Visser GH, Buitelaar JK. The effects of prenatal stress on temperament and problem behavior of 27-month-old toddlers. Eur Child Adolesc Psychiatry. 2005b; 14:41–51.
- Gutteling BM, de Weerth C, Zandbelt N, Mulder EJ, Visser GH, Buitelaar JK. 2006. Does maternal prenatal stress adversely affect the child's learning and memory at age six?. J Abnorm Child Psychol. 34:789–798.
- Hanson MA, Gluckman PD. 2008. Developmental origins of health and disease: New insights. Basic Clin Pharmacol Toxicol. 102:90–93.
- Harvison KW, Molfese DL, Woodruff-Borden J, Weigel RA. 2009. Neonatal auditory evoked responses are related to perinatal maternal anxiety. Brain Cogn. 71:369–374.
- Heinonen K, Räikkönen K, Pesonen AK, Kajantie E, Andersson S, Eriksson JG, Niemelä A, Vartia T, Peltola J, Lano A. 2008. Prenatal and postnatal growth and cognitive abilities at 56 months of age: A longitudinal study of infants born at term. Pediatrics. 121:e1325–e1333.
- Heinonen K, Räikkönen K, Pesonen AK, Andersson S, Kajantie E, Eriksson JG, Wolke D, Lano A. 2010. Behavioural symptoms of attention deficit/hyperactivity disorder in preterm and term children born small and appropriate for gestational age: A longitudinal study. BMC Pediatr. 15:10–91.
- Heinonen K, Räikkönen K, Pesonen AK, Andersson S, Kajantie E, Eriksson JG, Wolke D, Lano A. 2011. Longitudinal study of smoking cessation before pregnancy and children's cognitive abilities at 56 months of age. Early Hum Dev. 87 5: 353–359.
- Hellemans KG, Sliwowska JH, Verma P, Weinberg J. 2010. Prenatal alcohol exposure: Fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev. 34:791–807.
- Henrichs J, Schenk JJ, Schimdt HG, Velders FP, Hofman A, Jaddoe VWV, Verhulst FC, Tiemeier H. 2009. Maternal pre- and postnatal anxiety and infant temperament. The generation R study. Inf Child Dev. 18:556–572.
- Hernandez-Martinez C, Arija V, Balaguer A, Cavalle P, Canals J. 2008. Do the emotional states of pregnant women affect neonatal behaviour?. Early Hum Dev. 84:745–750.
- Hernandez-Reif M, Field T, Diego M, Ruddock M. 2006. Greater arousal and less attentiveness to face/voice stimuli by neonates of depressed mothers on the Brazelton Neonatal Behavioral Assessment Scale. Inf Child Dev. 29:594–598.
- Holmes MC, Abrahamsen CT, French KL, Paterson JM, Mullins JJ, Seckl JR. 2006. The mother or the fetus? 11beta-hydroxysteroid dehydrogenase type 2 null mice provide evidence for direct fetal programming of behavior by endogenous glucocorticoids. J Neurosci. 26:3840–3844.
- Huizink AC, de Medina PG, Mulder EJ, Visser GH, Buitelaar JK. 2002. Psychological measures of prenatal stress as predictors of infant temperament. J Am Acad Child Adolesc Psychiatry. 41:1078–1085.
- Huizink AC, Robles de Medina P, Mulder EJ, Visser GH, Buitelaar JK. 2003. Stress during pregnancy is associated with developmental outcome in infancy. J Child Psychol Psychiatry. 44:810–818.
- Huizink AC, Bartels M, Rose RJ, Pulkkinen L, Eriksson CJ, Kaprio J. 2008. Chernobyl exposure as stressor during pregnancy and hormone levels in adolescent offspring. J Epidemiol Community Health. 62:e5.
- IJzerman R, Stehouwer CD, de Geus EJ, van Weissenbruch MM, Delemarre-van de Waal HA, Boomsma DI. 2003. Low birth weight is associated with increased sympathetic activity: Dependence on genetic factors. Circulation. 108:566–571.
- Isbrucker RA, Burdock GA. 2006. Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul Toxicol Pharmacol. 46:167–192.
- Jones A, Godfrey KM, Wood P, Osmond C, Goulden P, Phillips DI. 2006. Fetal growth and the adrenocortical response to psychological stress. J Clin Endocrinol Metab. 91:1868–1871.
- Jones A, Beda A, Ward AM, Osmond C, Phillips DI, Moore VM, Simpson DM. 2007. Size at birth and autonomic function during psychological stress. Hypertension. 49:548–555.
- Kajantie E. 2008. Early-life events. Effects on aging. Hormones (Athens). 7:101–113.
- Kajantie E, Räikkönen K. 2010. Early life predictors of the physiological stress response later in life. Neurosci Biobehav Rev. 35:23–32.
- Kajantie E, Feldt K, Räikkönen K, Phillips DI, Osmond C, Heinonen K, Pesonen AK, Andersson S, Barker DJ, Eriksson JG. 2007. Body size at birth predicts hypothalamic–pituitary–adrenal axis response to psychosocial stress at age 60 to 70 years. J Clin Endocrinol Metab. 92:4094–4100.
- Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, Kenny LC, Mortensen PB. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry. 2008a; 65:146–152.
- Khashan AS, McNamee R, Abel KM, Pedersen MG, Webb RT, Kenny LC, Mortensen PB, Baker PN. Reduced infant birthweight consequent upon maternal exposure to severe life events. Psychosom Med. 2008b; 70:688–694.
- Kinney DK, Miller AM, Crowley DJ, Huang E, Gerber E. 2008. Autism prevalence following prenatal exposure to hurricanes and tropical storms in Louisiana. J Autism Dev Disord. 38:481–488.
- Knorr U, Vinberg M, Kessing LV, Wetterslev J. 2010. Salivary cortisol in depressed patients versus control persons: A systematic review and meta-analysis. Psychoneuroendocrinology. 35:1275–1286.
- Lahti J, Räikkönen K, Kajantie E, Heinonen K, Pesonen AK, Järvenpää AL, Strandberg T. 2006. Small body size at birth and behavioural symptoms of ADHD in children aged five to six years. J Child Psychol Psychiatry. 47:1167–1174.
- Lahti J, Räikkönen K, Sovio U, Miettunen J, Hartikainen AL, Pouta A, Taanila A, Joukamaa M, Järvelin MR, Veijola J. 2009. Early-life origins of schizotypal traits in adulthood. Br J Psychiatry. 195:132–137.
- Lahti J, Räikkönen K, Pesonen AK, Heinonen K, Kajantie E, Forsén T, Osmond C, Barker DJ, Eriksson JG. Prenatal growth, postnatal growth, and trait anxiety in late adulthood—the Helsinki Birth Cohort Study. Acta Psychiatr Scand. 2010a; 121:227–235.
- Lahti M, Räikkönen K, Wahlbeck K, Heinonen K, Forsen T, Kajantie E, Pesonen AK, Osmond C, Barker DJ, Eriksson JG. Prenatal origins of hospitalization for personality disorders: The Helsinki birth cohort study. Psychiatry Res. 2010b; 179:226–230.
- Langley-Evans SC, Phillips GJ, Benediktsson R, Gardner DS, Edwards CR, Jackson AA, Seckl JR. 1996. Protein intake in pregnancy, placental glucocorticoid metabolism and the programming of hypertension in the rat. Placenta. 17:169–172.
- Laplante DP, Barr RG, Brunet A, Galbaud du Fort G, Meaney ML, Saucier JF, Zelazo PR, King S. 2004. Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatr Res. 56:400–410.
- Laplante DP, Brunet A, Schmitz N, Ciampi A, King S. 2008. Project Ice Storm: Prenatal maternal stress affects cognitive and linguistic functioning in 5 1/2-year-old children. J Am Acad Child Adolesc Psychiatry. 47:1063–1072.
- Levitt NS, Lambert EV, Woods D, Hales CN, Andrew R, Seckl JR. 2000. Impaired glucose tolerance and elevated blood pressure in low birth weight, nonobese, young south african adults: Early programming of cortisol axis. J Clin Endocrinol Metab. 85:4611–4618.
- Li J, Vestergaard M, Obel C, Christensen J, Precht DH, Lu M, Olsen J. A nationwide study on the risk of autism after prenatal stress exposure to maternal bereavement. Pediatrics. 2009a; 123:1102–1107.
- Li J, Vestergaard M, Obel C, Precht DH, Christensen J, Lu M, Olsen J. Prenatal stress and cerebral palsy: A nationwide cohort study in Denmark. Psychosom Med. 2009b; 71:615–618.
- Lindsay RS, Lindsay RM, Edwards CR, Seckl JR. Inhibition of 11-beta-hydroxysteroid dehydrogenase in pregnant rats and the programming of blood pressure in the offspring. Hypertension. 1996a; 27:1200–1204.
- Lindsay RS, Lindsay RM, Waddell BJ, Seckl JR. Prenatal glucocorticoid exposure leads to offspring hyperglycaemia in the rat: Studies with the 11 beta-hydroxysteroid dehydrogenase inhibitor carbenoxolone. Diabetologia. 1996b; 39:1299–1305.
- Lou HC, Hansen D, Nordentoft M, Pryds O, Jensen F, Nim J, Hemmingsen R. 1994. Prenatal stressors of human life affect fetal brain development. Dev Med Child Neurol. 36:826–832.
- Lucassen PJ, Bosch OJ, Jousma E, Kromer SA, Andrew R, Seckl JR, Neumann ID. 2009. Prenatal stress reduces postnatal neurogenesis in rats selectively bred for high, but not low, anxiety: Possible key role of placental 11beta-hydroxysteroid dehydrogenase type 2. Eur J Neurosci. 29:97–103.
- MacArthur BA, Howie RN, Dezoete JA, Elkins J. 1982. School progress and cognitive development of 6-year-old children whose mothers were treated antenatally with betamethasone. Pediatrics. 70:99–105.
- Mairesse J, Lesage J, Breton C, Breant B, Hahn T, Darnaudery M, Dickson SL, Seckl J, Blondeau B, Vieau D, Maccari S, Viltart O. 2007. Maternal stress alters endocrine function of the feto-placental unit in rats. Am J Physiol Endocrinol Metab. 292:E1526–E1533.
- Martin RP, Noyes J, Wisenbaker J, Huttunen M. 1999. Prediction of early childhood negative emotionality and inhibition from maternal distress during pregnancy. Merrill-Palmer Q. 45:370–391.
- McGrath JM, Records K, Rice M. 2008. Maternal depression and infant temperament characteristics. Infant Behav Dev. 31:71–80.
- Meaney MJ. 2010. Epigenetics and the biological definition of gene × environment interactions. Child Dev. 81:41–79.
- Mennes M. 2008. Longitudinal study on the effects of maternal anxiety during pregnancy: Neuropsychological and neurophysiological examination of cognitive control in the adolescent offspring. Leuven: Katholieke Universiteit Leuven.
- Mennes M, Stiers P, Lagae L, Van den Bergh BR. 2006. Long-term cognitive sequelae of antenatal maternal anxiety: Involvement of the orbitofrontal cortex. Neurosci Biobehav Rev. 30:1078–1086.
- Mennes M, Van den Bergh BR, Lagae L, Stiers P. 2009. Developmental brain alterations in 17 year old boys are related to antenatal maternal anxiety. Clin Neurophysiol. 120:1116–1122.
- Niederhofer H, Reiter A. 2004. Prenatal maternal stress, prenatal fetal movements and perinatal temperament factors influence behavior and school marks at the age of 6 years. Fetal Diagn Ther. 19:160–162.
- Obel C. 2003. Epidemiological studies of stress during pregnancy and fetal brain development. Denmark: University of Aarhus.
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. 2008. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 3:97–106.
- O'Connor TG, Heron J, Golding J, Beveridge M, Glover V. 2002. Maternal antenatal anxiety and children's behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry. 180:502–508.
- O'Connor TG, Heron J, Golding J, Glover V. 2003. Maternal antenatal anxiety and behavioural/emotional problems in children: A test of a programming hypothesis. J Child Psychol Psychiatry. 44:1025–1036.
- O'Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. 2005. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biol Psychiatry. 58:211–217.
- O'Keane V, Lightman S, Marsh M, Pawlby S, Papadopoulos AS, Taylor A, Moore R, Patrick K. 2011. Increased pituitary–adrenal activation and shortened gestation in a sample of depressed pregnant women: A pilot study. J Affect Disord. 130:300–305.
- O'Regan D, Welberg LL, Holmes MC, Seckl JR. 2001. Glucocorticoid programming of pituitary–adrenal function: Mechanisms and physiological consequences. Semin Neonatol. 6:319–329.
- Pesonen AK, Räikkönen K, Strandberg TE, Järvenpää AL. 2005. Continuity of maternal stress from the pre- to the postnatal period: Associations with infant's positive, negative and overall temperamental reactivity. Inf Behav Devel. 28:36–47.
- Pesonen AK, Räikkönen K, Kajantie E, Heinonen K, Strandberg TE, Järvenpää AL. 2006. Fetal programming of temperamental negative affectivity among children born healthy at term. Dev Psychobiol. 48:633–643.
- Pesonen AK, Räikkönen K, Heinonen K, Kajantie E, Forsen T, Eriksson JG. 2007. Depressive symptoms in adults separated from their parents as children: A natural experiment during World War II. Am J Epidemiol. 166:1126–1133.
- Pesonen AK, Räikkönen K, Heinonen K, Andersson S, Hovi P, Järvenpää AL, Eriksson JG, Kajantie E. Personality of young adults born prematurely: The Helsinki study of very low birth weight adults. J Child Psychol Psychiatry. 2008a; 49:609–617.
- Pesonen AK, Räikkönen K, Heinonen K, Kajantie E, Forsen T, Eriksson JG. Reproductive traits following a parent-child separation trauma during childhood: A natural experiment during World War II. Am J Hum Biol. 2008b; 20:345–351.
- Pesonen AK, Räikkönen K, Matthews K, Heinonen K, Paavonen JE, Lahti J, Komsi N, Lemola S, Järvenpää AL, Kajantie E, Strandberg T. 2009. Prenatal origins of poor sleep in children. Sleep. 32:1086–1092.
- Pesonen AK, Räikkönen K, Feldt K, Heinonen K, Osmond C, Phillips DI, Barker DJ, Eriksson JG, Kajantie E. 2010. Childhood separation experience predicts HPA axis hormonal responses in late adulthood: A natural experiment of World War II. Psychoneuroendocrinology. 35:758–767.
- Pesonen AK, Räikkönen K, Kajantie E, Heinonen K, Osmond C, Barker DJ, Forsén T, Eriksson JG. 2011. Intergenerational social mobility following early life stress. Ann Med. 43 4: 320–328.
- Phillips DI, Jones A. 2006. Fetal programming of autonomic and HPA function: Do people who were small babies have enhanced stress responses?. J Physiol. 572:45–50.
- Phillips DI, Fall CHD, Whorwood CB, Seckl JR, Wood PJ, Barker DJP, . 1998. Elevated plasma cortisol concentrations: A link between low birth weight and the insulin resistance syndrome?. J Clin Endocrinol Metab. 83:757–760.
- Phillips DI, Walker BR, Reynolds RM, Flanagan DE, Wood PJ, Osmond C, Barker DJ, Whorwood CB. 2000. Low birth weight predicts elevated plasma cortisol concentrations in adults from 3 populations. Hypertension. 35:1301–1306.
- Ponirakis A, Susman EJ, Stifter CA. 1998. Negative emotionality and cortisol during adolescent pregnancy and its effects on infant health and autonomic nervous system reactivity. Dev Psychobiol. 33:163–174.
- Räikkönen K, Pesonen AK. 2009. Early life origins of psychological development and mental health. Scand J Psychol. 50:583–591.
- Räikkönen K, Pesonen AK, Kajantie E, Heinonen K, Forsen T, Phillips DI, Osmond C, Barker DJ, Eriksson JG. 2007. Length of gestation and depressive symptoms at age 60 years. Br J Psychiatry. 190:469–474.
- Räikkönen K, Pesonen A-K, Heinonen K, Lahti J, Kajantie E, Forsen T, Osmond C, Barker DJ, Eriksson JG. Infant growth and hostility in adult life. Psychosom Med. 2008a; 70:306–313.
- Räikkönen K, Pesonen AK, Heinonen K, Kajantie E, Hovi P, Järvenpää AL, Eriksson JG, Andersson S. Depression in young adults with very low birth weight: The Helsinki study of very low-birth-weight adults. Arch Gen Psychiatry. 2008b; 65:290–296.
- Räikkönen K, Forsén T, Henriksson M, Kajantie E, Laaksonen I, Leskinen JT, Laaksonen I, Osmond C, Barker DJ, Eriksson JG. Growth trajectories and intellectual abilities in young adulthood: The Helsinki Birth Cohort Study. Am J Epidemiol. 2009a; 15:447–455.
- Räikkönen K, Pesonen AK, Heinonen K, Lahti J, Komsi N, Eriksson JG, Seckl JR, Järvenpää AL, Strandberg TE. Maternal licorice consumption and detrimental cognitive and psychiatric outcomes in children. Am J Epidemiol. 2009b; 170:1137–1146.
- Räikkönen K, Lahti M, Heinonen K, Pesonen AK, Wahlbeck K, Kajantie E, Osmond C, Barker DJP, Eriksson JG. 2011. Risk of severe mental disorders in adults separated temporarily from their parents in childhood: The Helsinki birth cohort study. J Psychiatr Res. 45:332–338.
- Räikkönen K, Matthews KA, Pesonen AK, Pyhälä R, Paavonen EJ, Feldt K, Jones A, Phillips DI, Seckl JR, Heinonen K, Lahti J, Komsi N, Järvenpää AL, Eriksson JG, Strandberg TE, Kajantie E. Poor sleep and altered hypothalamic–pituitary–adrenocortical and sympatho–adrenal–medullary system activity in children. J Clin Endocrinol Metab. 2010b; 95:2254–2261.
- Räikkönen K, Seckl JR, Heinonen K, Pyhälä R, Feldt K, Jones A, Pesonen AK, Phillips DI, Lahti J, Järvenpää AL, Eriksson JG, Matthews KA, Strandberg TE, Kajantie E. Maternal prenatal licorice consumption alters hypothalamic–pituitary–adrenocortical axis function in children. Psychoneuroendocrinology. 2010c; 35:1587–1593.
- Reynolds RM, Walker BR, Syddall HE, Andrew R, Wood PJ, Whorwood CB, Phillips DI. 2001. Altered control of cortisol secretion in adult men with low birth weight and cardiovascular risk factors. J Clin Endocrinol Metab. 86:245–250.
- Reynolds RM, Godfrey KM, Barker M, Osmond C, Phillips DI. 2007. Stress responsiveness in adult life: Influence of mother's diet in late pregnancy. J Clin Endocrinol Metab. 92:2208–2210.
- Rieger M, Pirke KM, Buske-Kirschbaum A, Wurmser H, Papousek M, Hellhammer DH. 2004. Influence of stress during pregnancy on HPA activity and neonatal behavior. Ann N Y Acad Sci. 1032:228–230.
- Rodriguez A. 2010. Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. J Child Psychol Psychiatry. 51:134–143.
- Rodriguez A, Bohlin G. 2005. Are maternal smoking and stress during pregnancy related to ADHD symptoms in children?. J Child Psychol Psychiatry. 46:246–254.
- Roelofs K, van Peer J, Berretty E, Jong P, Spinhoven P, Elzinga BM. 2009. Hypothalamus–pituitary–adrenal axis hyperresponsiveness is associated with increased social avoidance behavior in social phobia. Biol Psychiatry. 65:336–343.
- Rogerson FM, Kayes KM, White PC. 1997. Variation in placental type 2 11beta-hydroxysteroid dehydrogenase activity is not related to birth weight or placental weight. Mol Cell Endocrinol. 128:103–109.
- Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP. 2001. Effects of prenatal exposure to the Dutch famine on adult disease in later life: An overview. Mol Cell Endocrinol. 185:93–98.
- Sarkar P, Bergman K, O'Connor TG, Glover V. 2008. Maternal antenatal anxiety and amniotic fluid cortisol and testosterone: Possible implications for foetal programming. J Neuroendocrinol. 20:489–496.
- Schlotz W, Jones A, Godfrey KM, Phillips DI. 2008. Effortful control mediates associations of fetal growth with hyperactivity and behavioural problems in 7- to 9-year-old children. J Child Psychol Psychiatry. 49:1228–1236.
- Schmidt LA, Miskovic V, Boyle MH, Saigal S. 2008. Shyness and timidity in young adults who were born at extremely low birth weight. Pediatrics. 122:e181–e187.
- Seckl JR. 2001. Glucocorticoid programming of the fetus; adult phenotypes and molecular mechanisms. Mol Cell Endocrinol. 185:61–71.
- Seckl JR. 2008. Glucocorticoids, developmental “programming” and the risk of affective dysfunction. Prog Brain Res. 167:17–34.
- Seckl JR, Meaney MJGlucocorticoid programmingBiobehavioral stress response: Protective and damaging effects. New York: Annals of the New York Academy of Sciences2004a63–84 Vol. 1032.
- Seckl JR, Meaney MJ. Glucocorticoid programming. Ann N Y Acad Sci. 2004b; 1032:63–84.
- Seckl JR, Meaney MJ. 2006. Glucocorticoid “programming” and PTSD risk. Ann N Y Acad Sci. 1071:351–378.
- Seckl JR, Holmes MC. 2007. Mechanisms of disease: Glucocorticoids, their placental metabolism and fetal “programming” of adult pathophysiology. Nat Clin Pract Endocrinol Metab. 3:479–488.
- Shenkin SD, Starr JM, Deary IJ. 2004. Birth weight and cognitive ability in childhood: A systematic review. Psychol Bull. 130:989–1013.
- Sloboda DM, Challis JR, Moss TJ, Newnham JP. 2005. Synthetic glucocorticoids: Antenatal administration and long-term implications. Curr Pharm Des. 11:1459–1472.
- Speirs HJ, Seckl JR, Brown RW. 2004. Ontogeny of glucocorticoid receptor and 11beta-hydroxysteroid dehydrogenase type-1 gene expression identifies potential critical periods of glucocorticoid susceptibility during development. J Endocrinol. 181:105–116.
- Stewart PM, Whorwood CB, Mason JI. 1995. Type 2 11 beta-hydroxysteroid dehydrogenase in foetal and adult life. J Steroid Biochem Mol Biol. 55:465–471.
- Strandberg TE, Järvenpää AL, Vanhanen H, McKeigue PM. 2001. Birth outcome in relation to licorice consumption during pregnancy. Am J Epidemiol. 153:1085–1088.
- Strandberg TE, Andersson S, Järvenpää AL, McKeigue PM. 2002. Preterm birth and licorice consumption during pregnancy. Am J Epidemiol. 156:803–805.
- Strang-Karlsson S, Räikkönen K, Kajantie E, Andersson S, Hovi P, Heinonen K, Pesonen AK, Järvenpää AL, Eriksson JG, Paavonen EJ. Sleep quality in young adults with very low birth weight—the Helsinki study of very low birth weight adults. J Pediatr Psychol. 2008a; 33:387–395.
- Strang-Karlsson S, Räikkönen K, Pesonen AK, Kajantie E, Paavonen EJ, Lahti J, Hovi P, Heinonen K, Järvenpää AL, Eriksson JG, Andersson S. Very low birth weight and behavioral symptoms of attention deficit hyperactivity disorder in young adulthood: The Helsinki study of very-low-birth-weight adults. Am J Psychiatry. 2008b; 165:1345–1353.
- Trautman PD, Meyer-Bahlburg HFL, Postelnek J, New MI. 1995. Effects of early prenatal dexamethasone on the cognitive and behavioral development of young children: Results of a pilot study. Psychoneuroendocrinology. 20:439–449.
- Tuovinen S, Räikkönen K, Kajantie E, Pesonen AK, Heinonen K, Osmond C, Barker DJ, Eriksson JG. 2010. Depressive symptoms in adulthood and intrauterine exposure to pre-eclampsia: The Helsinki Birth Cohort Study. Br J Obstet Gynaecol. 117:1236–1242.
- Tuovinen SH, Räikkönen K, Kajantie E, Lehtinen J, Henriksson M, Pesonen AK, Heinonen K, Osmond C, Barker D, Eriksson JG. Hypertensive disorders in pregnancy and intellectual abilities in the offspring in young adulthood: The Helsinki birth cohort study. Ann Med. 2011 Apr 15. [Epub ahead of print].
- Van den Bergh BR. 1990. The influence of maternal emotions during pregnancy on fetal and neonatal behavior, Win, 1990. J Prenatal Perinatal Psychol Health. 5:119–130.
- Van den Bergh BR. 1992. Maternal emotions during pregnancy and fetal and neonatal behavior. Oxford University Press: Oxford, UK.
- Van den Bergh BR, Marcoen A. 2004. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Dev. 75:1085–1097.
- Van den Bergh BR, Mennes M, Oosterlaan J, Stevens V, Stiers P, Marcoen A, Lagae L. High antenatal maternal anxiety is related to impulsivity during performance on cognitive tasks in 14- and 15-year-olds. Neurosci Biobehav Rev. 2005a; 29:259–269.
- Van den Bergh BR, Mulder EJ, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: Links and possible mechanisms. A review. Neurosci Biobehav Rev. 2005b; 29:237–258.
- Van den Bergh BR, Mennes M, Stevens V, van der Meere J, Borger N, Stiers P, Marcoen A, Lagae L. 2006. ADHD deficit as measured in adolescent boys with a continuous performance task is related to antenatal maternal anxiety. Pediatr Res. 59:78–82.
- van der Wal MF, van Eijsden M, Bonsel GJ. 2007. Stress and emotional problems during pregnancy and excessive infant crying. J Dev Behav Pediatr. 28:431–437.
- Van den Bergh BR, Van Calster B, Smits T, Van Huffel S, Lagae L. 2008. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: A prospective study on the fetal origins of depressed mood. Neuropsychopharmacology. 33:536–545.
- Vaughn B, Bradley C, Joffe L, Seifer R, Barglow P. 1987. Maternal characteristics measured prenatally are predictive of ratings of temperamental “difficulty” on the Carey Infant Temperament Questionnaire. Dev Psychol. 23:152–161.
- Wahlbeck K, Forsen T, Osmond C, Barker DJ, Eriksson JG. 2001. Association of schizophrenia with low maternal body mass index, small size at birth, and thinness during childhood. Arch Gen Psychiatry. 58:48–52.
- Warner MJ, Ozanne SE. Mechanisms involved in the developmental programming of adulthood disease. Biochem J. 2010427.
- Welberg LA, Seckl JR, Holmes MC. 2000. Inhibition of 11beta-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxiety-like behaviour in the offspring. Eur J Neurosci. 12:1047–1054.
- Wisborg K, Barklin A, Hedegaard M, Henriksen TB. 2008. Psychological stress during pregnancy and stillbirth: Prospective study. BJOG: Int J Obstet Gynaecol. 115:882–885.
- Wust S, Entringer S, Federenko IS, Schlotz W, Hellhammer DH. 2005. Birth weight is associated with salivary cortisol responses to psychosocial stress in adult life. Psychoneuroendocrinology. 30:591–598.
- Yeh TF, Lin YJ, Lin HC, Huang CC, Hsieh WS, Lin CH, Tsai CH. 2004. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med. 350:1304–1313.
- Yehuda R, Engel SM, Brand SR, Seckl J, Marcus SM, Berkowitz GS. 2005. Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the World Trade Center attacks during pregnancy. J Clin Endocrinol Metab. 90:4115–4118.
