Abstract
Lack of physical activity (PA) is a risk factor for Alzheimer's disease (AD), and PA interventions are believed to provide an effective non-pharmacological approach for attenuating the symptoms of this disease. However, the mechanism of action of these positive effects is currently unknown. It is possible that the benefits may be at least partially mediated by the effects on the neuroendocrine stress system. Chronic stress can lead to dysfunction of the hypothalamic–pituitary–adrenal (HPA) axis, leading to aberrant basal and circadian patterns of cortisol secretion and a cascade of negative downstream events. These factors have been linked not only to reduced cognitive function but also increased levels of amyloid-β plaques and protein tau “tangles” (the neuropathological hallmarks of AD) in the non-demented mouse models of this disease. However, there is evidence that PA can have restorative effects on the stress neuroendocrine system and related risk factors relevant to AD. We explore the possibility that PA can positively impact upon AD by restoring normative HPA axis function, with consequent downstream effects upon underlying neuropathology and associated cognitive function. We conclude with suggestions for future research to test this hypothesis in patients with AD.
Introduction
Alzheimer's disease (AD) is a neurodegenerative condition creating progressive deterioration of higher cognitive functioning in the areas of memory, problem solving, and thinking (Rimmer and Smith Citation2009). AD is the most common form of dementia and its pathological hallmarks in the brain are neuritic plaques (composed predominantly of amyloid-β peptides) and neurofibrillary tangles (formed by hyper-phosphorylated forms of tau protein; Cummings et al. Citation1998). AD is characterized by an inability to carry out everyday tasks or perform instrumental activities, and may be accompanied by behavioral disorders such as agitation, aggression, and wandering (Onor et al. Citation2007; Rimmer and Smith Citation2009). Mild cognitive impairment (MCI) often represents a prodromal form of dementia, conferring a 10–15% annual risk of converting to probable AD (Risacher et al. Citation2009). As the world's population ages the prevalence of this debilitating disease is increasing, which is becoming a social and economic concern important to families, caregivers, professionals, and others in public health systems (Haan and Wallace Citation2004). In 2006, the prevalence of AD worldwide was reported to be 26.6 million, and by 2050 this number is estimated to quadruple to 106.8 million (Brookmeyer et al. Citation2007). The estimated worldwide societal cost of AD in 2005 was US$315.4 billion (Wimo et al. 2007). Given the scale and impact of the problem, it is imperative that acceptable and inexpensive intervention strategies are identified in order to retard the onset or attenuate the progression of the disease. It has been estimated that if interventions could delay disease onset or progression of AD by as little as 1 year, nearly 9.2 million fewer patients would be expected by the year 2050 (Brookmeyer et al. Citation2007).
Risk factors for AD
Over 99% of AD cases are sporadic, not associated with any known genetic mutation, although the presence of one or two alleles of apolipoprotein (APOE) e4 as opposed to APOE e2 or APOE e3 increases disease risk by several fold (Corder et al. Citation1993). Aging is probably the biggest risk factor for non-AD-associated dementia and AD (Querfurth and LaFerla Citation2010). However, environmental, behavioral, and social factors can increase the risk of developing AD, indicating that disease progression is potentially modifiable. Such risk factors include head trauma (Rothman and Mattson Citation2010), alcohol abuse, addictive smoking, diet filled with high fat content (Gustaw-Rothenberg Citation2009), and lack of mental stimulation (Wilson et al. Citation2007) through the life span.
Chronic stress is a major risk factor for the development of AD, and there is evidence that it exacerbates the cognitive deficits and the accompanying brain pathological characteristics of the condition (see Rothman and Mattson Citation2010, for a review). People exposed to chronic stress have been estimated to be 2.7 times more likely to suffer from AD, and they are also more likely to experience more rapid disease progression (Wilson et al. Citation2006). Similarly, depression is considered a risk factor for AD (Green et al. Citation2003) since it may be strongly related to stress (Davidson et al. Citation2002). As effective social support is known to be a successful buffer to psychological stress (Cohen and Wills Citation1985), it is not surprising that a lack of social support and meaningful social networks may also contribute to the development of AD (Fratiglioni et al. Citation2004; Solfrizzi et al. Citation2008).
Chronic stress is related to increased risk of cardiovascular (CV) conditions (Björntorp Citation1997; Rosmond et al. Citation1998) which are also risk factors for AD, and play a role in the development of the disease (Gustafson et al. Citation2003; Arvanitakis et al. Citation2004; Kivipelto et al. Citation2005; Whitmer et al. Citation2005; Helzner et al. Citation2009). Furthermore, type 2 diabetes is associated with chronic stress (Nader et al. Citation2010) and a risk factor for AD (Ott et al. Citation1999; Messier Citation2003; Arvanitakis et al. Citation2004; Strachan et al. Citation2008; Maher and Schubert Citation2009). Indeed, type 2 diabetes and/or elevated fasting glucose are reported in up to 80% of patients with AD (Janson et al. Citation2004). A later systematic review of this literature found that individuals with diabetes had a higher incidence of AD in 8 of 13 studies (Biessels et al. Citation2006). Relevant to this review, lack of PA (along with other detrimental health behaviors such as smoking, poor diet, and sleep disruption) can be a behavioral product of chronic stress (Kyrou and Tsigos Citation2009), and is also consistently identified as a risk factor for AD (Laurin et al. Citation2001; Podewils et al. Citation2005; Rovio et al. Citation2005; Karp et al. Citation2006; Larson et al. Citation2006; Simons et al. Citation2006; Taaffe et al. Citation2008). See for a summary of the links between stress, risk factors for AD, and disease progression.
Figure 1. Summary diagram: arrows indicate pathways between psychosocial stress and AD (arrows indicate direction of causality). PA = physical activity and is located on arrows where it has been shown to attenuate that pathway (see text).
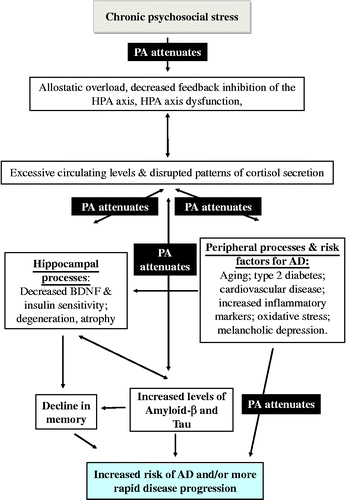
This wide range of risk factors is indicative of an underlying system (or systems) that interacts with the disease process to contribute to disease progression, and points to a role for the stress neuroendocrine system. We go on to explore the possible pathways by which psychological stress can affect cognitive function and the neuropathological bases of AD.
Evidence that stress impacts on cognitive function and AD
Chronic stress, allostatic overload, aging, and memory function
Excessive and repeated responses to stress and/or the inability to turn off the response when it is no longer needed are associated with increased activity of the sympathetic nervous system and a dysfunction of the hypothalamic–pituitary–adrenal (HPA; McEwen Citation2008) axis. The latter causes, among other things, aberrant patterns of cortisol secretion (Meerlo et al. Citation2002; Nader et al. Citation2010) and typically excessive levels of basal circulating cortisol (Lupien and Lepage Citation2001), which over time produces accumulative wear and tear on the body and brain (McEwen Citation2008). Under these circumstances, the ability to achieve stability through change (allostasis) fails, producing a condition of allostatic overload (Seeman et al. Citation1997; McEwen Citation1998; McEwen Citation2008).
Allostatic overload also has several behavioral sequelae known to be risk factors for AD, including disrupted sleep, unhealthy eating patterns, drinking too much alcohol, smoking, and lack of physical activity (PA; McEwen Citation2008; Kyrou and Tsigos Citation2009). Increasing age (the major risk factor for cognitive decline and AD) is associated with increases in the HPA axis response to the challenge in some studies [see Otte et al. (Citation2005) for a meta-analysis of 45 stress challenge studies of young vs. old healthy participants]. However, only four of these studies involved psychosocial challenge, and thus the ability of acute psychological stress changes to change the sensitivity of the HPA axis which remains to be firmly established. Furthermore, the finding that females changed more in response to increasing age may be more closely related to their known greater sensitivity to pharmacological challenge than psychosocial-related threat (Uhart et al. Citation2006). Other studies have reported enhanced HPA responsivity to psychosocial stress in older males and not older females (Kudielka et al. Citation2004). Moreover, some studies have shown no age-related effects on HPA axis reactivity; see Kudielka et al. (Citation2009) for a review of individual differences in salivary cortisol responses to challenge.
Increasing age in healthy participants is also associated with higher overall basal levels of cortisol secretion and flatter circadian profiles (van Cauter et al. Citation1996; Deuschle et al. Citation1997). However, some have suggested that an association is apparent only in depressed individuals (Kudielka et al. Citation2000). Other studies report aberrant cycles in sub-populations of the healthy old (Ice et al. Citation2004; Kumari et al. Citation2010). Interactions between increasing age, chronic stress exposure, and changing stress reactivity are a complex area recently reviewed and discussed by Gruenewald and Seeman (Citation2010). They conclude that the biological changes typically observed with advancing age may render older adults more susceptible to negative biological and health consequences of chronic stress.
Allostatic overload is consistently associated with reduced memory function; for example, in a longitudinal study of 194 participants aged 70–79 years, increased urinary free cortisol excretion was correlated with a decline in memory performance (Seeman et al. Citation1997). This study showed significant differences in memory decline only for females, while males participating in the study did not present any significant associations. However, in a different longitudinal study of 154 healthy men and women aged between 70 and 79 years, urinary excretion of epinephrine predicted cognitive decline in men but not in women (Karlamangla et al. Citation2005). Interestingly enough, Seeman et al. (Citation1997) found that the decline in women's memory was reversible: declines in cortisol were associated with improvements in memory. These data are consistent with evidence that elevated levels of cortisol are linked to impaired memory in healthy participants (Wolf et al. Citation2002a, Citation2009) and in patients with MCI (Wolf et al. Citation2002b; Arsenault-Lapierre et al. Citation2010).
Chronic stress and hippocampal function
Stress-associated decline in memory is attributed to hippocampal atrophy and degeneration caused by excessive levels of cortisol, as this area of the brain is characterized by high glucocorticoid sensitivity as a result of dense expression of glucocorticoid receptors (McEwen Citation1994; de Kloet et al. Citation2005). Furthermore, corticosteroids exert suppressive effects on cell proliferation (neurogenesis) in the dentate gyrus of the rat hippocampus (Cameron and Gould Citation1994). Thus, the inability to cope with stress may lead to a reduction in total hippocampal volume and loss of neurons in this region (Lupien et al. Citation1999; Warner-Schmidt and Duman Citation2006). The hippocampus is a key area for memory storage and processing, being one of the main areas affected by AD (Rothman and Mattson Citation2010). In fact, hippocampal atrophy is found in all stages of AD (Fox et al. Citation1996; Dickerson et al. Citation2001). Similar hippocampal atrophy is found in stressed patients and MCI (Lupien et al. Citation1999), and can be used as a predictor of conversion of MCI to AD (Apostolova et al. Citation2006). However, as noted in the recent review by Rothman and Mattson (Citation2010), there are still no studies reporting changes in hippocampal plasticity in AD after chronic stress.
Cardiovascular factors
Chronic stress is related to increased risk of CV conditions (Björntorp Citation1997; Rosmond et al. Citation1998) that are implicated in cognitive decline (Gustafson et al. Citation2003; Arvanitakis et al. Citation2004; Kivipelto et al. Citation2005; Whitmer et al. Citation2005; Helzner et al. Citation2009). Indeed, cerebrovascular disease is the second most common cause of acquired cognitive impairment and dementia, and contributes to cognitive decline in the neurodegenerative dementias (O'Brien et al. Citation2003). For example, higher pre-diagnosis total cholesterol and low-density lipoprotein concentrations (and history of diabetes, see below) were associated with faster cognitive decline in patients with AD (Helzner et al. Citation2009). There is accumulating evidence that increased plasma homocysteine level (an amino acid linked to CV disease) may play a role in cognitive decline and the onset of AD (Seshadri et al. Citation2002; CitationZhuo et al. in press). A recent study has found a link between elevated levels of homocysteine in non-demented patients with type 2 diabetes and hippocampal atrophy (Shimomura et al. Citation2011). The mechanism by which elevated homocysteine may affect cognitive function is still under investigation, but implicated pathways include increased oxidative stress, excitotoxic damage, and direct effects on amyloid-β and tau phosphorylation in the brain (see CitationZhuo et al. in press, for a summary).
Type 2 diabetes and the role of brain insulin
Chronic stress and allostatic overload are associated with a group of related disorders, including type 2 diabetes (Chrousos Citation2009; Kyrou and Tsigos Citation2009). For example, the prevalence of newly diagnosed type 2 diabetes is related to a high number of relatively common major life events during the preceding 5-year period (Mooy et al. Citation2000). This is relevant here, as type 2 diabetes is associated with cognitive decline (Wrighten et al. Citation2009). These deficits are reported to be more selective for hippocampal-related memory performance (Gold et al. Citation2007), and in animal models these deficits are attributed to deficiencies in the regulation of insulin within the hippocampus (Moosavi et al. Citation2007). Insulin mediates several brain functions including cognition and memory (Craft et al. Citation1996; Craft and Watson Citation2004), which may be associated with a high concentration of insulin receptors in the hippocampus (Craft et al. Citation1996; Craft and Watson Citation2004; Craft Citation2009). In the hippocampus, insulin appears to have some neuroprotective effects against memory loss in animal and human studies (Moosavi et al. Citation2007; Reagan Citation2007).
Insulin and insulin receptors are reduced in animal models of AD, suggesting that insulin-signaling pathways might be impaired (Takeda et al. Citation2010; Wang et al. Citation2010). Increased plasma insulin administration through intravenous infusion improved memory in AD patients (Craft et al. Citation1996). Furthermore, Wang et al. (Citation2010) demonstrated that diabetes induced by streptozotocin increased the amyloid load in a transgenic mouse model of AD. Another study using a mouse model of AD with diabetes indicates that the diabetic condition increases cognitive dysfunction, along with vascular inflammation and increased amyloid burden (Takeda et al. Citation2010). These changes were mediated by impaired brain insulin signaling. Furthermore, in this study, amyloid pathology seemed to negatively impact diabetes pathogenesis, once again pointing to the presence of deteriorating cycles of negatively interacting systems.
Brain-derived neurotrophic factor (BDNF)
Levels of BDNF are diminished in the hippocampus of patients with AD (Podewils et al. Citation2005; Querfurth and LaFerla Citation2010). There is compelling evidence from mice experiments on the negative impact of stress on BDNF production (reviewed in Duman and Monteggia Citation2006). Stress may play a key role in the growth factor's cascade due to altered levels of cortisol (Chao et al. Citation1998; Schaaf et al. Citation1998), serotonin (Vaidya et al. Citation1997), or interleukin-1β (Barrientos et al. Citation2003) in the hippocampus, interfering with growth factor signaling, reducing the BDNF availability in the hippocampus, and resulting in a decrease in neurogenesis and brain plasticity.
Inflammation
There is a strong correlation between inflammatory responses and the early stages of AD in humans (Parachikova et al. Citation2007). In rats, acute stress may facilitate innate immunity, and PA enhances this positive effect (Fleshner et al. Citation2002). However, chronic stress results in excessive levels of inflammatory markers (McEwen Citation2008). Furthermore, elevated levels of pro-inflammatory factors may be responsible for the decreased levels of growth factors, especially in the hippocampus [see Cotman et al. (Citation2007) for a review of human and animal literature]. Inflammation increases peripheral and central risk factors driving cognitive decline and neurodegeneration in humans and rodents (Yaffe et al. Citation2003; Cotman et al. Citation2007).
Melancholic depression
Hippocampus atrophy, insulin resistance, decreased BNDF levels, and increased inflammation are also shown in patients with melancholic depression (Rothermundt and Arolt Citation2001; Duman Citation2005; Kyrou and Tsigos Citation2009). In parallel, serotonin expression is inhibited by depression and chronic stress (Cameron and Gould Citation1994; Lopez et al. Citation1998). High corticosterone levels decrease the density of 5HT fibers or 5HT1A receptors in rat brain (Cameron and Gould Citation1994). Data derived from studies on postmortem human brain tissue indicate that serotonin may play an important role in learning and memory formation (Elliot et al. Citation2009), possibly through enhancing neurogenesis in the dentate gyrus via activation of the 5HT1A receptor (Gould Citation1999).
Oxidative stress
Oxidative stress, the imbalance in production of reactive oxygen species (ROS) and antioxidative defense (Gella and Durani Citation2009), is associated with both psychological stress and experimental models of AD (Rothman and Mattson Citation2010). ROS are physiological products of aerobic metabolism involved in cellular repair and adaptation. However, ROS overproduction is strongly implicated as a causal factor in the aging process, and occurs in neurodegenerative diseases such as Parkinson's and AD (Radak et al. Citation2005, Citation2008) and correlated with cognitive decline (Gella and Durani Citation2009). In experimental animal models of AD, oxidative damage precedes pathological changes in AD (Querfurth and LaFerla Citation2010), implicating ROS in disease onset. β-amyloid peptide (Aβ) is a potent generator of ROS, making it a prime generator of this damage and Aβ accumulation and a possible etiological factor in AD (Querfurth and LaFerla Citation2010; Wan et al. Citation2011). Experiments with male Wistar rats have shown that social stress (isolation) increases oxidative stress (Pajović et al. Citation2006). Moreover, other stressors (such as restraint or immobilization) increase the production of ROS (Kovács et al. Citation1996; Zaidi and Banu Citation2004). Not surprisingly, direct administration of corticosterone in rats promotes oxidative stress in the brain, suggesting that the stress neuroendocrine system plays a causal role in the oxidative stress process (Zafir and Banu Citation2009). At the same time, rats subjected to oxidative stress display serious damage to hippocampal pyramidal cells that is linked to impaired cognitive function (Sato et al. Citation2010).
Summary
Evidence from animal and human studies indicates that chronic psychological stress can impact upon a number of different interconnected routes relevant to cognition. It is also worth emphasizing that the hippocampus is involved in the regulation of the HPA axis (Jacobson and Sapolsky Citation1991; Lupien et al. Citation1998; Lupien and Lepage Citation2001). The hippocampus is rich in glucocorticoid receptors and many studies have demonstrated their role in HPA feedback regulation. In general, animal studies have shown that lesions to the hippocampus result in elevated corticosterone levels under basal and post-stress conditions (Wilson et al. Citation1980; Sapolsky et al. Citation1984). Stress-induced atrophy in this region can lead to further loss of the feedback inhibition of this system and a consequent reciprocal cycle of deterioration (Warner-Schmidt and Duman Citation2006).
The HPA axis in AD
HPA axis dysfunction is found in the early stages of patients with AD (Csernansky and Dong Citation2006), with reports of elevated basal levels of circulating cortisol (Swanwick et al. Citation1998; Wahbeh et al. Citation2008) and the inability to suppress cortisol after a dexamethasone (DEX) challenge (Hatzinger et al. Citation1995; Näsman et al. Citation1995). Furthermore, there is a relationship between hypercortisolemia and progression of the disease in humans (Weiner et al. Citation1993, Citation1997). In both of these studies, higher values of midday serum cortisol concentrations were associated with a more rapid cognitive decline. However, the sample size in these studies was small: just 12 patients followed up for 12 months in the first study, and 9 patients followed longitudinally for 2–3 years in the second study. Consistent with these findings, higher baseline morning cortisol levels are associated with a greater level of cognitive impairment in 27 patients diagnosed with AD (Miller et al. Citation1998). Similarly, higher morning levels of plasma cortisol are linked to rapid disease progression and decreased performance in neuropsychological tests in patients with mild or very mild AD (Csernansky and Dong Citation2006). This latter study was a longitudinal design, in which 33 community-dwelling participants with very mild and mild Alzheimer-type dementia [scores of 0.5 and 1 on the five-point Clinical Dementia Rating Scale (CDRS)] and 21 participants without dementia provided plasma for determination of morning cortisol concentration at the start of the study. Subsequently, they performed a battery of neuropsychological tests and the CDRS annually for up to 4 years. In both groups of dementia participants combined, but not in those without dementia; higher plasma cortisol levels were associated with more rapidly increasing symptoms and more rapidly decreasing performance on neuropsychological tests. The results support the idea of a negative impact of elevated cortisol levels on cognitive performance and thus on the progression of AD. However, this small study was not able to distinguish very mild from no dementia in terms of morning plasma cortisol levels at the start of the study.
Some of the most persuasive data linking psychological stress and AD are evidence of increased formation of amyloid-β plaques and protein tau “tangles,” alongside a decrease in their degradation, following stress exposure in mice models of AD (Green et al. Citation2006; Jeong et al. Citation2006; Dong et al. Citation2008; Lee et al. Citation2009). These neuropathological features are considered the two main hallmarks of AD and implicate stress in the disease process, rather than just cognitive function. However, it is also possible that elevated levels of amyloid-β plaques in the hippocampus may precede elevations in basal levels of plasma corticosterone, which could imply that the observed alteration in the HPA axis in AD may be subsequent to neuropathology (Green et al. Citation2006; Nuntagij et al. Citation2009; Rothman and Mattson Citation2010). Further work is required to elucidate the extent to which these pathways are causally associated in AD.
Converging evidence from human and animal studies indicates that chronic stress and AD cause similar cognitive impairments and pathological hallmarks, specifically in the hippocampus, and that stress may both increase the risk of developing AD and cause a more rapid disease progression (Rothman and Mattson Citation2010; see ).
The stress–AD link implies that stress management could be a key component of AD prevention and treatment. Within non-pharmacological approaches, PA could be an inexpensive option for reducing the effects of stress, with many proven health benefits and very little or no side effects. However, to our knowledge, the mechanism of the effect of PA on disease progression in AD patients has not been explored.
PA and stress: current evidence of interactions and mechanisms
In the UK, it is recommended that adults should participate in a minimum of 30 min of at least moderate intensity activity (such as brisk walking, cycling, or climbing the stairs) on 5 or more days of the week (Department of Health Citation2004) to maintain health and well-being. However, only 39% of men and 29% of women in England meet this recommended level (CitationCraig et al. 2008). PA participation has remained low despite its benefits being widely publicized. Guidelines for older adults are provided by the CitationWorld Health Organisation (2010). It is recommended that older adults (65 years plus) should do at least 150 min of moderate intensity, or 75 min of vigorous intensity, aerobic PA throughout a typical week or do an equivalent combination of moderate- and vigorous-intensity activity. Promoting PA for older adults is deemed especially important because this population is the least physically active of any age group.
Evidence of the effects of PA on stress response systems is complicated by the wide array of PA and stressors investigated. For ease of reference, relevant studies are summarized in .
Table I. Effects of PA on stress responding human studies.
Table II. Effects of PA on stress responding rodent studies.
The tables indicate that there is increasing evidence of the benefits of PA to cope with psychological/exercise stress from both human (for example, Sinyor et al. Citation1983; Holmes and McGilley Citation1987; Deuster et al. Citation1989; Brown Citation1991; Korkushko et al. Citation1995; Unger et al. Citation1997; Broocks et al. Citation2001; Salmon Citation2001; Traustadóttir et al. Citation2004; Rimmele et al. Citation2007; Rimmele et al. Citation2009) and animal studies (for example, Watanabe et al. Citation1991; Droste et al. Citation2003, Citation2006, Citation2007; Greenwood et al. Citation2007; Hill et al. Citation2010; Kannangara et al. Citation2011). However, there are significant inconsistencies in both literatures (humans: de Geus et al. Citation1993, see meta-analysis by Jackson and Dishman Citation2006; Heiden et al. Citation2007; rodents: Moraska et al. Citation2000; Fediuc et al. Citation2006).
There are several potential reasons for identified inconsistencies, including the use of different PA training protocols and different stressors (Rimmele et al. Citation2007). It seems that the effects of PA may vary depending on factors such as intensity, type, and the duration of the exercise. In human studies, population characteristics, such as age and gender, are also important, as these factors are associated with activity of the stress neuroendocrine system and may interact with the effects of PA. Another factor that needs to be taken into consideration, for both human and rodent studies, is whether the interaction being studied is between PA and a physical exercise stressor (which may or may not be novel) or psychological stressor, which is typically novel (see Dickerson and Kemeny Citation2004). Considering all these possibly interacting variables, it is not surprising that, alongside the controversy about the interaction of PA with psychological stress, the underlying mechanisms that mediate the claimed effects remain unclear.
The effects of PA on responses to physical exercise stress
It is clear that the effects of a single bout of PA are different than those seen with regular PA. Acute bursts of PA induce a strong neuroendocrine stress response in rodents (Timofeeva et al. Citation2003; Campbell et al. Citation2009). Similarly, short bursts of PA increase levels of adrenocorticotrophic hormone (ACTH) and glucocorticoids in humans (Kiive et al. Citation2004), especially if the exercise is of high intensity (Viru et al. Citation1992). In rodents, these changes are attenuated after long-term adaptation to exercise [Campbell et al. Citation2009; unless absolute intensity is gradually increased (Park et al. Citation2005)]. In humans, if the intensity of the exercise is moderate or light, acute exercise may not increase cortisol secretion (Nieman et al. Citation2005).
Long-term regular physical training in rodents may reduce or enhance the stress response, depending on whether exposure is to a familiar physical stressor, a novel physical stressor, or a psychological stressor (Droste et al. Citation2003, Citation2006, Citation2007). There is evidence from both human and rodent studies that exercise training produces adaptations in HPA axis reactivity to familiar acute exercise stress. This includes evidence from rodents of higher threshold of activation and attenuated ACTH and corticosterone responses (Watanabe et al. Citation1991; White-Welkley et al. Citation1995; Dishman et al. Citation2000). Greater maximal capacity of the adrenal glands is reported in both human (Luger et al. Citation1987; Kjær Citation1992; Deuster et al. Citation1998) and rodent studies (Watanabe et al. Citation1991). Similar adaptations are also reported in older humans (Korkushko et al. Citation1995). However, in rats, physical training increases HPA axis reactivity to a novel physical stressor (White-Welkley et al. Citation1996; Droste et al. Citation2007). The higher cortisol responses after a novel physical stress may be adaptive, as novel physical stressors can be seen as life-threatening challenges, requiring increased glucocorticoid levels to successfully respond to the physical challenge (Droste et al. Citation2007).
The effects of PA on responses to psychological stress
The interaction between PA training and psychological stress is called “cross-stress adaptation.” Studies have reported inconsistent findings, especially in human populations, with most of them focusing on CV changes (Rimmele et al. Citation2007). Some studies focusing on HPA axis reactivity did not show lower levels of cortisol after a psychological stress for aerobically fit human participants (Sinyor et al. Citation1983; Moyna et al. Citation1999). However, trained men and elite athletes are reported to have lower cortisol and heart rate responses to an acute psychological stressor than untrained men (Rimmele et al. Citation2007; Rimmele et al. Citation2009). More work is required to clarify the strength of these findings (for example, just how much exercise is required to generate an advantageous effect) and to examine the impact of potential interacting factors such as age and gender.
In studies using mice and rats, results are also inconsistent. However, in these studies, it is important to distinguish forced exercise from voluntary exercise (Droste et al. Citation2003; Dishman et al. Citation2006). Long-term forced exercise produces negative adaptations associated with chronic stress such as increased adrenal weight, thymic involution, and decreased serum corticosteroid-binding globulin (Moraska et al. Citation2000; Fediuc et al. Citation2006). However, long-term voluntary exercise may positively impact HPA axis regulation (Hill et al. Citation2010), contributing to a reversal of the effects of uncontrolled stress (Greenwood et al. Citation2007) and showing appropriate glucocorticoid responses to stress in mice (Droste et al. Citation2003, Citation2006, Citation2007; Kannangara et al. Citation2011).
Greenwood et al. (Citation2007) demonstrated that 6 weeks of voluntary wheel running after stressor exposure can reverse the long-lasting behavioral consequences of uncontrolled stress (shuttle box escape) in rats (although 2 weeks of exercise was not sufficient to show positive results). Four weeks of voluntary running increases the glucocorticoid response to a novel physical stress (potentially life-threatening challenge, such as forced swimming), but decreases by 50% the glucocorticoid response to a mild psychological stressor such as a novel environment (Droste et al. Citation2007). In this study, plasma ACTH levels remained unchanged in both exercising and control mice, suggesting adaptive changes at the level of the adrenal cortex, for example, changes in sympathoadrenomedullary input (Droste et al. Citation2007).
These studies suggest that in young animals, the response to psychological stress is different after voluntary, as opposed to forced, physical training, and is dependent on the duration of the training regime. The benefits of PA for stress in aged animals and humans have received less attention. Traustadóttir et al. (Citation2004) found that older women had a higher HPA axis reactivity to a psychological stressor than younger women, but this age-related change was attenuated by physical fitness. Consistent with this finding, 11 days of voluntary running significantly lowered plasma corticosterone levels in socially housed aged mice, when measured at the onset of the mouse active cycle (Kannangara et al. Citation2011). However, more research is required to establish the benefits of PA upon the aging HPA axis and its responses to psychological stress. These studies are needed to inform the development of intervention strategies relevant to the aging population, as well as enhance the theoretical understanding of “cross-stress adaptation.”
PA and corticosterone levels in the hippocampus
Rodent studies have shown that peripheral corticosterone levels do not necessarily predict corticosterone levels in the hippocampus (Droste et al. Citation2007, Citation2009), the brain area of interest for this review. Indeed, corticosteroid levels were not attenuated in the hippocampus after 4 weeks of voluntary running (Droste et al. Citation2009). The results need to be replicated and extended in healthy and AD mouse models and also in human populations to explore whether longer periods of exercise are needed to reduce corticosteroid levels in the hippocampus. Furthermore, due to the many pathways and interconnections existing between PA, stress, and AD, it is important to determine whether hippocampal amyloid load, BDNF, oxidative stress, CV factors, depression, and inflammatory processes (as described below) are beneficially affected by PA.
PA, AD, and stress: pathways and interconnections
PA and cognitive function
There is compelling evidence from longitudinal studies in humans to show that PA is protective against MCI, dementia, and AD (Laurin et al. Citation2001; Abbott et al. Citation2004; Podewils et al. 2004; Rovio et al. Citation2005; Karp et al. Citation2006; Larson et al. Citation2006; Simons et al. Citation2006; Taaffe et al. Citation2008; Rovio et al. Citation2010). Exercise seems to improve cognitive function, especially in older adults (Colcombe and Kramer Citation2003; Heyn et al. Citation2004; Weuve et al. Citation2004), and enhance learning and memory in mice (van Praag et al. Citation1999, Citation2005).
Voluntary exercise enhances memory in transgenic mouse models of AD (Adlard et al. Citation2005; Parachikova et al. Citation2008; Nichol et al. Citation2009), suggesting that PA may inhibit the normal progression of the disease. Equivalent evidence is currently scarce in humans, although studies show that AD patients' health and functioning are positively influenced by participating in a variety of physical activities (Palleschi et al. Citation1996; Arkin Citation2003; Teri et al. Citation2003; Rolland et al. Citation2007; Williams and Tappen Citation2007). However, the mechanisms of the benefits of PA on cognition in healthy and AD populations remain unclear. Interestingly enough, most accepted theories such as reduction in amyloid load (Adlard et al. Citation2005), reduction in peripheral risk factors (Kramer and Erickson Citation2007), increased neurotrophic factors (Cotman and Berchtold Citation2002; Cotman and Engesser-Cesar Citation2002; Garza et al. Citation2004; Berchtold et al. Citation2005; Vaynman et al. Citation2006; Kramer and Erickson Citation2007), reduction in the negative effects of inflammation (Cotman et al. Citation2007), improved depression symptoms (Blumenthal et al. Citation1999; De Moor et al. Citation2006; Zheng et al. Citation2006; Blumenthal et al. Citation2007), increased serotonin expression (Ivy et al. Citation2003; Greenwood et al. Citation2005), and a protective effect against oxidative stress (Vaynman et al. Citation2006; Radak et al. Citation2008) may be linked to stress reduction (Cameron and Gould Citation1994; Björntorp Citation1997; Lopez et al. Citation1998; Traustadóttir et al. Citation2005; Duman and Monteggia Citation2006; Green et al. Citation2006; Jeong et al. Citation2006; Dong et al. Citation2008; McEwen Citation2008; Chrousos Citation2009; Kyrou and Tsigos Citation2009; Lee et al. Citation2009; Zafir and Banu Citation2009), suggesting that stress reduction could mediate some of the benefits attributed to PA for AD.
PA and amyloid-β plaques
PA has direct effects on the neuropathological signs of AD in animal models. Voluntary wheel running for 5 months, in the TgCRND8 mouse model of AD, produced an improvement in cognition and a reduction in the amyloid load in both cortical and hippocampal regions of the brain (Adlard et al. Citation2005). The mechanisms involved may be related to a change in the processing of the amyloid precursor protein (APP) seen after 1 month of exercise, with a decrease in the proteolytic fragments of APP. The reduction in the amyloid load was considered to be responsible for the improvement in learning and memory shown in Morris water maze.
Again, duration of the exercise appears to be a key factor. In another study, a short-term voluntary wheel running intervention (3 weeks, as opposed to 5 months) was not able to reduce the amyloid load, although it did improve cognition, of a Tg2576 mouse model of AD (Parachikova et al. Citation2008). Again, more studies into the links between PA and the neuropathological underpinnings of AD, exploring the causal mechanisms, are warranted.
PA and CV factors
There is compelling evidence that PA attenuates CV risk factors and vascular chronic diseases in humans (Blair and Connelly Citation1996; Dela et al. Citation1999; Ivy et al. Citation1999; Thomas et al. Citation2006; Mora et al. Citation2007; Mueller Citation2007), especially if we substitute sedentary time by light PA (Healy et al. Citation2008). This evidence could partially explain the preventive effects of PA on cognitive dysfunction, as cerebral CV events can be one cause of dementia. Another possible mechanism underlying this protective effect could be increased cerebral flow with exercise, resulting in the development of new capillaries (angiogenesis) in the hippocampus (see Kramer and Erickson Citation2007). In addition, the reduction in sympathetic outflow shown with exercise training may be important, as stress and CV diseases are both associated with overactivity of the sympathetic nervous system (Dishman et al. Citation2002; Mueller Citation2007). Increased activity of the SNS might also increase glucocorticoid secretion (Droste et al. Citation2007). However, it seems that the positive effects of PA on CV disease are not mediated through reductions in plasma homocysteine levels in humans (Mora et al. Citation2007). Thus, PA has direct effects on a key AD risk factor, one that is exacerbated by chronic psychological stress, providing the opportunity for interacting loops and pathways and a rationale for the development and testing of PA intervention strategies.
PA and type 2 diabetes
Another possible mechanism that deserves further attention is the role of PA in attenuating the onset of yet another stress-related condition in humans: type 2 diabetes (Blair and Connelly Citation1996; Dela et al. Citation1999; Ivy et al. Citation1999; Thomas et al. Citation2006). It is clear that regular PA reduces dyslipidemia and insulin resistance (Blair and Connelly Citation1996; Thomas et al. Citation2006) enhancing glucose uptake into cells (Dela et al. Citation1999; Ivy et al. Citation1999). Since insulin therapy slows cognitive decline in patients with AD (Plastino et al. Citation2010), it is predicted that PA may have a positive impact on AD through reducing insulin resistance. Yet again, more work in this area is warranted.
PA and neurotrophic factors
Rodent studies indicate that exercise increases (as opposed to stress, which decreases) neurotrophic factors in the hippocampus, which is associated with neurogenesis and neuroprotection (Cotman and Berchtold Citation2002; Cotman and Engesser-Cesar Citation2002; Garza et al. Citation2004; Berchtold et al. Citation2005; Vaynman et al. Citation2006; Kramer and Erickson Citation2007). Berchtold et al. (Citation2005) showed voluntary daily, and also intermittent, exercise increased the expression of BDNF, enhancing brain health and function in Sprague-Dawley rats. Furthermore, BDNF levels remained elevated for several days after cessation of exercise, and a brief re-exposure to exercise rapidly re-induced BDNF expression to levels that would normally take weeks of exercise to achieve. Consistent with these findings, Nichol et al. (Citation2009) found that exercise increased BDNF levels in the hippocampus of APOE3 and APOE4 transgenic mice. Cognitive function was particularly improved in APOE4 carriers. Interestingly, a 10-week strength training program (as opposed to the aerobic exercise regimes used in the rodent studies described above) in untrained human participants did not induce any changes in serum BDNF (Goekint et al. Citation2010). Thus, the relationship between different types and duration of exercise in human studies and availability of neurotropic factors is another relevant candidate for the examination of the interactive effects of stress and PA.
PA and inflammatory processes
Another possible mechanism underlying the effects of PA on the brain and cognition is attenuation of the negative effects of inflammation (see Cotman et al. Citation2007 for a review). Although exercise can produce a short-term inflammatory response, moderate regular exercise causes more sustained anti-inflammatory effect (Kasapis and Thompson Citation2005). For example, in a longitudinal study of 870 human participants aged 70–79 years, high levels of recreational PA were related to lower levels of pro-inflammatory markers, such as interleukin-6 (Il-6) and C-reactive protein (Reuben et al. Citation2003). Voluntary PA for 6 weeks attenuated the stress-induced suppression of natural killer cell cytotoxicity in rats produced by a foot-shock stress in rats (Dishman et al. Citation2000). Similarly, 4 weeks of voluntary wheel running prevented the stress-induced suppression of the antibody response in rats (Moraska and Fleshner Citation2001).
It is proposed (Parachikova et al. Citation2008) that the benefits of exercise on cognition in the Tg2576 mouse model of AD were partially mediated by the alteration of the expression of inflammatory molecules, including increased levels of the chemokines CXCL1 (Groα) and CXCL12 (SDF1), which are involved in cognitive restoration (Watson and Fan Citation2005; Parachikova and Cotman Citation2007; Parachikova et al. Citation2008).
PA and depression
Anti-depressant drug treatment may block or reverse the effects of stress and depression on BDNF levels, neurogenesis, and brain volume (see Warner-Schmidt and Duman Citation2006 for a review). PA may have similar effects as anti-depressive treatment, improving depressive symptoms in humans (Blumenthal et al. Citation1999; De Moor et al. Citation2006; Zheng et al. Citation2006; Blumenthal et al. Citation2007) and a range of indicators in rats [for example, increasing expression of BDNF (Russo-Neustadt et al. Citation1999), IGF-1 (Trejo et al. Citation2001), and serotonin expression (Ivy et al. Citation2003; Greenwood et al. Citation2005)]. Furthermore, aerobic exercise (of relatively low intensity and which depends primarily on the aerobic energy system) in untrained healthy participants is associated with an attenuated cortisol response to the serotonergic agonist meta-chlorophenylpiperazine (m-CPP), possibly reflecting a downregulation of central 5-HT2C receptors (Broocks et al. Citation2001). Animal studies implicate serotonin in the mechanisms by which PA attenuates memory loss (Ivy et al. Citation2003) and the consequences of uncontrollable stress (Greenwood et al. Citation2005). 5-HT1A receptor activation seems to be especially influential for PA-induced effects on cognition in one region of the hippocampus, CA4 (Ivy et al. Citation2003).
PA and oxidative stress
In addition to the pathways described above, PA may also have positive effects on oxidative stress. While low concentrations of ROS are beneficial, excessive concentrations cause apoptosis and necrosis (see Radak et al. Citation2008 for a review). PA induces an increase in adenosine-5′-triphosphate demands (indicating increased intracellular energy transport) and increased aerobic and/or anaerobic metabolic activity, resulting in more ROS. However, regular PA is believed to enhance the resistance to oxidative stress, providing increased protection in rodent studies (Vaynman et al. Citation2006; Radak et al. Citation2008) by enhancing the antioxidant defense mechanisms, including enzymes such as superoxide dismutase, catalase, and glutathione peroxidase (Ji Citation1999). This paradox could be explained by the hormesis theory, which basically states that small doses of toxic substances can enhance the body and brain protection against larger doses of those same substances (Radak et al. Citation2001, Citation2005, Citation2008). Animal studies indicate that this adaptation may be due to increased proteosome activity with PA (Ogonovsky et al. Citation2005). The proteosome is primarily responsible for disposing of oxidatively modified proteins (Grune et al. Citation1997) and increasing protein breakdown (Ogonovsky et al. Citation2005), both of which may contribute to reduced amyloid load (Celsi et al. Citation2004; Adlard et al. Citation2005). Another possible explanation is the increase in PA of the uncoupling protein 2 (UCP2), which is abundantly expressed in the hippocampus and is related to calcium homeostasis, ATP synthesis, and free radical management. UCP2 may also modulate BDNF production in the hippocampus, improving memory and learning (Dishman et al. Citation2006). The effects of exercise on oxidative stress depend on the intensity of the exercise, as moderate intensity increases oxidative stress protection while high-intensity exercise possibly increases oxidative stress (Goto et al. Citation2003). The duration of the exercise program is also a factor that needs to be considered, as an 8-week program of aerobic exercise did not reduce oxidative stress in patients with type 2 diabetes (Mori et al. Citation1999), whereas 12 months of endurance exercise was able to decrease oxidative stress markers in another sample of type 2 diabetes patients (Nojima and Watanabe Citation2008; ).
Conclusions
A range of physiological systems are activated by both psychological stress and AD, and evidence suggests their effects can be synergistic. The cascade of shared events includes HPA axis dysfunction, hippocampal atrophy, impaired memory, accumulation of amyloid load, increased inflammatory markers, increased insulin resistance, decreased BDNF availability, decreased serotonin expression, and excessive levels of free radicals causing oxidative stress.
Evidence from animal and human studies suggests that PA might play an important moderating role in this cascade, as voluntary regular PA decreases HPA axis responses to psychological stress, promotes angiogenesis and neurogenesis within the hippocampus, improves cognitive function, reduces amyloid load, inflammatory markers, insulin resistance, and oxidative stress while increasing BDNF and serotonin function (see ).
Although the evidence reviewed here suggests that PA interventions might be a potentially useful strategy to reduce risk factors for AD and even retard disease progression, the evidence of the effects of PA on AD itself is patchy. More research needs to be performed to explore the multiple interacting factors. For example, to our knowledge, the hypotheses that PA influences HPA axis reactivity, reduces oxidative stress, and/or reduces insulin resistance in AD patients or mouse models of AD have yet to be directly tested. Further work is required to tease apart the specific and interacting effects of PA and psychological stress on a range of biological pathways relevant to cognitive function and AD (Iqbal and Grundke-Iqbal Citation2010). Research is also urgently required to establish the appropriate type, intensity, frequency, and duration of PA that will be acceptable to, and achievable, by AD patients. These preliminary studies should be followed by clinical trials to evaluate their efficacy in terms of disease progression so that policy guidelines can be established and implemented. Given the scale of the problem and the relative weight of the evidence described here, we would propose that such investigations are clearly justified.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. 2004. Walking and dementia in physically capable elderly men. JAMA. 292:1447–1453.
- Adlard PA, Perreau VM, Pop V, Cotman CW. 2005. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer's disease. J Neurosci. 25:4217–4221.
- Apostolova LG, Dutton RA, Dinov ID, Hayashi KM, Toga AW, Cummings JL, Thompson PM. 2006. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol. 63:693–699.
- Arkin SM. 2003. Student-led exercise sessions yield significant fitness gains for Alzheimer's patients. Am J Alzheimers Dis Other Demen. 18 3: 159–170.
- Arsenault-Lapierre G, Chertkow H, Lupien S. 2010. Seasonal effects on cortisol secretion in normal aging, mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 31:1051–1054.
- Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. 2004. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 61:661–666.
- Barrientos R, Sprunger DB, Campeau S, Higgins EA, Watkins LR, Rudy JW, Maier SF. 2003. Brain-derived neurotrophic factor mRNA downregulation induced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience. 121:847–853.
- Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. 2005. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 133:853–861.
- Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. 2006. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol. 5:64–74.
- Björntorp P. 1997. Obesity. Lancet. 350:423–426.
- Blair SN, Connelly JC. 1996. How much physical activity should we do? The case for moderate amounts and intensities of physical activity. RQES. 67:193–205.
- Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, Herman S, Craighead WE, Brosse AL, Waugh R, Hinderliter A, Sherwood A. 2007. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 69:587–596.
- Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, Khatri P, Waugh R, Napolitano MA, Forman LM, Appelbaum M, Doraiswamy PM, Krishnan KR. 1999. Effects of exercise training on older patients with major depression. Arch Int Med. 159:2349–2356.
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. 2007. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 3:186–191.
- Broocks A, Meyer T, Gleiter CH, Hillmer-Vogel U, George A, Bartmann U, Bandelow B. 2001. Effect of aerobic exercise on behavioral and neuroendocrine responses to meta-chlorophenylpiperazine and to ipsapirone in untrained healthy subjects. Psychopharmacology. 155:234–241.
- Brown JD. 1991. Staying fit and staying well: Physical fitness as a moderator of life stress. J Pers Soc Psychol. 60:555–561.
- Cameron HA, Gould E. 1994. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 61:203–209.
- Campbell JE, Rakhshani N, Fediuc S, Bruni S, Riddell MC. 2009. Voluntary wheel running initially increases adrenal sensitivity to adrenocorticotrophic hormone, which is attenuated with long-term training. J Appl Physiol. 106 1: 66–72.
- Celsi F, Ferri A, Casciati A, D'Ambrosi N, Rotilio G, Costa A, Volonté C, Carrì MT. 2004. Overexpression of superoxide dismutase 1 protects against beta-amyloid peptide toxicity: Effect of estrogen and copper chelators. Neurochem Int. 44:25–33.
- Chao H, Sakai RR, Ma LY, McEwen BS. 1998. Adrenal steroid regulation of neurotrophic factor expression in the rat hippocampus. Endocrinology. 139:3112–3118.
- Chrousos GP. 2009. Stress and disorders of the stress system. Nat Rev Endocrinol. 5:374–381.
- Cohen S, Wills TA. 1985. Stress, social support, and the buffering hypothesis. Psychol Bull. 98 2: 310–357.
- Colcombe S, Kramer A. 2003. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci. 14 2: 125–130.
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. 1993. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 261:921–923.
- Cotman CW, Berchtold NC. 2002. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 25:295–301.
- Cotman CW, Berchtold NC, Christie L-A. 2007. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 30 9: 464–472.
- Cotman CW, Engesser-Cesar C. 2002. Exercise enhances and protects brain function. Exerc Sport Sci Rev. 30:75–79.
- Craft S. 2009. The role of metabolic disorders in Alzheimer disease and vascular dementia: Two roads converged. Arch Neurol. 66 3: 300–305.
- Craft S, Newcomer J, Kanne S, Dagogo-Jack S, Cryer P, Sheline Y, Luby J, Dagogo-Jack A, Alderson A. 1996. Memory improvement following induced hyperinsulinemia in Alzheimer's disease. Neurobiol Aging. 17:123–130.
- Craft S, Watson GS. 2004. Insulin and neurodegenerative disease: Shared and specific mechanisms. Lancet Neurol. 3:169–178.
- Craig R, Mindell J, Hirani V. 2008. Health survey for england – 2008: Physical activity and fitness. Summary of key findings, The NHS Information Centre, available at: http://www.ic.nhs.uk/pubs/hse08physicalactivity.
- Csernansky JG, Dong H. 2006. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am J Psychiatry. 163:2164–2169.
- Cummings JL, Vinters HV, Cole GM, Khachaturian ZS. 1998. Alzheimer's disease: Etiologies, pathophysiology, cognitive reserve, and treatment opportunities. Neurology. 51:2–17.
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. 2002. Depression: Perspectives from affective neuroscience. Annu Rev Psychol. 53:545–574.
- de Geus EJ, van Doornen LJ, Orlebeke JF. 1993. Regular exercise and aerobic fitness in relation to psychological make-up and physiological stress reactivity. Psychosom Med. 55:347–363.
- de Kloet ER, Joëls M, Holsboer F. 2005. Stress and the brain: From adaptation to disease. Nat Rev Neurosci. 6:463–475.
- De Moor MHM, Beem AL, Stubbe JH, Boomsma DI, De Geus EJC. 2006. Regular exercise, anxiety, depression and personality: A population-based study. Prev Med. 42:273–279.
- Dela F, Mikines KJ, Larsen JJ, Galbo H. 1999. Glucose clearance in aged trained skeletal muscle during maximal insulin with superimposed exercise. J Appl Physiol. 87:2059–2067.
- Department of Health. 2004. At least five a week: Evidence on the impact of physical activity and its relationship to health. A report from the chief medical officerLondon.
- Deuschle M, Gotthardt U, Schweiger U, Weber B, Körner A, Schmider J, Standhardt H, Lammers CH, Heuser I. 1997. With aging in humans the activity of the HPA system increases and its diurnal amplitude flattens. Life Sci. 61:2239–2246.
- Deuster PA, Chrousos GP, Luger A, DeBolt JE, Bernier LL, Trostmann UH, Kyle SB, Montgomery LC, Loriaux DL. 1989. Hormonal and metabolic responses of untrained, moderately trained, and highly trained men to three exercise intensities. Metabolism. 38:141–148.
- Deuster PA, Petrides JS, Singh A, Lucci EB, Chrousos GP, Gold PW. 1998. High intensity exercise promotes escape of adrenocorticotropin and cortisol from suppression by dexamethasone: Sexually dimorphic responses. J Clin Endocrinol Metab. 83 9: 3332–3338.
- Dickerson BC, Goncharova I, Sullivan MP, Forchetti C, Wilson RS, Bennett DA, Beckett LA, deToledo-Morrell L. 2001. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer's disease. Neurobiol Aging. 22:747–754.
- Dickerson SS, Kemeny ME. 2004. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bull. 130:355–391.
- Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH, Kramer AF, Levin BE, Moran TH, Russo-Neustadt AA, Salamone JD, Van Hoomissen JD, Wade CE, York DA, Zigmond MJ. 2006. Neurobiology of exercise. Obesity. 14 3: 345–356.
- Dishman RK, Jackson EM, Nakamura Y. 2002. Influence of fitness and gender on blood pressure responses during active or passive stress. Psychophysiology. 39:568–576.
- Dishman RK, Renner KJ, White-Welkley JE, Bunnell BN. 2000. Treadmill exercise training augments brain norepinephrine response to familiar and novel stress. Brain Res Bull. 52:337–342.
- Dong H, Yuede CM, Yoo HS, Martin MV, Deal C, Mace AG, Csernansky JG. 2008. Corticosterone and related receptor expression are associated with increased β-amyloid plaques in isolated Tg2576 mice. Neuroscience. 155:154–163.
- Droste SK, Chandramohan Y, Hill LE, Linthorst AC, Reul JMHM. 2007. Voluntary exercise impacts on the rat hypothalamic-pituitary-adrenocortical axis mainly at the adrenal level. Neuroendocrinology. 86:26–37.
- Droste SK, Collins A, Lightman SL, Linthorst AC, Reul JM. 2009. Distinct, time-dependent effects of voluntary exercise on circadian and ultradian rhythms and stress responses of free corticosterone in the rat hippocampus. Endocrinology. 150 9: 4170–4179.
- Droste SK, Gesing A, Ulbricht S, Müller MB, Linthorst AC, Reul JM. 2003. Effects of long-term voluntary exercise on the mouse hypothalamic-pituitary-adrenocortical axis. Endocrinology. 144 7: 3012–3023.
- Droste SK, Schweizer MC, Ulbricht S, Reul JMHM. 2006. Long-term voluntary exercise and the mouse hypothalamic-pituitary-adrenocortical axis: Impact of concurrent treatment with the antidepressant drug tianeptine. J Neuroendocrinol. 18:915–925.
- Duman RS. 2005. Neurotrophic factors and regulation of mood: Role of exercise, diet and metabolism. Neurobiol Aging. 26:88–93.
- Duman RS, Monteggia LM. 2006. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 59:1116–1127.
- Elliot MSJ, Ballard CG, Kalaria RN, Perry R, Hortobágyi T, Francis PT. 2009. Increased binding to 5-HT1A and 5-HT2A receptors is associated with large vessel infarction and relative preservation of cognition. Brain. 132:1858–1865.
- Fediuc S, Campbell JE, Riddell MC. 2006. Effect of voluntary wheel running on circadian corticosterone release and on HPA axis responsiveness to restraint stress in Sprague-Dawley rats. J Appl Physiol. 100:1867–1875.
- Fleshner M, Campisi J, Deak T, Greenwood BN, Kintzel JA, Leem TH, Smith TP, Sorensen B. 2002. Acute stressor exposure facilitates innate immunity more in physically active than in sedentary rats. Am J Physiol Regul Integr Comp Physiol. 282:1680–1686.
- Fox NC, Warrington EK, Freeborough PA, Hartikainen P, Kennedy AM, Stevens JM, Rossor MN. 1996. Presymptomatic hippocampal atrophy in Alzheimer's disease. A longitudinal MRI study. Brain. 119:2001–2007.
- Fratiglioni L, Paillard-Borg S, Winblad B. 2004. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 3:343–353.
- Garza AA, Ha TG, Garcia C, Chen MJ, Russo-Neustadt AA. 2004. Exercise, antidepressant treatment, and BDNF mRNA expression in the aging brain. Pharmacol Biochem Behav. 77:209–220.
- Gella A, Durani N. 2009. Oxidative stress in Alzheimer disease. Cell Adh Migr. 3 1: 88–93.
- Goekint M, De Pauw K, Roelands B, Njemini R, Bautmans I, Mets T, Meeusen R. 2010. Strength training does not influence serum brain-derived neurotrophic factor. Eur J Appl Physiol. 110:285–293.
- Gold SM, Dziobek I, Sweat V, Tirsi A, Rogers K, Bruehl H, Tsui W, Richardson S, Javier E, Convit A. 2007. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 50:711–719.
- Goto C, Higashi Y, Kimura M, Noma K, Hara K, Nakagawa K, Kawamura M, Chayama K, Yoshizumi M, Nara I. 2003. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: Role of endothelium-dependent nitric oxide and oxidative stress. Circulation. 108:530–535.
- Gould E. 1999. Serotonin and hippocampal neurogenesis. Neuropsychopharmacology. 21:46–51.
- Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. 2006. Glucocorticoids increase amyloid-β and tau pathology in a mouse model of Alzheimer's disease. J Neurosci. 26:9047–9056.
- Green RC, Cupples LA, Kurz A, Auerbach S, Go R, Sadovnick D, Duara R, Kukull WA, Chui H, Edeki T, Griffith PA, Friedland RP, Bachman D, Farrer Ll. 2003. Depression as a risk factor for Alzheimer disease: The MIRAGE study. Arch Neurol. 60:753–759.
- Greenwood BN, Foleyac TE, Day HEW, Burhans D, Brooks L, Campeau S, Fleshner M. 2005. Wheel running alters serotonin (5-HT) transporter, 5-HT1A, 5-HT1B, and alpha1b-adrenergic receptor mRNA in the rat raphe nuclei. Biol Psychiatry. 57 5: 559–568.
- Greenwood BN, Strong PV, Dorey AA, Fleshner M. 2007. Therapeutic effects of exercise: Wheel running reverses stress-induced interference with shuttle box escape. Behav Neurosci. 121 5: 992–1000.
- Gruenewald TL, Seeman TE. 2010. Stress and aging: A biological double jeopardy?. Annu Rev Gerontol Geriatr. 30:155–177.
- Grune T, Reinheckel T, Davies KJA. 1997. Degradation of oxidized proteins in mammalian cells. FASEB J. 11:526–534.
- Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. 2003. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 163:1524–1528.
- Gustaw-Rothenberg K. 2009. Dietary patterns associated with Alzheimer's disease: Population based study. Int J Environ Res Public Health. 6:1335–1340.
- Haan MN, Wallace R. 2004. Can dementia be prevented? Brain aging in a population-based context. Annu Rev Public Health. 25:1–24.
- Hatzinger M, Z'Brun A, Hemmeter U, Seifritz E, Baumann F, Holsboer-Trachsler E, Heuser IJ. 1995. Hypothalamic-pituitary-adrenal system function in patients with Alzheimer's disease. Neurobiol Aging. 16 2: 205–220.
- Healy GN, Wijndaele K, Dunstan DW, Shaw JE, Salmon J, Zimmet PZ, Owen N. 2008. Objectively measured sedentary time, physical activity, and metabolic risk the Australian diabetes, obesity and lifestyle study (AusDiab). Diabetes Care. 31 2: 369–371.
- Heiden M, Lyskov E, Nakata M, Sahlin K, Sahlin T, Barnekow-Bergkvist M. 2007. Evaluation of cognitive behavioural training and physical activity for patients with stress-related illnesses: A randomized controlled study. J Rehabil Med. 39 5: 366–373.
- Helzner EP, Luchsinger JA, Scarmeas N, Cosentino S, Brickman AM, Glymour MM, Stern Y. 2009. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch Neurol. 66 3: 343–348.
- Heyn P, Abreu BC, Ottenbacher KJ. 2004. The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis. Arch Phys Med Rehabil. 85:1694–1704.
- Hill LE, Droste SK, Nutt DJ, Linthorst ACE, Reul JMHM. 2010. Voluntary exercise alters GABAA receptor subunit and glutamic acid decarboxylase-67 gene expression in the rat forebrain. J Psychopharmacol. 24:745–756.
- Holmes DS, McGilley BM. 1987. Influence of a brief aerobic training program on heart rate and subjective response to a psychologic stressor. Psychosom Med. 49 4: 366–374.
- Ice GH, Katz-Stein A, Himes J, Kane RL. 2004. Diurnal cycles of salivary cortisol in older adults. Psychoneuroendocrinology. 29:355–370.
- Iqbal K, Grundke-Iqbal I. 2010. Alzheimer's disease, a multifactorial disorder seeking multitherapies. Alzheimers Dement. 6:420–424.
- Ivy AS, Rodriguez FG, Garcia C, Chen MJ, Russo-Neustadt AA. 2003. Noradrenergic and serotonergic blockade inhibits BDNF mRNA activation following exercise and antidepressant. Pharmacol Biochem Behav. 75 1: 81–88.
- Ivy JL, Zderic TW, Fogt DL. 1999. Prevention and treatment of non-insulin-dependent diabetes mellitus. Exerc Sport Sci Rev. 27:1–35.
- Jackson EM, Dishman RK. 2006. Cardiorespiratory fitness and laboratory stress: A meta-regression analysis. Psychophysiology. 43:57–72.
- Jacobson L, Sapolsky R. 1991. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocrine Rev. 12:118–134.
- Janson J, Laedtke T, Parisi JE, O'Brien P, Petersen RC, Butler PC. 2004. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 53:474–481.
- Jeong YH, Park CH, Yoo J, Shin KY, Ahn SM, Kim HS, Lee SH, Emson PC, Suh YH. 2006. Chronic stress accelerates learning and memory impairments and increases amyloid deposition in APPV7171-CT100 transgenic mice, an Alzheimer's disease model. FASEB J. 20:729–731.
- Ji LL. 1999. Antioxidants and oxidative stress in exercise. Proc Soc Exp Biol Med. 222:283–292.
- Kannangara TS, Lucero MJ, Gil-Mohapel J, Drapala RJ, Simpson JM, Christie BR, van Praag H. 2011. Running reduces stress and enhances cell genesis in aged mice. Neurobiol Aging. 32 12: 2279–2286.
- Karlamangla AS, Singer BH, Greendale GA, Seeman TE. 2005. Increase in epinephrine excretion is associated with cognitive decline in elderly men: MacArthur studies of successful aging. Psychoneuroendocrinology. 30:453–460.
- Karp A, Paillard-Borg S, Wang HX, Silverstein M, Winblad B, Fratiglioni L. 2006. Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dement Geriatr Cogn Disord. 21:65–67.
- Kasapis C, Thompson PD. 2005. The effects of physical activity on serum creactive protein and inflammatory markers: A systematic review. J Am Coll Cardiol. 45:1563–1569.
- Kiive E, Maaroos J, Shlik J, To˜ru I, Harro J. 2004. Growth hormone, cortisol and prolactin responses to physical exercise: Higher prolactin response in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 28:1007–1013.
- Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kåreholt I, Winblad B, Helkala EL, Tuomilehto J, Soininen H, Nissinen A. 2005. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 62:1556–1560.
- Kjær M. 1992. Regulation of hormonal and metabolic responses during exercise in humans. Exerc Sport Sci Rev. 20:161–184.
- Korkushko OV, Frolkis MV, Shatilo VB. 1995. Reaction of pituitary-adrenal and autonomic nervous systems to stress in trained and untrained elderly people. J Auton Nerv Syst. 54:27–32.
- Kovács P, Juránek I, Stankovičová T, Švec P. 1996. Lipid peroxidation during acute stress. Pharmazie. 51:51–53.
- Kramer AF, Erickson KI. 2007. Capitalizing on cortical plasticity: Influence of physical activity on cognition and brain function. Trends Cogn Sci. 11:342–348.
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. 2004. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: Impact of age and gender. Psychoneuroendocrinology. 29:83–98.
- Kudielka BM, Hellhammer D, Wüst S. 2009. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 34:2–18.
- Kudielka BM, Schmidt-Reinwald AK, Hellhammer DH, Schurmeyer T, Kirschbaum C. 2000. Psychosocial stress and HPA functioning: No evidence for a reduced resilience in healthy elderly men. Stress. 3:229–240.
- Kumari M, Badrick E, Sacker A, Kirschbaum C, Marmot M, Chandola T. 2010. Identifying patterns in cortisol secretion in an older population. Findings from the Whitehall II study. Psychoneuroendocrinology. 35:1091–1099.
- Kyrou I, Tsigos C. 2009. Stress hormones: Physiological stress and regulation of metabolism. Curr Opin Pharmacol. 9:787–793.
- Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W. 2006. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 144:73–81.
- Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. 2001. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 58 3: 498–504.
- Lee KW, Kim JB, Seo JS, Kim TK, Im JY, Baek IS, Kim KS, Lee JK, Han PL. 2009. Behavioral stress accelerates plaque pathogenesis in the brain of Tg2576 mice via generation of metabolic oxidative stress. J Neurochem. 108:165–175.
- Lopez JF, Chalmers DT, Little KY, Watson SJ. 1998. Regulation of serotonin1A, glucorticoid, and mineralocorticoid receptor in rat and human hippocampus: Implications for the neurobiology of depression. Biol Psychiatry. 43:547–573.
- Luger A, Deuster PA, Kyle SB, Gallucci WT, Montgomery LC, Gold PW, Loriaux DL, Chrousos GP. 1987. Acute hypothalamic-pituitary-adrenal response to the stress of treadmill exercise. N Engl J Med. 316:1309–1315.
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ. 1998. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neurosci. 1:69–73.
- Lupien SJ, Lepage M. 2001. Stress, memory, and the hippocampus: Can't live with it, can't live without it. Behav Brain Res. 127:137–158.
- Lupien SJ, Nair NP, Brière S, Maheu F, Tu MT, Lemay M, McEwen BS, Meaney MJ. 1999. Increased cortisol levels and impaired cognition in human aging: Implication for depression and dementia in later life. Rev Neurosci. 10 2: 117–139.
- Maher P, Schubert DR. 2009. Metabolic links between diabetes and Alzheimer's disease. Expert Rev Neurother. 9 5: 617–630.
- McEwen BS. 1994. The plasticity of the hippocampus is the reason for its vulnerability. Semin Neurosci. 6:239–246.
- McEwen BS. 1998. Protective and damaging effects of stress mediators. New Engl J Med. 338:171–179.
- McEwen BS. 2008. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 583:174–185.
- Meerlo P, Sgoifo A, Turek FW. 2002. The effects of social defeat and other stressors on the expression of circadian rhythms. Stress. 5:15–22.
- Messier C. 2003. Diabetes, Alzheimer's disease and apolipoprotein genotype. Exp Gerontol. 8:941–946.
- Miller TP, Taylor J, Rogerson S, Mauricio M, Kennedy Q, Schatzberg A, Tinklenberg J, Yesavage J. 1998. Cognitive and noncognitive symptoms in dementia patients: Relationship to cortisol and dehydroepiandrosterone. Int Psychogeriatr. 10:85–96.
- Moosavi M, Naghdi N, Maghsoudi N, Zahedi Asl S. 2007. Insulin protects against stress-induced impairments in water maze performance. Behav Brain Res. 176:230–236.
- Mooy JM, de Vries H, Grootenhuis PA, Bouter LM, Heine RJ. 2000. Major stressful life events in relation to prevalence of undetected type 2 diabetes: The Hoorn study. Diabetes Care. 23:197–201.
- Mora S, Cook N, Buring JE, Ridker PM, Lee I-M. 2007. Physical activity and reduced risk of cardiovascular events: Potential mediating mechanisms. Circulation. 116:2110–2118.
- Moraska A, Deak T, Spencer RL, Roth D, Fleshner M. 2000. Treadmill running produces both positive and negative physiological adaptations in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol. 279:1321–1329.
- Moraska A, Fleshner M. 2001. Voluntary physical activity prevents stress induced behavioral depression and anti-KLH antibody suppression. Am J Physiol Regul Integr Comp Physiol. 281:484–489.
- Mori TA, Dunstan DW, Burke V, Croft KD, Rivera JH, Beilin LJ, Puddey IB. 1999. Effect of dietary fish and exercise training on urinary F2-isoprostane excretion in non-insulin-dependent diabetic patients. Metabolism. 48:1402–1408.
- Moyna NM, Bodnar JD, Goldberg HR, Shurin MS, Robertson RJ, Rabin BS. 1999. Relation between aerobic fitness level and stress induced alterations in neuroendocrine and immune function. Int J Sports Med. 20:136–141.
- Mueller PJ. 2007. Exercise training and sympathetic nervous system activity: Evidence for physical activity dependent neural plasticity. Clin Exp Pharmacol Physiol. 34:377–384.
- Nader N, Chrousos GP, Kino T. 2010. Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrinol Metab. 21 5: 277–286.
- Näsman B, Olsson T, Viitanen M, Carlstrom K. 1995. A subtle disturbance in the feedback regulation of the hypothalamic-pituitary-adrenal axis in the early phase of Alzheimer's disease. Psychoneuroendocrinology. 20:211–220.
- Nichol KE, Deeny SP, Seif J, Camaclang K, Cotman CW. 2009. Exercise improves cognition and hippocampal plasticity in APOE 34 mice. Alzheimers Dement. 5:287–294.
- Nieman DC, Henson DA, Austin MD, Brown VA. 2005. Immune response to a 30-minute walk. Med Sci Sports Exerc. 37 1: 57–62.
- Nojima H, Watanabe H. 2008. Effect of aerobic exercise training on oxidative stress in patients with type 2 diabetes mellitus. Metabolism. 57:170–176.
- Nuntagij P, Oddo S, LaFerla FM, Kotchabhakdi N, Ottersen OP, Torp R. 2009. Amyloid deposits show complexity and intimate spatial relationship with dendrosomatic plasma membranes: An electron microscopic 3D reconstruction analysis in 3xTg-AD mice and aged canines. J Alzheimers Dis. 16:315–323.
- O'Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, Bowler JV, DeKosky ST. 2003. Vascular cognitive impairment. Lancet Neurol. 2:89–98.
- Ogonovsky H, Berkes I, Kumagai S, Kaneko T, Tahara S, Goto S, Radák Z. 2005. The effects of moderate-, strenuous- and over-training on oxidative stress markers, DNA repair and memory, in rat brain. Neurochem Int. 46:635–640.
- Onor ML, Trevisol M, Negro C, Signorini A, Saina M, Aguglia E. 2007. Impact of a multimodal rehabilitative intervention on demented patients and their caregivers. Am J Alzheimers Dis Other Demen. 22:261–272.
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. 1999. Diabetes mellitus and the risk of dementia: The Rotterdam study. Neurology. 53:1937–1942.
- Otte C, Hart S, Neylan TC, Marmar CR, Yaffe K, Mohr DC. 2005. A meta-analysis of cortisol response to challenge in human aging: Importance of gender. Psychoneuroendocrinology. 30:80–91.
- Pajović SB, Pejić S, Stojiljković V, Gavrilović L, Dronjak S, Kanazir DT. 2006. Alterations in hippocampal antioxidant enzyme activities and sympatho-adrenomedullary system of rats in response to different stress models. Physiol Res. 55:453–460.
- Palleschi L, Vetta F, De Gennaro E, Idone G, Sottosanti G, Gianni W, Marigliano V. 1996. Effect of aerobic training on the cognitive performance of elderly patients with senile dementia of Alzheimer type. Arch Gerontol Geriatr. 5:47–50.
- Parachikova A, Agadjanyan MG, Cribbs DH, Blurton-Jones M, Perreau V, Rogers J, Beach TG, Cotman CW. 2007. Inflammatory changes parallel the early stages of Alzheimer disease. Neurobiol Aging. 28 12: 1821–1833.
- Parachikova A, Cotman CW. 2007. Reduced CXCL12/CXCR4 results in impaired learning and is downregulated in a mouse model of Alzheimer disease. Neurobiol Dis. 28:143–153.
- Parachikova A, Nichol KE, Cotman CW. 2008. Short-term exercise in aged Tg2576 mice alters neuroinflammation and improves cognition. Neurobiol Dis. 30:121–129.
- Park E, Chan O, Li Q, Kiraly M, Matthews SG, Vranic M, Riddell MC. 2005. Changes in basal hypothalamo-pituitary-adrenal activity during exercise training are centrally mediated. Am J Physiol Regul Integr Comp Physiol. 289 5: 1360–1371.
- Plastino M, Fava A, Pirritano D, Cotronei P, Sacco N, Sperlì T, Spanò A, Gallo D, Mungari P, Consoli D, Bosco D. 2010. Effects of insulinic therapy on cognitive impairment in patients with Alzheimer disease and diabetes mellitus type-2. J Neurol Sci. 288:112–116.
- Podewils LJ, Guallar E, . 2005. Physical activity, APOE genotype, and dementia risk: findings from the cardiovascular health cognition study. Am J Epidemiol. 161:639–651.
- Querfurth HW, LaFerla FM. 2010. Mechanisms of disease. Alzheimer's disease. N Engl J Med. 362:329–344.
- Radak Z, Chung HY, Goto S. 2005. Exercise and hormesis: Oxidative stress-related adaptation for successful aging. Biogerontology. 6:71–75.
- Radak Z, Chung HY, Koltai E, Taylor AW, Goto S. 2008. Exercise, oxidative stress and hormesis. Ageing Res Rev. 7:34–42.
- Radak Z, Taylor AW, Ohno H, Goto S. 2001. Adaptation to exercise-induced oxidative stress: From muscle to brain. Exerc Immunol Rev. 7:90–107.
- Reagan LP. 2007. Insulin signaling effects on memory and mood. Curr Opin Pharmacol. 7:633–637.
- Reuben DB, Judd-Hamilton L, Harris TB, Seeman TE. 2003. The Associations Between Physical Activity and Inflammatory Markers in High-Functioning Older Persons: MacArthur Studies of Successful Aging. J Am Geriatr Soc. 51 8: 1125–1130.
- Rimmele U, Zellweger BC, Marti B, Seiler R, Mohiyeddini C, Ehlert U, Heinrichs M. 2007. Trained men show lower cortisol, heart rate and psychological responses to psychosocial stress compared with untrained men. Psychoneuroendocrinology. 32:627–635.
- Rimmele U, Seiler R, Marti B, Wirtz PH, Ehlert U, Heinrichs M. 2009. The level of physical activity affects adrenal and cardiovascular reactivity to psychosocial stress. Psychoneuroendocrinology. 34 2: 190–198.
- Rimmer JH, Smith DL. 2009. Alzheimer's disease. In: Durstine JL, Moore GE, Painter PL, Roberts SD. editors. ACSM's management for persons with chronic diseases and disabilities. Champaign, IL: Human Kinetics368–374.
- Risacher SL, Saykin AJ, West JD, Shen L, Firpi HA, McDonald BC. 2009. Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort. Curr Alzheimer Res. 6:347–361.
- Rolland Y, Pillard F, Klapouszczak A, Reynish E, Thomas D, Andrieu S, Rivière D, Vellas B. 2007. Exercise program for nursing home residents with Alzheimer's disease: A 1-year randomized, controlled trial. J Am Geriatr Soc. 55:158–165.
- Rosmond R, Dallman MF, Björntorp P. 1998. Stress-related cortisol secretion in men: Relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab. 83:1853–1859.
- Rothermundt M, Arolt V. 2001. Inflammatory markers in major depression and melancholia. J Affect Disord. 63:93–102.
- Rothman SM, Mattson MP. 2010. Adverse stress, hippocampal networks, and Alzheimer's disease. Neuromolecular Med. 12 1: 56–70.
- Rovio S, Kåreholt I, Helkala EL, Viitanen M, Winblad B, Tuomilehto J, Soininen H, Nissinen A, Kivipelto M. 2005. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol. 4 11: 690–691.
- Rovio S, Spulber G, Nieminen LJ, Niskanen E, Winblad B, Tuomilehto J, Nissinen A, Kivipelto M. 2010. The effect of midlife physical activity on structural brain changes in the elderly. Neurobiol Aging. 31:927–936.
- Russo-Neustadt A, Beard RC, Cotman CW. 1999. Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology. 21:679–682.
- Salmon P. 2001. Effects of physical exercise on anxiety, depression, and sensitivity to stress: A unifying theory. Clin Psychol Rev. 21:33–61.
- Sapolsky RM, Krey LC, McEwen BS. 1984. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc Nat Acad Sci. 81:6174–6177.
- Sato H, Takahashi T, Sumitani K, Takatsu H, Urano S. 2010. Glucocorticoid generates ROS to induce oxidative injury in the hippocampus, leading to impairment of cognitive function of rats. J Clin Biochem Nutr. 47:224–232.
- Schaaf M, de Jong J, de Kloet ER, Vreugdenhil E. 1998. Downregulation of BDNF mRNA and protein in the rat hippocamppus by corticosterone. Brain Res. 813:112–120.
- Seeman TE, McEwen BS, Singer BK, Albert MS, Rowe JW. 1997. Increase in urinary cortisol excretion and memory declines: MacArthur studies of successful aging. J Clin Endocrinol Metab. 82 8: 2458–2465.
- Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D'Agostino RB, Wilson PWF, Wolf PA. 2002. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med. 346:476–483.
- Shimomura T, Anan F, Masaki T, Umeno Y, Eshima N, Saikawa T, Yoshimatsu H, Kobayashi H. 2011. Homocysteine levels are associated with hippocampus volume in type 2 diabetic patients. Eur J Clin Invest. 41:751–758.
- Simons LA, Simons J, McCallum J, Friedlander Y. 2006. Lifestyle factors and risk of dementia: Dubbo study of the elderly. MJA. 184:68–70.
- Sinyor D, Schwartz SG, Peronnet F, Brisson G, Seraganian P. 1983. Aerobic fitness level and reactivity to psychosocial stress: Physiological, biochemical, and subjective measures. Psychosom Med. 45 3: 205–217.
- Solfrizzi V, Capurso C, D'Introno A, Colacicco AM, Santamato A, Ranieri M, Fiore P, Capurso A, Panza F. 2008. Lifestyle-related factors in predementia and dementia syndromes. Expert Rev Neurother. 8:133–158.
- Strachan MWJ, Reynolds RM, Frier BM, Mitchell RJ, Price JF. 2008. The relationship between type 2 diabetes and dementia. Br Med Bull. 88:131–146.
- Swanwick GRJ, Kirby M, Bruce I, Buggy F, Coen RF, Coakley D, Lawlor BA. 1998. Hypothalamic-pituitary-adrenal axis dysfunction in Alzheimer's disease: Lack of association between longitudinal and cross-sectional findings. Am J Psychiatry. 155:286–289.
- Taaffe DR, Irie F, Masaki KH, Abbott RD, Petrovitch H, Ross GW, White LR. 2008. Physical activity, physical function, and incident dementia in elderly men: The Honolulu-Asia aging study. J Gerontol A Biol Sci Med Sci. 63 5: 529–535.
- Takeda S, Naoyuki S, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, Kurinami H, Shinohara M, Rakugi H, Morishita R. 2010. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Aβ deposition in an Alzheimer mouse model with diabetes. PNAS. 107 15: 7036–7041.
- Teri L, Gibbons LE, McCurry SM, Logsdon RG, Buchner DM, Barlow WE, Kukull WA, LaCroix AZ, McCormick W, Larson EB. 2003. Exercise plus behavioral management in patients with Alzheimer disease: A randomized controlled trial. JAMA. 290 15: 2015–2022.
- Thomas DE, Elliott EJ, Naughton GA. 2006. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 3:1–41.
- Timofeeva E, Huang Q, Richard D. 2003. Effects of treadmill running on brain activation and the corticotropin-releasing hormone system. Neuroendocrinology. 77:388–405.
- Traustadóttir T, Bosch PR, Cantu T, Matt KS. 2004. Hypothalamic-pituitary-adrenal axis response and recovery from high-intensity exercise in women: Effects of aging and fitness. J Clin Endocrinol Metab. 89 7: 3248–3254.
- Traustadóttir T, Bosch PR, Matt KS. 2005. The HPA axis response to stress in women: Effects of aging and fitness. Psychoneuroendocrinology. 30:392–402.
- Trejo J, Cairo E, Torres-Aleman I. 2001. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 21:1628–1634.
- Uhart M, Chong RY, Oswald L, Lin P-I, Wand GS. 2006. Gender differences in hypothalamic-pituitary-adrenal (HPA) axis reactivity. Psychoneuroendocrinology. 31:642–652.
- Unger JB, Johnson CA, Marks G. 1997. Functional decline in the elderly: Evidence for direct and stress-buffering protective effects of social interactions and physical activity. Ann Behav Med. 19 2: 152–160.
- Vaidya V, Marek GJ, Aghajanian GA, Duman RS. 1997. 5-HT2A receptormediated regulation of BDNF mRNA in the hippocampus and the neocortex. J Neurosci. 17:2785–2795.
- van Cauter E, Leproult R, Kupfer D. 1996. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab. 81:2468–2473.
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. 1999. Running enhances neurogenesis, learning and long-term potentiation in mice. Proc Natl Acad Sci USA. 96:13427–13431.
- van Praag H, Shubert T, Zhao C, Gage FH. 2005. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 25:8680–8685.
- Vaynman SS, Ying Z, Yin D, Gomez-Pinilla F. 2006. Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res. 1070:124–130.
- Viru A, Karelson K, Smirnova K. 1992. Stability and variability in hormonal responses to prolonged exercise. Int J Sports Med. 13 3: 230–235.
- Wahbeh H, Kishiyama S, Zajdel D, Oken B. 2008. Salivary cortisol awakening response in mild Alzheimer disease, caregivers, and noncaregivers. Alzheimer Dis Assoc Disord. 22 2: 181–183.
- Wan L, Nie G, Zhang J, Luo Y, Zhang P, Zhang Z, Zhao B. 2011. β-Amyloid peptide increases levels of iron content and oxidative stress in human cell and Caenorhabditis elegans models of Alzheimer disease. Free Rad Biol Med. 50:122–129.
- Wang X, Zheng W, Xie JW, Wang T, Wang SL, Teng WP, Wang ZY. 2010. Insulin deficiency exacerbates cerebral amyloidosis and behavioral deficits in an Alzheimer transgenic mouse model. Mol Neurodegener. 5:305–310.
- Warner-Schmidt JL, Duman RS. 2006. Hippocampal neurogenesis: Opposing effects of stress and antidepressant treatment. Hippocampus. 16 3: 239–249.
- Watanabe T, Morimoto A, Sakata Y, Wada M, Murakami N. 1991. The effect of chronic exercise on the pituitary-adrenocortical responses in conscious rats. J Physiol. 439:691–699.
- Watson K, Fan GH. 2005. Macrophage inflammatory protein 2 inhibits beta-amyloid peptide (1-42)-mediated hippocampal neuronal apoptosis through activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase signaling pathways. Mol Pharmacol. 67:757–765.
- Weiner MF, Vobach S, Olsson K, Svetlik D, Risser RC. 1997. Cortisol secretion and Alzheimer's disease progression. Biol Psychiatry. 42:1030–1038.
- Weiner MF, Vobach S, Svetlik D, Risser RC. 1993. Cortisol secretion and Alzheimer's disease progression: A preliminary report. Biol Psychiatry. 34:158–161.
- Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. 2004. Physical activity, including walking, and cognitive function in older women. JAMA. 292:1454–1461.
- White-Welkley JE, Bunnell BN, Mougey EH, Meyerhoff JL, Dishman RK. 1995. Treadmill exercise training and estradiol differentially modulate hypothalamic-pituitary-adrenal cortical responses to acute running and immobilization. Physiol Behav. 57 3: 533–540.
- White-Welkley J, Warren G, Bunnell BN, Mougey EH, Meyerhoff JL, Dishman RK. 1996. Treadmill exercise training and estradiol increase plasma ACTH and prolactin after novel footshock. J Appl Physiol. 80 3: 931–939.
- Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. 2005. Midlife cardiovascular risk factors and risk of dementia in late-life. Neurology. 64:277–281.
- Williams CL, Tappen RM. 2007. Effect of exercise on mood in nursing home residents with Alzheimer's disease. Am J Alzheimers Dis Other Demen. 22:389–397.
- Wilson MM, Greer SE, Greer MA, Roberts L. 1980. Hippocampal inhibition of pituitary-adrenocortical function in female rats. Brain Res. 197:433–441.
- Wilson RS, Arnold SE, Schneider JA, Kelly JF, Tang Y, Bennett DA. 2006. Chronic psychological distress and risk of Alzheimer's disease in old age. Neuroepidemiology. 27:143–153.
- Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. 2007. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 69:1911–1920.
- Wolf OT. 2009. Stress and memory in humans: Twelve years of progress?. Brain Res. 1293:142–154.
- Wolf OT, Convit A, de Leon MJ, Caraos C, Qadri SF. Basal hypothalamo-pituitary-adrenal axis activity and corticotropin feedback in young and older men: Relationship to magnetic resonance imaging derived hippocampus and cingulate gyrus volumes. Neuroendocrinology. 2002a; 75:241–249.
- Wolf OT, Convit A, Thorn E, de Leon MJ. Salivary cortisol day profiles in elderly with mild cognitive impairment. Psychoneuroendocrinology. 2002b; 27:777–789.
- World Health Organisation. 2010. Global recommendations on physical activity and health, 65 years and above, available at http://www.who.int/dietphysicalactivity/physical-activity-recommendations-65years.pdf.
- Wrighten SA, Piroli GG, Grillo CA, Reagan LP. 2009. A look inside the diabetic brain: Contributors to diabetes-induced brain aging. Biochim Biophys Acta. 1792:444–453.
- Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, Harris T. 2003. Inflammatory markers and cognition in wellfunctioning African-American and white elders. Neurology. 61:76–80.
- Zafir A, Banu N. 2009. Modulation of in vivo oxidative status by exogenous corticosterone and restraint stress in rats. Stress. 12 2: 167–177.
- Zaidi SMKR, Banu N. 2004. Antioxidant potential of vitamins A, E and C in modulating oxidative stress in rat brain. Clin Chim Acta. 340:229–233.
- Zheng H, Liu Y, Li W, Yang B, Chen D, Wang X, Jiang Z, Wang H, Wang Z, Cornelisson G, Halberg F. 2006. Beneficial effects of exercise and its molecular mechanisms on depression in rats. Behav Brain Res. 168:47–55.
- Zhuo J-M, Wang H, Praticò D. 2011. Is hyperhomocysteinemia an Alzheimer's disease (AD) risk factor, an AD marker, or neither?. Trends Pharmacol Sci. 32 9: 562–571.
