Abstract
Stress modulates vital aspects of immune functioning in both human and non-human animals, including tissue repair. For example, dermal wounds heal more slowly and are associated with prolonged inflammation and increased bacterial load in mice that experience chronic physical restraint. Social stressors also negatively affect healing; however, previous studies suggest that the affected healing mechanisms may be stress model-specific. Here, the effects of either social isolation or physical restraint on dermal wound healing (3.5 mm wounds on the dorsum) were compared in hairless male mice. Social isolation beginning 3 weeks prior to wounding delayed healing comparably to physical restraint (12 h/day for eight days), in spite of marked differences in metabolic and hormonal consequences (i.e. body mass) between the two stress models. Additionally, isolated mice exhibited reductions in wound bacterial load and inflammatory gene expression (interleukin-1beta [IL-1β], monocyte chemoattractant protein [MCP]), whereas restraint significantly increased both of these parameters relative to controls. Experimentally augmenting bacterial concentrations in wounds of isolated mice did not ameliorate healing, whereas this treatment accelerated healing in controls. This work indicates that social isolation and restraint stressors comparably impair healing, but do so through disparate mechanisms and at different phases of healing.
Introduction
A growing body of basic and clinical research indicates that social environment profoundly modulates mental and physical health in social species (Cacioppo et al., Citation2011; Karelina & DeVries, Citation2011). In general, social support or positive social interactions improve health and mood outcomes, whereas social isolation or negative social interactions lead to mental and physical health impairments. In humans, perceived social isolation (i.e. loneliness) is associated with a pro-inflammatory phenotype (Cole et al., Citation2007), increased blood pressure (Hawkley et al., Citation2010), and poor stroke outcomes (Boden-Albala et al., Citation2005). The behavioral and physiological consequences of social isolation in humans are remarkably similar to those observed following social isolation (single-housing) in laboratory rodents. For example, socially isolating rodents precipitates depressive-like behavior (Martin & Brown, Citation2010), autonomic and hypothalamic-pituitary-adrenal (HPA) axis dysregulation (Grippo et al., Citation2007; Weiss et al., Citation2004; Williams et al., Citation2009), and altered heart rate (Grippo et al., Citation2007). These physiological effects translate into negative stroke (Craft et al., Citation2005) and cardiac arrest outcomes (Norman et al., Citation2011), and impaired dermal wound healing (Detillion et al., Citation2004). How isolation alters the kinetics of tissue repair was the focus of this study.
Wound healing is an intricately synchronized immune process characterized by three overlapping phases: inflammation, proliferation, and remodeling (Chen et al., Citation2010). During dermal healing in mice, the inflammatory phase (∼first 3 days of healing) is characterized by neutrophil and macrophage recruitment to the wound site for bacterial clearance. The proliferative phase (∼3–7 days post-wounding) consists of re-epithelialization of the wound, collagen formation, and angiogenesis. The final phase lasts weeks to months and is characterized by scar formation and extracellular matrix restructuring (Engeland & Marucha, Citation2009).
Many factors have been shown to modulate healing in humans including: sex, age, metabolic disease, nutrition, and psychological stress (Engeland et al., Citation2006; Engeland & Graham, Citation2011; Guo & Dipietro, Citation2010). For example, stress associated with the responsibility of caring for loved ones with Alzheimer’s disease prolongs dermal wound closure by 24% compared to age-matched non-caregiver controls (Kiecolt-Glaser et al., Citation1995). Such negative modulators of healing can increase the risk of wound bacterial infection and post-operative complications (Robson, Citation1997). In mice, a repeated physical restraint stress paradigm similarly impairs dermal healing. In-depth characterization of this model has determined that restraint induces prolonged inflammation, increases wound bacterial load and impairs bacterial clearance (Mercado et al., Citation2002a; Padgett et al., Citation1998; Rojas et al., Citation2002). This increase in wound bacterial load following restraint is thought to be the primary mechanism by which stress delays healing. Social isolation stress also impairs dermal healing in rodents (Detillion et al., Citation2004; Glasper & Devries, Citation2005; Levine et al., Citation2008), although the healing mechanisms by which this less physical and more psychosocial stressor exerts its effects remain unclear. In addition, isolation of research animals is a standard practice used in many biomedical studies; therefore it is important to understand the consequences of isolation on immunity.
In this study, the mechanisms by which social isolation delays dermal wound healing in mice were examined and compared to those of the well-established physical restraint stress model. The influence of these stressors on wound closure, gene expression for factors necessary for the inflammatory (i.e. pro-inflammatory cytokines, chemokines) or the proliferative (i.e. wound contraction, re-epithelialization, and angiogenesis) phases of tissue repair, and wound bacterial load were determined. We predicted that social isolation would impair dermal tissue repair, albeit more modestly than physical restraint, through similar deficits in healing mechanisms. In contrast, the results indicate that social isolation impairs healing in mice to the same magnitude as a physical stressor (restraint) but through divergent healing mechanisms.
Methods
Animals
Virus-antibody-free SKH-1 male mice (6–8 weeks of age) were obtained from Charles River, Inc (Wilmington, MA). SKH-1 mice were chosen because their skin is largely hairless (similar to human skin) and their wound healing process has been well-characterized (Eijkelkamp et al., Citation2007; Mercado et al., Citation2002a, Citationb; Padgett et al., Citation1998). Mice were housed in polypropylene cages (27.8 × 7.5 × 13 cm) with corncob bedding and microisolator lids in a vivarium at a temperature of 21 ± 1 °C under a 14:10 h light:dark cycle (lights off at 6 pm) and had access to food (Harlan 7912 rodent chow) and water ad libitum (unless otherwise noted). The vivarium is accredited by The American Association for the Accreditation of Laboratory Animal Care (AAALAC) and all procedures were approved by the Office of Animal Care and Institutional Biosafety at the University of Illinois at Chicago (UIC) and conform to the NIH Guide for the Care and Use of Laboratory Animals.
Experiment 1: Comparison between chronic social isolation and restraint on physiology and behavior
Forty mice were used for this experiment to compare the effects of social isolation and restraint on body mass, HPA axis reactivity, and behavior. Mice were either (1) isolated for three weeks before and throughout assessments (ISOLATION); (2) group-housed controls (GROUP; 5/cage); (3) physically restrained (12 h/day) for three days prior to the HPA axis assessment (RESTRAINT; see below); or (4) respective controls which were food- and water-deprived (but free to roam their cage) for the same 12-h period (FWD). Body mass was recorded prior to isolation, after three weeks of isolation (just before restraint for RESTRAINT group), and 3 days later (after 3 days of restraint for RESTRAINT group).
Chronic restraint group
Three days before HPA axis reactivity assessment, RESTRAINT mice were placed in well-ventilated 50 ml polypropylene tubes daily for 12–13 h per day within their homecages (18:00–06:00 h) (Padgett et al., Citation1998).
HPA axis reactivity to acute stressor
Circulating corticosterone concentrations were determined before and after an acute stressor (50-min bout of restraint) to compare HPA axis feedback function among treatment groups. Within two minutes of isoflurane anesthetization, retro-orbital blood samples (100 µl) were collected: (1) before a 50-min bout of physical restraint (baseline), (2) immediately after restraint (post-acute stressor), and (3) 55 minutes after the end of restraint (recovery). All mice in a cage were anesthetized simultaneously. Mice were returned to their homecage between the collection of the post-stressor and recovery blood samples. The acute stressor was the same as that described for the chronic RESTRAINT group, except the immobilized mice were placed on a counter under standard ambient lighting for 50 min. Corticosterone was measured in duplicate in all blood samples via EIA according to the manufacturer’s instructions (Enzo Life Sciences, Plymouth Meeting, PA) after 1:30 dilution. Intrassay variation was 9% and interassay variation for the three plates was 2.4%.
Anxiety-like behavior
As a follow-up measure to the HPA assessment, total locomotor activity was measured using an open field test in isolated and group-housed mice. Increased total locomotor activity and velocity in an open field are indicative of anxiety-like behavior (Crawley, Citation2000). One week after HPA axis reactivity assessment and after 30 min of acclimation to the dark testing room, mice were individually placed in a clear 27 × 27 cm acrylic box inside a ventilated cabinet using dim red light (during active dark phase: 20:00–23:00 h). A frame at the base of the box consisting of 24 photobeams in a 12 × 12 arrangement detected the location of horizontal movement (Med Associates Inc, St. Albans, VT). Total locomotor activity and average velocity were tracked for 5 min.
Experiment 2: Comparison between social isolation and restraint on wound healing
Eighty mice were used for this experiment to determine the effects of chronic social isolation on dermal wound healing relative to group-housed controls and to compare these findings to those of a well-established model of chronic restraint stress. Mice were either (1) isolated for three weeks before and throughout healing (ISOLATION); (2) group-housed controls (GROUP; 5/cage); (3) physically restrained three days prior to wounding and five days after wounding for 12 h/day (RESTRAINT); or (4) respective controls which were food- and water-deprived (but free to roam their cage) for the same 12-h period (FWD). Three days before wounding and five days after wounding mice were restrained as described in Experiment 1 for chronic restraint group. Based on pilot studies in our lab, three weeks of isolation prior to wounding was determined to exert significant and repeatable healing impairments. In separate groups of mice, wounds were biopsied prior to sacrifice on Days 1, 3, or 5 post-wounding to measure gene expression for factors important for the inflammatory and proliferative phases of healing, and to quantify bacterial load.
Dermal wounding
Mice (at 8–9 weeks of age) were anesthetized (100 mg/kg ketamine and 10 mg/kg xylazine; i.p.), the skin was cleaned with alcohol, and two full-thickness 3.5 mm excisional wounds were placed on the dorsum using sterile biopsy punches (Miltex Instrument Company, Plainsboro, NJ) just caudal to the shoulder blades. In one cohort of mice, wound closure was captured by daily photographs (through Day 5 post-wounding) and images were analyzed by a single investigator blind to the treatments (L.Y.). Photographs of the biopsy sites were taken with a 3.5 mm standard-sized dot placed beside the wound to control for variations in photograph angle and distance. Wound size was measured using Canvas 9 software (ACD Systems, Seattle, WA; Horan et al., Citation2005) and expressed as the ratio of the wound area to the standard dot measurement, then as a ratio to the original wound size on Day 0 (Horan et al., Citation2005). In separate cohorts of mice, both wounds were harvested on Days 1, 3, or 5 post-wounding by sterile 6.0 mm punch biopsies (Miltex Instrument Company, Plainsboro, NJ) following deep anesthetization (ketamine/xylazine). One wound was used to quantify bacteria (n = 4–5/group) and the other wound was used for genomic comparison of factors known to regulate wound healing using quantitative real-time PCR (qRT-PCR; n = 9–12/group).
Bacterial quantification
Wounds harvested for bacterial quantification were weighed and then homogenized on ice in 1 ml chilled PBS. The homogenates were serially diluted 1:10 six times with PBS, and 100 μl of each dilution was plated in duplicate on brain-heart infusion agar (Becton Dickinson, Franklin Lakes, NJ). Following overnight incubation at 37 °C with 5% CO2, colonies were counted to determine initial colony forming units (CFU) per gram of wound tissue (Rojas et al., Citation2002).
qRT-PCR
Gene expression for various factors involved in the inflammatory and proliferative phases of wound healing were determined (see for gene function and primer/probe sequences). Wounds harvested for qRT-PCR were immediately placed in 1 ml Trizol (Invitrogen, Carlsbad, CA), flash frozen in liquid nitrogen, and stored at −80 °C. Total RNA was extracted from wound tissue, and reverse transcribed to create cDNA, as previously described (Horan et al., Citation2005; Mercado et al., Citation2002a). All TaqMan primers and probes (Biosearch Technologies, Navato, CA) were designed using Primer Express software (Applied Biosystems, Carlsbad, CA) with the exception of GAPDH and KGF (off-the-shelf primer/probe sets, Applied Biosystems). Using a 7000 Sequence Detection System (Applied Biosystems), relative gene expression of individual samples were calculated by the comparative CT method (2−ΔCT) with GAPDH used as a housekeeping gene.
Table 1. Primer and TaqMan probe sequences for qRT-PCR.
Experiment 3: Effects of social isolation on bacterial clearance
Based on the finding in Experiment 2, that wound bacterial burden was significantly lower in isolated mice compared with group-housed controls, 35 mice were used for a follow-up experiment to determine whether supplementation of wound bacteria would ameliorate healing in isolates. Appropriate skin bacterial concentrations and species promote dermal healing (Gutierrez-Garcia & Contreras, 2009; Hasen et al., 2010). Bacteria were harvested from intact dorsal skin of group-housed mice via skin swabs and grown in culture; such cultures primarily consist of aerobic proteobacteria species (Bikowski, Citation1999; Grice et al., Citation2008). Mice were either isolated for three weeks before and throughout wounding (n = 15) or group-housed (5/cage; n = 10). 1.6 × 108 bacteria (in 25 µl sterile PBS) or PBS for controls (n = 5–10/group) were applied topically once to freshly excised wounds and allowed to air-dry while mice recovered from the anesthesia. This quantity of bacteria corresponds to the average number of bacteria in wounds of restrained mice on Day 5 post-wounding as determined in Experiment 2. Wound size was recorded daily.
Statistical analysis
Differences in wound closure, bacterial quantification, and mRNA expression were analyzed using repeated measures and 2-way ANOVA using SPSS v.19.0 software (Chicago, IL). PCR data were square-root transformed to achieve a normal distribution. Data were determined to be statistically significant when p < 0.05. Error bars represent standard error of the mean (SEM).
Results
Experiment 1: Effects of social isolation and restraint on physiology and behavior
Body mass
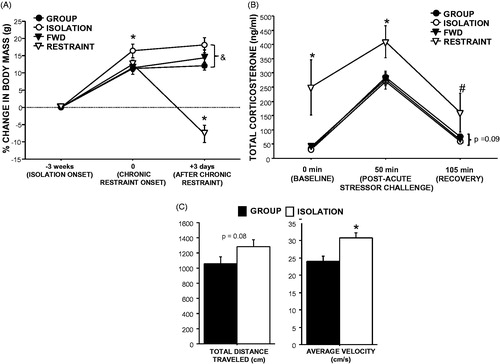
Three weeks of social isolation increased body mass relative to group-housing (GROUP, RESTRAINT, and FWD were combined because these three groups were treated identically up to this point; t1,38 = 2.7, p = 0.01; ). Three days of chronic restraint decreased body mass relative to all other groups (F1,36 = 28.8, p < 0.001; ). Body mass of isolated mice remained higher than group-housed controls at this time (t1,18 = 7.7, p < 0.001).
Figure 1. Effects of social isolation and chronic restraint on physiology and behavior. (A) Three weeks of social isolation (ISOLATION) increased body mass compared with group-housed controls (GROUP), whereas daily 12–13-h restraint for three days (RESTRAINT) decreased body mass relative to controls that were food- and water-deprived during the same time period (FWD). *p < 0.05 compared with all other groups; &p < 0.05 between group and isolation treatments. (B) Three days of chronic restraint (12 h/day) increased circulating corticosterone at rest (baseline), immediately after a 30-min acute restraint challenge, and 55 min after the challenge (recovery) compared with FWD controls and three weeks of social isolation or group housing. Isolation tended to decrease recovery corticosterone concentrations relative to group-housed controls. *p < 0.05 relative to all other groups, #p < 0.05 relative to all other groups except group-housed controls, p = 0.09 between ISOLATION and GROUP. (C) Four weeks of social isolation increased total distance traveled and average locomotor velocity during a 5-min open field test compared with group-housed controls. *p < 0.05. n = 10/group.
Circulating corticosterone concentrations
Circulating corticosterone concentrations were higher in RESTRAINT mice than all other groups at baseline (F3,32 = 5.8, p = 0.003), post-challenge (F3,34 = 3.6, p = 0.02), and recovery (F3,31 = 2.2, p = 0.1; ), except compared with group-housed controls at recovery due to high variability (p = 0.2). At recovery, isolated mice tended to have higher circulating corticosterone concentrations compared with group-housed controls (t1,16 = 1.78, p = 0.09).
Anxiety-like behavior
Isolation increased locomotor velocity in an open field relative to group-housing (t1,18 = 3.2, p = 0.005; ). Similarly, total locomotor activity was increased in isolated mice, although this difference was not statistically significant (t1,18 = 1.8, p = 0.08; ). While data were not shown for the restraint group because the daily restraint treatment had ceased for one week by the time of the behavioral testing, neither locomotor activity nor velocity was altered in this group relative to controls (p < 0.05).
Experiment 2: Effects of social isolation and restraint on wound healing
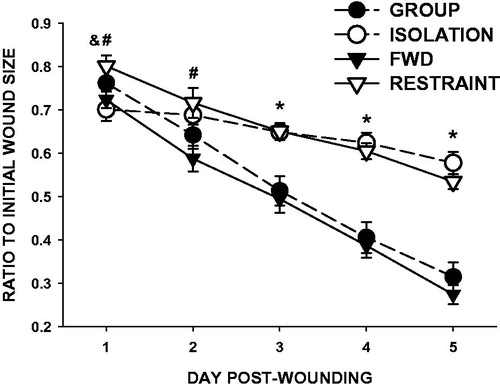
Both isolation and restraint delayed wound closure (; F1,28 = 10.451, p < 0.01; F1,28 = 30.590, p < 0.001, respectively) and altered the pattern of healing over time (F4,112 =40.446, p < 0.001; F4,112 = 11.977, p < 0.001, respectively) compared with respective controls. Specifically, isolation delayed wound healing from Days 3 to 5 compared with group-housed controls (p < 0.001 on each day) and restraint delayed wound healing from Days 1 to 5 relative to food- and water-deprived controls (FWD; p < 0.05 or better; ). Wound closure did not differ between the two control groups at any time point (group-housed and FWD; p > 0.05). Wounds in restrained mice were significantly larger than those of isolates on Day 1 post-wounding only (p < 0.05).
Figure 2. Social isolation and restraint comparably impair dermal wound closure. Three weeks of social isolation (open circles) reduced wound closure (relative to initial wound area) compared with group-housed controls (closed circles) on Days 3–5 post-wounding. Daily 12-h restraint (open triangles) 3 days prior and throughout wound healing decreased wound closure compared with food- and water-deprived controls (closed triangles) on Days 1–5 post-wounding. n = 15/group; #p < 0.05 for FWD versus RESTRAINT, *p < 0.001 for both stress groups versus respective controls, &p < 0.05 RESTRAINT versus ISOLATION.
Bacterial quantification
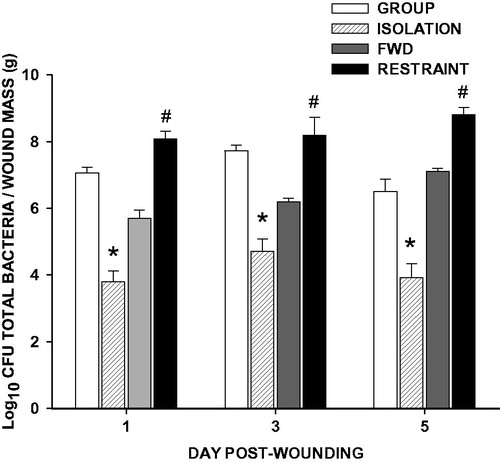
Despite inducing comparable wound closure impairments, social isolation decreased wound bacterial load whereas restraint increased wound bacterial load relative to the appropriate control groups on Days 1, 3, and 5 post-wounding (; p < 0.05 in all cases).
Figure 3. Social isolation reduces, and restraint increases, total bacterial load. Three weeks of social isolation (ISOLATION) decreased wound total bacterial load on Days 1, 3, and 5 post-wounding compared with group-housed controls (GROUP). Conversely, daily 12-h restraint 3 days prior and throughout wound healing (RESTRAINT) increased wound total bacterial load on Days 1, 3, and 5 post-wounding compared with food- and water-deprived controls (FWD). n = 4–5/group; *p < 0.05 compared with GROUP, #p < 0.05 compared with FWD.
Wound gene expression of factors that regulate healing
Isolation decreased wound tissue gene expression of proinflammatory cytokine, interleukin-1β (IL-1β), and chemokine, monocyte chemoattractant protein-1 (MCP-1/CCL2), on Day 1 post-wounding (; p < 0.05), whereas restraint increased IL-1β, TNF-α, and MIP-1α/CCL3 mRNA on Day 1 (; ). Both social isolation and restraint decreased gene expression for keratinocyte growth factor (KGF) on Days 1 (isolation) and 3 (isolation and restraint), as well as, α-smooth muscle actin (α-SMA) on Days 3 (isolation and restraint) and 5 (restraint) (; p < 0.05). Additionally, isolation reduced keratinocyte chemoattractant (KC/CXCL1) on Day 3 post-wounding (; p < 0.05), whereas restraint had no effect on gene expression of this factor involved in both the inflammatory and proliferative phase. Conversely, restraint singularly decreased Day 5 mRNA expression of vascular endothelial growth factor (VEGF) relative to FWD controls (; p < 0.05). No differences in IL-6 were observed using either stress paradigm.
Figure 4. Social isolation alters healing gene expression differently than restraint. Three weeks of social isolation decreased wound (A) interleukin-1β (IL-1β) and (B) monocyte chemoattractant protein-1 (MCP-1) mRNA on Day 1, decreased (C) keratinocyte growth factor (KGF) mRNA on Days 1 and 3, and decreased (D) keratinocyte chemoattractant (KC) and (E) α-smooth muscle actin (αSMA) mRNA on Day 3 post-wounding as measured by qRT-PCR. Daily 12-h restraint 3 days prior and throughout wound healing increased IL-1β on Day 1, decreased KGF and KC on Day 3, and decreased αSMA on Days 3 and 5 post-wounding. n = 9–12/group; *p < 0.05 compared with GROUP, #p < 0.05 compared with FWD.
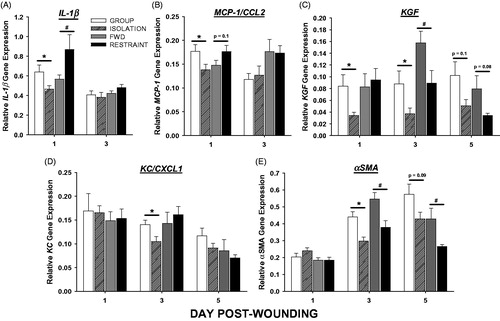
Table 2. Effects of social isolation and restraint stress on wound gene expression for factors that regulate healing.
Experiment 3: Effects of social isolation on bacterial clearance
Based on the findings in Experiment 1, that wound bacterial burden was significantly lower in isolated mice compared with controls while wound closure was impaired, this experiment was designed to determine whether supplementation of wound bacteria would ameliorate healing in isolates, given that appropriate skin bacterial concentrations and species promote dermal healing by stimulating appropriate macrophage recruitment (Gutierrez-Garcia & Contreras, 2009; Hasen et al., 2010).
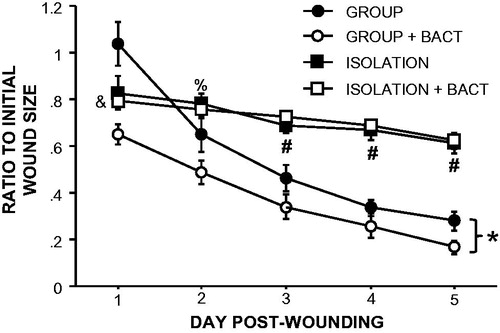
Similar to the previous experiment, isolation alone delayed wound closure (F4,32 = 39.4, p < 0.001; ). The addition of indigenous skin bacteria to wounds improved healing rates in group-housed control mice compared with PBS-treated group-housed controls (F4,32 = 12.6, p < 0.001), driven primarily by the immediate reduction in wound size on Day 1 post-wounding (p < 0.05). In contrast, bacterial supplementation did not affect healing rates in isolated mice (F4,52 = 1.6, p > 0.05). As such, the wound sizes of isolated mice with bacterial treatment remained larger than those of bacteria-treated group-housed mice (F4,52 = 33.8, p < 0.001) on Days 1–5 post-wounding and non-treated group-housed mice on Days 1 and 3–5 (p < 0.05 for each day).
Figure 5. Supplementation of bacteria on wounds does not improve healing in socially isolated mice. Supplementation with indigenous bacteria to wounds at the time of wounding improved healing in group-housed but not isolated mice. n = 5–10/group; *p < 0.05 repeated measures between GROUP and GROUP + BACT; #p < 0.05 between GROUP, GROUP + BACT and ISOLATION, ISOLATION + BACT; &p < 0.05 between ISOLATION + BACT and both GROUP and GROUP + BACT; %p < 0.05 between ISOLATION + BACT and GROUP + BACT.
Discussion
Previous studies demonstrate that both models of stress, social isolation (Detillion et al., Citation2004; Glasper & Devries, Citation2005; Levine et al., Citation2008) and restraint (Mercado et al., 2002a; Padgett et al., 1998; Tymen et al., 2013), independently impair healing, findings which were corroborated by the present experiments. However, different types of stressors (e.g. psychosocial versus physical) can have varied effects on physiological processes (Gutierrez-Garcia & Contreras, Citation2009; Santha et al., Citation2013). This is the first study to directly compare the magnitude of the healing impairment and the underlying healing mechanisms affected by these two stressor paradigms. In contrast to our prediction, that isolation would have a more modest effect on wound closure than restraint, we observed that three weeks of social isolation was sufficient to induce healing deficits of a similar magnitude as those reported following eight daily sessions of 12-h restraint in mice. Thus the physiological consequences of social isolation on wound healing appear comparable to that of a more physical stressor.
The temporal dynamics of the observed healing deficits differed between the two stressors. Wound closure impairments began on Day 1 (inflammatory phase) in restrained mice and on Day 3 (proliferative phase) in isolated mice, relative to controls. This timing discrepancy suggests that the early differences in wound closure of restrained mice were initially driven by changes in bacterial/inflammatory mechanisms and later by effects on proliferative phase mechanisms, whereas the relatively later healing impairment observed in isolated mice were driven by effects on proliferative phase mechanisms only. In support of this, social isolation decreased gene expression for factors important in the proliferative phase of healing: keratinocyte growth factor (KGF) and α-smooth muscle actin (α-SMA). These decreases in KGF and α-SMA suggest that re-epithelialization (Raja et al., Citation2007) and wound contraction (Desmouliere et al., Citation2005) are negatively affected by isolation. Similar results were seen during restraint, and indeed wound contraction is impaired in restrained mice (Horan et al., Citation2005). Isolation also decreased keratinocyte chemoattractant (KC) gene expression (important for re-epithelialization), whereas restraint had no such effect. Conversely, gene expression for VEGF (critical for angiogenesis) was reduced with restraint but not with isolation. Taken together, these data suggest that isolation and restraint affect different phases of wound healing. A more detailed examination of the influence of isolation on tissue repair mechanisms through the later remodeling phase of healing is warranted.
Based on previous studies using the restraint stress model, high bacterial load has been hypothesized to mediate stress-impaired healing in rodents (Rojas et al., Citation2002). However, the present results in male mice support recent findings in female mice (Pyter et al., Citation2014) indicating that while the healing rates of isolated and restrained mice were similar, wounds from isolated mice had very low bacterial counts, whereas those of restrained mice were high compared to controls. This difference likely reflects, in part, the relatively higher frequency with which wounds from group-housed mice came into direct contact with surfaces containing microbes (e.g. littermates or restraint tubes). Isolated mice, in contrast, were not exposed to bacteria from the skin or feces of other mice. Alternatively, such differences in microbiology may be due to differences in the physiological consequences of these two stressors, with physical restraint causing the skin to be more amenable to bacterial growth. A definitive understanding of the causes underlying the disparate wound microbiology between these two stressors remains to be determined.
Predictably, inflammatory gene expression in the wound corresponded with the aforementioned wound bacterial burden. Compared to controls, wounds of restrained mice (high bacterial load) displayed increased proinflammatory gene expression (IL-1β, TNFα, MIP-1α), whereas wounds of isolated mice (low bacterial load) displayed decreased proinflammatory expression (IL-1β, MCP-1). This is consistent with previously reported increases in neutrophil activity and proinflammatory cytokine production in wounds of restrained mice (Mercado et al., Citation2002a; Rojas et al., Citation2002; Tymen et al., Citation2013). The combination of elevated proinflammatory responses and circulating glucocorticoid concentrations in restrained mice may reflect glucocorticoid insensitivity of immune cells, as is observed in other chronic stress models (O'Connor et al., Citation2003; Sheridan et al., Citation2000).
To determine the relevance of the reduced bacterial load observed in isolated mice on wound closure, wounds were supplemented with bacteria in a subset of isolated and control mice. The beneficial effects of bacteria on wound closure have been shown previously in unmanipulated mice (Levenson et al., Citation1983; Tenorio et al., Citation1976). In the present study, supplementing bacteria onto wounds resulted in healing improvements for group-housed control mice only. In contrast, isolated mice that had wounds treated with bacteria healed just as slowly as non-treated isolates, suggesting that lowered bacterial burden is not the primary mechanism by which slower healing occurs during isolation. Rather, increased wound bacteria may be responsible for the relatively earlier wound healing impairments observed in restrained mice, but is not seemingly related to the later healing impairments in isolated mice. This is consistent with findings in restrained mice, whose healing rates also remain unchanged when supplemented with bacteria (Mercado et al., Citation2002a; Padgett et al., Citation1998; Rojas et al., Citation2002). Together, these results indicate that bacterial load and wound closure rates can be dissociated.
Some potential confounding factors inherent to group-housing (e.g. grooming, huddling) that might modulate wound repair are lacking in social isolation paradigms. While grooming was not directly assessed in this study, previous work (Vegas et al., Citation2012) and our observations (unpublished) indicate that group-housed mice do not groom the wounds of cagemates. Single-housed mice also display compensatory thermoregulatory mechanisms (Himms-Hagen & Villemure, Citation1992) which make potential differences in body temperature unlikely to contribute to the observed effects on tissue repair. This work supports previous studies that demonstrate the negative effects of social isolation in rodents on other physical health outcomes (Advani et al., Citation2007; Hasen et al., Citation2010; Norman et al., Citation2011). The potential impact of isolation on immune processes, such as tissue repair, reinforces the need for thoughtful consideration and reporting of social housing conditions in biomedical studies.
The effects of two common rodent models of stress on wound healing were compared in this study. Stressors are generally validated by the physiological (e.g. HPA axis response), behavioral (e.g. anxiety-like behavior), and/or neurobiological (e.g. hippocampal damage) effects they elicit. For example, restraint is characterized as a physical stressor and reliably elicits elevated HPA axis output (glucocorticoid release) (Barlow et al., Citation1975; Glavin et al., Citation1994), whereas isolation is considered more of a psychological stressor and mixed results have been reported on HPA axis output following chronic isolation (Sanchez et al., Citation1998; Scaccianoce et al., Citation2006; Vegas et al., Citation2012). Using the present restraint and isolation paradigms, several physiological and behavioral effects were observed. Body mass decreased in restrained mice and increased in isolated mice as previously reported by our lab and others’ (Hotchkiss et al., Citation2004; Jeong et al., Citation2013; Martin & Brown, Citation2010; Pyter et al., Citation2014). The reduction in body mass of restrained mice was presumably independent of food and water deprivation, as food- and water-deprived controls maintained their body mass.
Three days of chronic restraint resulted in consistently elevated circulating corticosterone concentrations over an assessment of HPA axis reactivity to an acute stressor. These elevations appear to conflict with other reports in which chronic restraint renders the HPA axis less sensitive to subsequent novel stressors (Buwalda et al., Citation1999; Deak et al., Citation1999). However, in the present study, they likely reflect (1) a sustained corticosterone release for several hours following this prolonged restraint paradigm (12–13 h) and (2) a corroboration of previous observations that mice do not habituate to repeated exposure to the same stressor (e.g. restraint) (Hotchkiss et al., Citation2004; Tuli et al., Citation1995). Elevated corticosterone has been associated with impaired wound healing in rodents and humans (Glaser et al., Citation1999; Padgett et al., Citation1998), although other stress-induced changes likely contribute as well (Eijkelkamp et al., Citation2007; Padgett et al., Citation1998).
In contrast, isolation had little influence on circulating corticosterone concentrations and responses to an acute stressor. Isolation has been reported to have mixed effects on HPA axis output ranging from corticosterone increases (Vegas et al., Citation2012), to corticosterone decreases (Boggiano et al., Citation2008; Martin & Brown, Citation2010), to eliciting no change (Arndt et al., Citation2009; Scaccianoce et al., Citation2006). These mixed results are likely due to differences in housing conditions, strain, sex and circadian timing of blood sampling. Based on a previous study from our lab, females of this same strain (SKH-1) exhibit decreased corticosterone concentrations both at baseline and during recovery from a similar acute stressor (Pyter et al., Citation2014). Although corticosterone has been shown to be partially responsible for restraint stress-induced delays in wound closure (Detillion et al., Citation2004; Mercado et al., Citation2002a; Padgett et al., Citation1998; Rojas et al., Citation2002) this is unlikely to be relevant to isolation-induced healing impairments in males given the observed lack of changes in corticosterone following isolation. Alternatively, endogenous oxytocin may play a key role in isolation-impaired healing, as central oxytocin levels are reduced during social isolation (Karelina & DeVries, Citation2011) and central treatment with an oxytocin agonist ameliorates wound healing in socially-isolated animals (shown in hamsters; Detillion et al., Citation2004).
To assess potential behavioral consequences of isolation, total locomotor activity was determined one week after HPA axis responses were measured. Isolation increased overall activity and speed, which are suggestive of altered psychomotor systems or a deficit in locomotor habituation, and is consistent with other studies of isolated rodents (Naert et al., Citation2011; Rilke et al., Citation1998; Voikar et al., Citation2005). Taken together, both restraint and isolation distinctly disrupted various physiological and behavioral processes.
Conclusions
These data are consistent with the growing evidence that social interactions garner significant health benefits for social animals, whereas social isolation is consistently detrimental (Karelina & DeVries, Citation2011). How social environment affects health has potential implications for decisions about standard rodent housing conditions in research and the value of social environment (e.g. social support) in the field of medicine. In addition, this work indicates that tissue repair is an excellent paradigm by which to understand how different stressors can negatively affect immunity through potentially disparate mechanisms. By identifying stressor-specific mechanisms, there is a better potential for individualizing health treatments and thereby optimizing healing outcomes.
Declaration of interest
The authors declare no conflicts of interest. This work was supported by UIC College of Dentistry start-up funds to CGE.
Acknowledgements
The authors thank Dr. Charles Zhou, Dr. Lin Tao, Zong Juan Fang, Yan Zang, Dr. Stephanie Tymen, Dr. Yasmin Husain, and Dr. Phillip Marucha for technical assistance and advice.
References
- Advani T, Hensler JG, Koek W. (2007). Effect of early rearing conditions on alcohol drinking and 5-HT1A receptor function in C57BL/6J mice. Int J Neuropsychopharmacol 10:595–607
- Arndt SS, Laarakker MC, van Lith HA, van der Staay FJ, Gieling E, Salomons AR, van't Klooster J, Ohl F. (2009). Individual housing of mice – impact on behavior and stress responses. Physiol Behav 97:385–93
- Barlow SM, Morrison PJ, Sullivan FM. (1975). Effects of acute and chronic stress on plasma corticosterone levels in the pregnant and non-pregnant mouse. J Endocrinol 66:90–9
- Bikowski J. (1999). Secondarily infected wounds and dermatoses: a diagnosis and treatment guide. J Emerg Med 17:197–206
- Boden-Albala B, Litwak E, Elkind MS, Rundek T, Sacco RL. (2005). Social isolation and outcomes post stroke. Neurology 64:1888–92
- Boggiano MM, Cavigelli SA, Dorsey JR, Kelley CE, Ragan CM, Chandler-Laney PC. (2008). Effect of a cage divider permitting social stimuli on stress and food intake in rats. Physiol Behav 95:222–8
- Buwalda B, de Boer SF, Schmidt ED, Felszeghy K, Nyakas C, Sgoifo A, Van der Vegt BJ, et al. (1999). Long-lasting deficient dexamethasone suppression of hypothalamic-pituitary-adrenocortical activation following peripheral CRF challenge in socially defeated rats. J Neuroendocrinol 11:513–20
- Cacioppo JT, Hawkley LC, Norman GJ, Berntson GG. (2011). Social isolation. Ann N Y Acad Sci 1231:17–22
- Chen L, Arbieva ZH, Guo S, Marucha PT, Mustoe TA, DiPietro LA. (2010). Positional differences in the wound transcriptome of skin and oral mucosa. BMC Genomics 11:471
- Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. (2007). Social regulation of gene expression in human leukocytes. Genome Biol 8:R189.1–R189.13
- Craft TK, Glasper ER, McCullough L, Zhang N, Sugo N, Otsuka T, Hurn PD, DeVries AC. (2005). Social interaction improves experimental stroke outcome. Stroke 36:2006–11
- Crawley JN. (2000). What's wrong with my mouse? Behavioral phenotyping of transgenic and knockout mice. New York: Wiley–Liss
- Deak T, Nguyen KT, Cotter CS, Fleshner M, Watkins LR, Maier SF, Spencer RL. (1999). Long-term changes in mineralocorticoid and glucocorticoid receptor occupancy following exposure to an acute stressor. Brain Res 847:211–20
- Desmouliere A, Chaponnier C, Gabbiani G. (2005). Tissue repair, contraction, and the myofibroblast. Wound Repair Regen 13:7–12
- Detillion CE, Craft TK, Glasper ER, Prendergast BJ, DeVries AC. (2004). Social facilitation of wound healing. Psychoneuroendocrinology 29:1004–11
- Eijkelkamp N, Engeland CG, Gajendrareddy PK, Marucha PT. (2007). Restraint stress impairs early wound healing in mice via alpha-adrenergic but not beta-adrenergic receptors. Brain Behav Immun 21:409–12
- Engeland CG, Bosch JA, Cacioppo JT, Marucha PT. (2006). Mucosal wound healing: the roles of age and sex. Arch Surg 141:1193–7 (discussion 1198)
- Engeland CG, Graham JE. (2011). Psychoneuroimmunological aspects of wound healing and the role of pain. In: Upton D, editor. Psychological impact of pain in patients with wounds. London: Wounds UK. p 87–114
- Engeland CG, Marucha PT. (2009). Wound healing and stress. In: Grantstein RD, Luger TA, editors. Neuroimmunology of the skin: basic science to clinical relevance. Berlin: Springer. p 233–47
- Glaser R, Kiecolt-Glaser JK, Marucha PT, MacCallum RC, Laskowski BF, Malarkey WB. (1999). Stress-related changes in proinflammatory cytokine production in wounds. Arch Gen Psychiatry 56:450–6
- Glasper ER, Devries AC. (2005). Social structure influences effects of pair-housing on wound healing. Brain Behav Immun 19:61–8
- Glavin GB, Pare WP, Sandbak T, Bakke HK, Murison R. (1994). Restraint stress in biomedical research: an update. Neurosci Biobehav Rev 18:223–49
- Grice EA, Kong HH, Renaud G, Young AC, Bouffard GG, Blakesley RW, Wolfsberg TG, et al. (2008). A diversity profile of the human skin microbiota. Genome Res 18:1043–50
- Grippo AJ, Lamb DG, Carter CS, Porges SW. (2007). Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviors. Biol Psychiatry 62:1162–70
- Guo S, Dipietro LA. (2010). Factors affecting wound healing. J Dent Res 89:219–29
- Gutierrez-Garcia AG, Contreras CM. (2009). Stressors can affect immobility time and response to imipramine in the rat forced swim test. Pharmacol Biochem Behav 91:542–8
- Hasen NS, O'Leary KA, Auger AP, Schuler LA. (2010). Social isolation reduces mammary development, tumor incidence, and expression of epigenetic regulators in wild-type and p53-heterozygotic mice. Cancer Prev Res 3:620–9
- Hawkley LC, Thisted RA, Masi CM, Cacioppo JT. (2010). Loneliness predicts increased blood pressure: 5-year cross-lagged analyses in middle-aged and older adults. Psychol Aging 25:132–41
- Himms-Hagen J, Villemure, C. (1992). Number of mice per cage influence uncoupling protein content of brown adipose tissue. Curr Biol 200:502–6
- Horan MP, Quan N, Subramanian SV, Strauch AR, Gajendrareddy PK, Marucha PT. (2005). Impaired wound contraction and delayed myofibroblast differentiation in restraint-stressed mice. Brain Behav Immun 19:207–16
- Hotchkiss AK, Pyter LM, Neigh GN, Nelson RJ. (2004). Nyctohemeral differences in response to restraint stress in CD-1 and C57BL/6 mice. Physiol Behav 80:441–7
- Jeong JY, Lee DH, Kang SS. (2013). Effects of chronic restraint stress on body weight, food intake, and hypothalamic gene expressions in mice. Endocrinol Metab 28:288–96
- Karelina K, DeVries AC. (2011). Modeling social influences on human health. Psychosom Med 73:67–74
- Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. (1995). Slowing of wound healing by psychological stress. Lancet 346:1194–6
- Levenson SM, Kan-Gruber D, Gruber C, Molnar J, Seifter E. (1983). Wound healing accelerated by Staphylococcus aureus. Arch. Surg 118:310–20
- Levine JB, Leeder AD, Parekkadan B, Berdichevsky Y, Rauch SL, Smoller JW, Konradi C, et al. (2008). Isolation rearing impairs wound healing and is associated with increased locomotion and decreased immediate early gene expression in the medial prefrontal cortex of juvenile rats. Neuroscience 151:589–603
- Martin AL, Brown RE. (2010). The lonely mouse: verification of a separation-induced model of depression in female mice. Behav Brain Res 207:196–207
- Mercado AM, Padgett DA, Sheridan JF, Marucha PT. (2002a). Altered kinetics of IL-1 alpha, IL-1 beta, and KGF-1 gene expression in early wounds of restrained mice. Brain Behav Immun 16:150–62
- Mercado AM, Quan N, Padgett DA, Sheridan JF, Marucha PT. (2002b). Restraint stress alters the expression of interleukin-1 and keratinocyte growth factor at the wound site: an in situ hybridization study. J Neuroimmunol 129:74–83
- Naert A, Callaerts-Vegh Z, D'Hooge R. (2011). Nocturnal hyperactivity, increased social novelty preference and delayed extinction of fear responses in post-weaning socially isolated mice. Brain Res Bull 85:354–62
- Norman GJ, Morris JS, Karelina K, Weil ZM, Zhang N, Al-Abed Y, Brothers HM, et al. (2011). Cardiopulmonary arrest and resuscitation disrupts cholinergic anti-inflammatory processes: a role for cholinergic alpha7 nicotinic receptors. J Neurosci 31:3446–52
- O'Connor KA, Johnson JD, Hammack SE, Brooks LM, Spencer RL, Watkins LR, Maier SF. (2003). Inescapable shock induces resistance to the effects of dexamethasone. Psychoneuroendocrinology 28:481–500
- Padgett DA, Marucha PT, Sheridan JF. (1998). Restraint stress slows cutaneous wound healing in mice. Brain Behav Immun 12:64–73
- Pyter LM, Yang L, da Rocha JM, Engeland CG. (2014). The effects of social isolation on wound healing mechanisms in mice. Physiol Behav 127:64–70
- Raja, Sivamani K, Garcia MS, Isseroff RR. (2007). Wound re-epithelialization: modulating keratinocyte migration in wound healing. Front Biosci 12:2849–68
- Rilke O, Jahkel M, Oehler J. (1998). Dopaminergic parameters during social isolation in low- and high-active mice. Pharmacol Biochem Behav 60:499–505
- Robson MC. Wound infection. (1997). A failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am 77:637–50
- Rojas IG, Padgett DA, Sheridan JF, Marucha PT. (2002). Stress-induced susceptibility to bacterial infection during cutaneous wound healing. Brain Behav Immun 16:74–84
- Sanchez MM, Aguado F, Sanchez-Toscano F, Saphier D. (1998). Neuroendocrine and immunocytochemical demonstrations of decreased hypothalamo-pituitary-adrenal axis responsiveness to restraint stress after long-term social isolation. Endocrinology 139:579–87
- Santha P, Pakaski M, Fodor EK, Fazekas OC, Kalman S, Kalman J, Jr Janka Z, et al. (2013). Cytoskeletal protein translation and expression in the rat brain are stressor-dependent and region-specific. PLoS One 8:e73504
- Scaccianoce S, Del Bianco P, Paolone G, Caprioli D, Modafferi AM, Nencini P, Badiani A. (2006). Social isolation selectively reduces hippocampal brain-derived neurotrophic factor without altering plasma corticosterone. Behav Brain Res 168:323–5
- Sheridan JF, Stark JL, Avitsur R, Padgett DA. (2000). Social disruption, immunity, and susceptibility to viral infection. Role of glucocorticoid insensitivity and NGF. Ann N Y Acad Sci 917:894–905
- Tenorio A, Jindrak K, Weiner M, Bella E, Enquist IF. (1976). Accelerated healing in infected wounds. Surg Gynecol Obstet 142:537–43
- Tuli JS, Smith JA, Morton DB. (1995). Effects of acute and chronic restraint on the adrenal gland weight and serum corticosterone concentration of mice and their faecal output of oocysts after infection with Eimeria apionodes. Res Vet Sci 59:82–6
- Tymen SD, Rojas IG, Zhou X, Fang ZJ, Zhao Y, Marucha PT. (2013). Restraint stress alters neutrophil and macrophage phenotypes during wound healing. Brain Behav Immun 28:207–17
- Vegas O, VanBuskirk J, Richardson S, Parfitt D, Helmreich D, Rempel M, Moynihan J, Tausk F. (2012). Effects of psychological stress and housing conditions on the delay of wound healing. Psicothema 24:581–6
- Voikar V, Polus A, Vasar E, Rauvala H. (2005). Long–term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav 4:240–52
- Weiss IC, Pryce CR, Jongen-Relo AL, Nanz-Bahr NI, Feldon J. (2004). Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res 152:279–95
- Williams JB, Pang D, Delgado B, Kocherginsky M, Tretiakova M, Krausz T, Pan D, et al. (2009). A model of gene-environment interaction reveals altered mammary gland gene expression and increased tumor growth following social isolation. Cancer Prev Res 2:850–61

