Abstract
Hormesis is the process by which small stresses build resilience to large stresses. We pre-exposed rats to various parameters of mild-to-moderate stress prior to traumatic stress in the present experiments to assess the potential benefits of hormetic training on resilience to traumatic, uncontrollable stress. Rats underwent varying stress pre-training parameters prior to exposure to uncontrollable traumatic stress in the learned helplessness procedure. The ability to prevent the exaggerated fear responding and escape deficits that normally follow experience with traumatic stress were used as a measure of the benefits of hormetic training. Four experiments examined the effects of number of training sessions, stressor severity and pattern of rest between pre-training stress sessions. Repeated exposure to mild restraint stress or moderate shock stress eliminated both the enhanced fear conditioning and shuttle-escape deficits that result from exposure to traumatic, inescapable shock. The pattern of rest did not contribute to resilience when the pre-exposure stressor was mild, but was vital when the pre-exposure stressor was moderate, with an alternation of stress and rest being the most effective procedure. The data also suggest that the level of resilience may increase with the number of pre-exposure sessions.
Introduction
Hormesis is the process by which small stresses build resilience to large stresses. The pharmacologist Hugo Schulz originally coined the term in 1888 in a discussion of the immunity to poisoning that develops when an individual ingests a small amount of the toxin over an extended period of time (Calabrese et al., Citation2007; Southam & Erhlich, Citation1943). The term has been used more recently to describe the benefits of exercise and oxidative stress in preventing bodily disease and improving emotional health (Li & He, Citation2009; Radak et al., Citation2008). Hormesis is probably best conceptualized in modern parlance as an increased capacity for allostasis – the process of adapting to an environmental challenge – as the result of repeated exposure to uncontrollable, but otherwise mild stress (Sterling & Eyer, Citation1988). Allostatic load refers to the cumulative damage that occurs as a consequence of allostasis when recovery is inadequate or incomplete (McEwen & Gianaros, Citation2011; McEwen & Stellar, Citation1993; Schulkin, Citation2003).
The present experiments examined the potential hormetic benefits of stress pre-exposure in the learned helplessness paradigm. This procedure is a traditional method for analyzing the effects of acute, traumatic stress and modeling related symptoms of post-traumatic stress disorder (PTSD) and comorbid major depression in rats (Başoğlu et al., Citation1997; Hammack et al., Citation2012; Minor et al., Citation1991, Citation2011). The procedure consists of two phases. Rats initially are exposed to a large number of unsignaled, inescapable tail shocks in tubes over an extended period (2–4 h). A control group is simply restrained in tubes for the same time period in the absence of shock. All rats are tested for shuttle-escape performance 24 h later. Rats pre-exposed to inescapable shock enter the test situation in an anxious/agitated state and show exaggerated fear responding during initial escape testing. Inescapably shocked rats rapidly transition to an unresponsive, depression-like state, termed conservation-withdrawal, as testing progresses. The transition to conservation-withdrawal is evidenced as a profound deficit in escape performance (Minor et al., Citation1994a; Plumb et al., Citation2013). More generally, experience with uncontrollable shock results in disturbances in sleep (Kant et al., Citation1995), exaggerated startle (Servatius et al., Citation1995), hypervigilance (McAuley et al., Citation2009), anorexia (Dess et al., Citation1989), anhedonia (Zacharko & Anisman, Citation1991), reinstatement of drug seeking (Figueroa-Guzman et al., Citation2011) and attentional/cognitive deficits in rats (Jackson et al., Citation1980; Minor et al., Citation1984; Shors, Citation2004).
We pre-exposed rats to various parameters of mild-to-moderate stress prior to traumatic stress in the present experiments to assess the potential benefits of pre-training stress exposure. The most severe pre-training stressor used in the present experiments (i.e. 25 shocks) is not sufficient to induce the helplessness effect alone (Minor et al., Citation1994b). The enhanced fear conditioning (Maier, Citation1990; Minor, Citation1990) and escape deficits (Maier et al., Citation1973) normally observed 24 h after experience with inescapable shock should be greatly diminished by pre-exposure to comparatively mild stress.
Methods
Experiment 1
The majority of work on stress resilience has focused on early-life experiences and the subsequent effect on stress coping techniques as an adult. Exposure to intermittent mild stress during infancy builds resilience during adulthood only if the individual is allowed time to recover from the stressor prior to the next stress experience (Boyce & Chesterman, Citation1990; Denenberg, Citation1967; Hunt, Citation1965; Khoshaba & Maddi, Citation1999; Levine, Citation1960). In addition, the more exposure to mild stresses during infancy, the greater the protection against stress as an adult (Denenberg & Haltmeyer, Citation1967). The benefits of the previous stress, however, are overwhelmed when the target challenge is too severe (Bateson et al., Citation2004; Macrì et al., Citation2011; Minor et al., Citation1994a).
Whereas the available evidence indicates that mild-to-moderate stress early in life can benefit the individual in adulthood, it is less clear that adults are equally malleable. Adult resilience may be established during a critical developmental period (Denenberg, Citation1967; Denenberg & Haltmeyer, Citation1967) or may be subject to mother-offspring interactions that are only available during infancy (Bateson et al., Citation2004; Macrì & Wuerbel, Citation2006; Macrì et al., Citation2011; Meaney et al., Citation1989).
Some evidence that resilience is enhanced in adults comes from the classic work on “toughening-up” by Miller, Weiss and their colleagues (Anisman Citation1978; Miller, Citation1976; Weiss et al., Citation1976, Citation1981). Rats were exposed to an increasing intensity of shock stress over a 2-week period in these experiments. This initial training eliminated symptoms of behavioral depression following exposure to uncontrollable traumatic stress in the learned helplessness procedure. Even though these data provide evidence that changes in resilience can be achieved in adulthood, the paradigm has limited value due to the severity of the pre-exposure stressor.
Experiment 1 examined whether pre-exposure to a number of days (3 or 5) of simple restraint stress (30 min) mitigated the exaggerated fear conditioning and shuttle-escape deficits that are normally observed 24 h after exposure to traumatic, uncontrollable shock.
Subjects
Forty-eight male Sprague–Dawley albino rats (290–320 g) from Harlan Laboratories (Indianapolis, IN) were housed in individual cages with free access to food and water in a room maintained on a 12:12-h light/dark cycle for 1 week prior to experimental treatment. Experimentation occurred during the light portion of the cycle. All protocols in this article were pre-approved by the UCLA IACUC.
Apparatus
Restraint and tail shock pre-treatments occurred in clear Plexiglas restraining tubes, measuring 23 cm in length and 6 cm in diameter. Adjustable front walls prevented the rats from moving forward in the tubes. A rat's tail extended through the rear door of each tube and was taped to a plastic rod. Unscrambled electric shocks were delivered from one of four constant-current shock generators (Lafayette Instrument Co., Model 82400, Lafayette, IN) through electrodes attached to the rat's tail with electrode paste and tape. Each tube was housed in a sound-attenuating enclosure containing an exhaust fan that masked extraneous noises. A 7 W house light located in the center of the rear wall of the attenuating enclosure’s rear wall provided constant illumination.
Escape testing occurred in a (45 cm × 20 cm × 20 cm) shuttle box (BRS-LVE model 146-40). The shuttle box was divided into two equal compartments by a metal barrier that had an 8 × 7 cm center opening flush with the grid floor. The floor consisted of 2-mm diameter stainless-steel rods spaced 1.1 cm apart center to center. Continuous scrambled shock was delivered to the grid floor from a Grason-Stadler (Series 700, West Concord, MA) shock generator. The floor pivoted in the center and a response was recorded when a 300 g rat's front paws touched the center grid in a compartment. Two 6 W lamps located in the center of each end wall provided constant illumination. The shuttle box was housed in a sound-attenuating chest, containing an exhaust fan that masked extraneous noise.
Procedure
Rats were assigned randomly to one of six groups of eight rats each. Two groups received no pre-training (Groups S and R). They received either exposure to 100, 1.0 mA variable-duration (mean = 8.0 s; range: 3–15 s) inescapable tail shocks on a variable-time 60 s schedule (range: 20–150 s) in restraining tubes over 1.83 h (Group S) or simple restraint in restraining tubes with no tail shock for the same amount of time (Group R) during the stress treatment session. These groups served to define the boundaries of the learned helplessness effect. Two other groups received either three or five 30-min sessions of restraint stress in tubes with a day of interpolated rest occurring after each of these sessions. These groups then were exposed to the traumatic shock stressor during the treatment session (Groups r-r-r-S and r-r-r-r-r-S). Two other groups (Groups r-r-r-R and r-r-r-r-r-R) also received either three or five 30-min sessions of restraint stress in tubes with a day of interpolated rest occurring between each of these sessions. These groups received simple restraint during the treatment session.
Shuttle-escape testing occurred 24 h later. The test consisted of five trials during which a rat had to cross from one side of the central barrier to the other to terminate shock (FR-1 trials). These trials occurred on a fixed-time 60-s schedule. A trained observer scored defensive freezing, defined as the absence of all bodily and vibrissae movement except for that related to respiration, during each inter-trial interval using a time-sampling procedure every 5 s. FR-1 trials were followed by 25 FR-2 trials during which a rat had to cross from one side of the central barrier and then return to terminate shock. Shock terminated automatically if the appropriate response contingency was not met within 40 s of shock onset on a given trial. Escape latencies were recorded on each trial. Shock intensity was set at 0.6 mA with FR-2 trials occurring on a variable time 60-s schedule (range: 20–230 s); however, 3 min intervened between FR-1 and FR-2 trials (Minor & LoLordo, Citation1984).
Experiment 2
McEwen and his colleagues (McEwen & Gianaros, Citation2011; McEwen & Stellar, Citation1993) argue that rest is important in repairing the damaging effects of stress and building resilience. The rationale for this proposal is that tissue damage associated with a rise in catabolic hormones is repaired by a nocturnal rise in anabolic hormones. Failure to achieve adequate rest (or sleep) following stress results in an accumulation of stress-related damage (allostatic load) and impedes the ability to respond adaptively to future stressors.
Whether rest is necessary or sufficient for resilience is not clear. Experiment 1 clearly demonstrated that as few as 3 d of restraint stress with interpolated rest has hormetic benefits. This experiment examined whether rest and the pattern of rest influence that outcome.
Subjects and apparatus
Forty male Sprague–Dawley albino rats (290–320 g) were housed as in Experiment 1. The apparatus was the same as described above.
Procedure
Rats were randomly assigned to one of five groups of eight rats each. Two groups received no pre-training prior to exposure to traumatic shock (Group S) or simple restraint (Group R) during the stress treatment session. The other three groups received three sessions of 30-min restraint in tubes prior to exposure to traumatic stress. These groups differed with respect to the pattern of restraint and rest: Group rrr—S received 3 consecutive days of restraint stress followed by 3 consecutive days of rest; Group —rrrS received 3 d of rest followed by 3 consecutive days of restraint stress; and Group r-r-r-S received 3 d of restraint stress with 3 d of interpolated rest. Shuttle-escape testing occurred 24 h after the stress treatment session.
Experiment 3
Experiment 3 determined whether pattern of rest is critical when the pre-training stressor is more severe. This experiment utilized the same general design as Experiment 2; however, the pre-training stressor was 25 inescapable tail shocks rather than restraint.
Subjects and apparatus
Forty male Sprague–Dawley albino rats (290–320 g) were housed as in Experiment 1. The apparatus was the same as above.
Procedure
Rats were randomly assigned to one of five groups of eight rats each. Two groups received no pre-training prior to exposure to traumatic shock (Group S) or simple restraint (Group R) during the stress treatment session. The other three groups received three 30-min sessions of 25, 1.0 mA variable-duration (mean = 8.0 s; range: 3–15 s) inescapable tail shocks on a variable-time 60-s schedule (range: 20–150 s) in restraining tubes prior to exposure to traumatic stress. These groups differed with respect to the pattern of shock and rest: Group sss—S received 3 consecutive days of shock followed by 3 consecutive days of rest; Group —sssS received 3 d of rest followed by 3 consecutive days of shock; and Group s-s-s-S received 3 d of shock with 3 d of interpolated rest. Shuttle-escape testing occurred 24 h after the stress treatment session.
Experiment 4
Experiment 1 provided some evidence that more stress pre-training yields greater resilience against traumatic stress. In Experiment 4, we used the same general design as in Experiment 1, but tried to amplify the benefits of stress pre-training by increasing the severity of the stressor.
Subjects and apparatus
Thirty-two male Sprague–Dawley albino rats (290–320 g) were housed as in Experiment 1. The apparatus was the same as above.
Procedure
Rats were randomly assigned to one of four groups of eight rats each. Two groups received no pre-training prior to exposure to traumatic shock (Group S) or simple restraint (Group R) during the stress treatment session. Two other groups received either three or five 30-min sessions of 25 inescapable tail shocks with interpolated days of rest prior to exposure to traumatic shock (Group s-s-s-S and Group s-s-s-s-s-S). Shuttle-escape testing occurred 24 h after the stress treatment session.
Results
Experiment 1
The left panel of shows mean percent post-trial freezing in each group. Rats exposed to inescapable shock without prior training (Group S) showed substantial higher levels of freezing from the outset of training and generally increased over trials relative to the restrained control (Group R). All pre-training groups performed similarly to the restraint control, with some indication that a greater amount of pre-training yielded lower freezing levels.
Figure 1. Percent freezing (left panel) and shuttle escape latencies (right panel) for 6 groups in Experiment 1. Two groups were exposed to traumatic shock stress (Group S) or simple restraint (Group R). Two other groups were pre-exposed to 3 d of restraint with interpolated days of rest followed by either restraint or traumatic shock (Groups r-r-r-R and r-r-r-S). Two other groups were pre-exposed to 5 d of restraint with interpolated days of rest followed by either restraint or traumatic shock (Groups r-r-r-r-r-R and r-r-r-r-r-S). Shuttle-escape testing occurred 24 h later. Freezing was measured over five trials (FR-1) at the start of the shuttle-escape test. Impaired escape performance was assessed over the next 25 trials (FR-2).
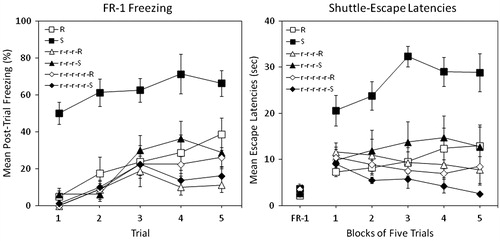
A mixed-design analysis of variance (ANOVA: Stress Condition × Pre-training Condition × Trial) yielded significant main effects of stress, F(1, 42) = 17.87, p < 0.001, Pre-training, F(2, 42) = 23.49, p < 0.001, and Trial, F(4, 168) = 24.23, p < 0.001 and significant interactions of Stress × Pre-training, F(2, 42) = 10.95, p < 0.001, and Stress × Pre-training × Trial, F(8, 168) = 2.02, p = 0.05. The interactions between Stress × Trial and Pre-training × Trial were not statistically significant. Newman–Keuls post-hoc contrasts (α = 0.05) on grand mean freezing suggested the following ordered relation among group means: S > R = r-r-r-S = r-r-r-R = r-r-r-r-r-S = r-r-r-r-r-R.
The right panel of shows mean escape latencies across blocks of five trials in each group. FR-1 escape latencies did not differ among groups, F < 1. The standard helplessness effect is defined by the difference between FR-2 escape latencies of Groups S and R. All pre-training groups performed similarly to the restraint control, with some indication that a greater amount of pre-training afforded slightly greater protection.
A mixed-design ANOVA (Stress Condition × Pre-training Condition × Trial Block) on FR-2 escape latencies yielded significant main effects of Stress, F(1, 42) = 6.89, p = 0.012, and Pre-training, F(2, 42) = 10.18, p < 0.001, and significant interactions of Stress × Pre-training, F(2, 42) = 7.75, p = 0.002, and Pre-training × Trial Block, F(8, 168) = 4.48, p < 0.001. The main effect of Trial Block and the other potential interactions were not statistically significant. Newman–Keuls post-hoc contrasts (α = 0.05) on grand mean FR-2 escape latencies suggested the following ordered relation among group means: S > R = r-r-r-S = r-r-r-R = r-r-r-r-r-S = r-r-r-r-r-R.
Experiment 2
The left panel of shows mean percent post-trial freezing in each of the five groups in Experiment 2. Group S showed excessive levels of freezing from the outset of testing relative to the restrained control (Group R). Stress training prior to traumatic stress mitigated fearfulness at the time of testing, regardless of the pattern of rest.
Figure 2. Percent freezing (left panel) and shuttle escape latencies (right panel) for five groups in Experiment 2. Two groups were exposed to traumatic shock stress (Group S) or simple restraint (Group R). Three other groups were pre-exposed to 3 d of restraint followed by traumatic shock. These three groups received 3 d of rest that either preceded training (Group —rrrS), followed training (Group rrr—S), or was interpolated with training (Group r-r-r-S). Shuttle-escape testing occurred 24 h later. Freezing was measured over five trials (FR-1) at the start of the shuttle-escape test. Impaired escape performance was assessed over the next 25 trials (FR-2).
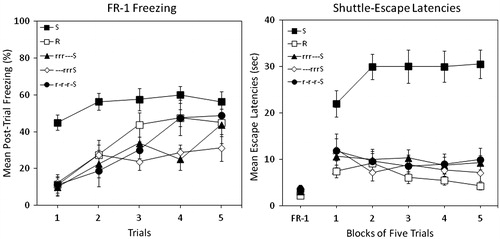
A mixed-design ANOVA (Group x Trial) yielded significant main effects of Group, F(4, 35) = 7.44, p < 0.001, and Trial, F(4, 140) = 23.32, p < 0.001, and a significant Group × Trial interaction, F(16, 140) = 1.93, p < 0.03. Newman–Keuls post-hoc contrasts (α = 0.05) on grand mean freezing suggested the following ordered relation among group means: S > R = r-r-r-S = rrr—S = —rrrS.
The right panel of shows mean escape latencies across blocks of five trials in each group. FR-1 escape latencies did not differ, F < 1. Escape latencies were similar to freezing behavior. A large deficit in FR-2 escape performance occurred in Group S relative to Group R. Stress pre-training dramatically improved escape performance, regardless of the pattern of rest.
A mixed-design ANOVA (Group × Trial Block) yielded a significant main effect of Group, F(4, 35) = 22.15, p < 0.001, and a significant Group × Trial Block interaction, F(16, 140) = 3.045, p < 0.001, indicating that escape latencies increased in Group S as they decreased in all other groups across trial blocks. The main effect of Trial Block was not statistically significant. Newman–Keuls post-hoc contrasts (α = 0.05) on grand mean FR-2 escape latencies suggested the following ordered relation among group means: S > R = r-r-r-S = rrr—S = —rrrS.
Experiment 3
The left panel of shows mean percent post-trial freezing in each of the groups in Experiment 3. There is considerable overlap in freezing behavior among all groups. A consistent pattern did not emerge among those that received stress pre-training and those that did not.
Figure 3. Percent freezing (left panel) and shuttle escape latencies (right panel) for five groups in Experiment 3. Two groups were exposed to traumatic shock stress (Group S) or simple restraint (Group R). Three other groups were pre-exposed to 3 d of 25 inescapable tail shocks followed by traumatic shock. These three groups received 3 d of rest that either preceded training (Group —sssS), followed training (Group sss—S), or was interpolated with training (Group s-s-s-S). Shuttle-escape testing occurred 24 h later. Freezing was measured over 5 trials (FR-1) at the start of the shuttle-escape test. Impaired escape performance was assessed over the next 25 trials (FR-2).
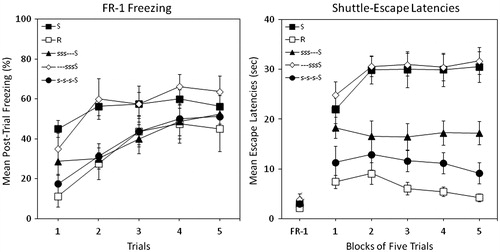
A mixed-design ANOVA (Group × Trial) yielded significant main effects of Group, F(4, 35) = 3.46, p < 0.02, and Trial, F(4, 140) = 22.82, p < 0.001. The interaction of Group and Trial was not statistically significant. Newman–Keuls post-hoc contrasts (α = 0.05) on grand mean freezing identified a marginally significant difference (α = 0.054) between groups and suggested the following ordered relation among group means: S = —sssS > sss—S = s-s-s-S = R.
The right panel of shows mean escape latencies across blocks of five trials. FR-1 escape latencies did not differ, F < 1. A large deficit in FR-2 escape performance occurred in Group S relative to Group R. The benefits of stress pre-training clearly depended on the pattern of rest. Three days of pre-training shock stress prior to traumatic shock afforded no protection (Group —sssS). In contrast, 3 d of shock stress with interpolated rest yielded the greatest protection such that Group s-s-s-S performed similarly to the restrained control. Three consecutive days of shock stress followed by three days of rest yielded an intermediate amount of resilience (Group sss—S).
A mixed-design ANOVA (Group × Trial Block) yielded significant main effects of Group, F(4, 35) = 22.86, p < 0.000, and Trial Block, F(4, 140) = 3.12, p < 0.02, and a significant Group × Trial Block interaction, F(16, 140) = 2.49, p < 0.01. Newman–Keuls post-hoc contrasts (α = 0.05) on grand mean FR-2 escape latencies suggested the following ordered relation among group means: S = —sssS > sss—S > s-s-s-S = R.
Experiment 4
The left panel of shows mean percent post-trial freezing as a function of trial. Group S showed excessive levels of freezing from the outset of testing relative to the restrained control (Group R). Both stress pre-training groups performed similarly to the restraint control.
Figure 4. Percent freezing (left panel) and shuttle escape latencies (right panel) for four groups in Experiment 4. Two groups were exposed to traumatic shock stress (Group S) or simple restraint (Group R). Two other groups were trained with 3 or 5 d of 25 inescapable tail shocks with interpolated days of rest followed by traumatic shock (Groups s-s-s-S and s-s-s-s-s-S). Shuttle-escape testing occurred 24 h later. Freezing was measured over five trials (FR-1) at the start of the shuttle-escape test. Impaired escape performance was assessed over the next 25 trials (FR-2).
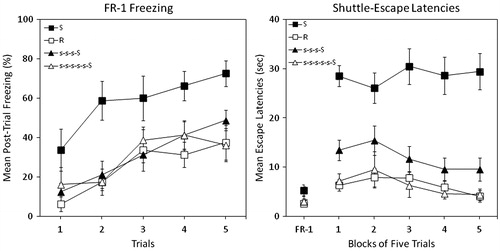
A mixed-design ANOVA (Group × Trial) yielded significant main effects of Group, F(3, 28) = 6.72, p < 0.002, and Trial, F(4, 112) = 22.35, p < 0.001. The interaction between Group × Trial was not statistically significant. Newman–Keuls post-hoc contrasts (α = 0.05) on grand mean freezing suggested the following ordered relation among group means: S > R = s-s-s-S = s-s-s-s-s-S.
The right panel of shows mean escape latencies across blocks of five trials. FR-1 escape latencies did not differ, F < 1. A large deficit in FR-2 escape performance occurred in Group S relative to Group R. Both stress pre-training groups performed similarly to the restrained control, with evidence that 5 d of stress pre-training afforded slightly greater protection than 3 d.
A mixed-design ANOVA (Group × Trial Block) yielded a significant main effect of Group, F(3, 28) = 34.93, p < 0.001, and Trial Block, F(4,112) = 3.13, p < 0.02. The interaction between Group × Trial Block was not statistically significant. Newman–Keuls post-hoc contrasts (α = 0.05) on grand mean FR-2 escape latencies suggested the following ordered relation among group means: S > R = s-s-s-S = s-s-s-s-s-S.
Discussion
The present experiments indicate that repeated exposure to severe stress is not necessary to build resilience in adult rats. Exposure to mild or moderate stress with interpolated rest is sufficient to block the exaggerated fear conditioning and shuttle escape deficits that normally follow experience with traumatic, uncontrollable shock. The pattern of rest surrounding the mild pre-training stress sessions had no effect on escape latencies; however, the pattern of rest becomes critical when the initial stress sessions are more severe. When rest is allowed between stress sessions, it allows the animal to recover physically from the damaging effects of each hormetic stress session (McEwen & Gianaros, Citation2011; McEwen & Stellar, Citation1993). Exposure to 3 d of shock stress immediately before traumatic stress did not provide adequate recovery time following each stress session, resulting in no benefit of stress pre-treatment and subsequent helplessness. Allowing for 3 d of rest following pre-training stress provided some benefit, but was less effective than if rest was allowed after each session. There is also some evidence that an increased number of pre-training stress sessions may be more beneficial, though this needs to be explored in greater detail.
The helplessness effect has both associative and non-associative mediators – both are necessary, neither is sufficient (Minor et al., Citation1991; Weiss & Simson, Citation1985). One way that pre-training stress sessions might impact the helplessness effect is by impacting one or the other set of mediators. For instance, repeated exposure to the treatment context prior to traumatic stress might facilitate discrimination between treatment and test contexts. This discrimination is severely impaired following traumatic stress and leads to a limited form of associative transfer based on common odors (Minor, Citation1990; Minor & LoLordo, Citation1984). Manipulations that limit associative transfer across contexts eliminate the helplessness effect.
Pre-training stress sessions also might impact a non-associative mediator. An early example was provided by Weiss et al. (Citation1976) in their studies of “toughening-up”. These researchers attributed the helplessness effect to a depletion of brain catecholamines following traumatic stress. Repeated exposure to the traumatic stressor eventually upregulated the synthesis of tyrosine hydroxylase, the rate-limiting enzyme for catecholamine synthesis. The upregulation prevented catecholamine depletion and therefore behavioral impairment.
A more recent example of a potential non-associative mediator involves brain metabolic regulation via adenosine signaling (Plumb et al. Citation2013). We have linked the onset of escape deficits in this paradigm to an increase in adenosine signaling in the nucleus accumbens. Adenosine is a critical modulator of neural activation that links cellular excitability to energy availability. The effect of enhanced adenosine signaling in this region is to uncouple dopamine from its receptor and undermine the motivation for ongoing behavior. Performance deficits ensue.
This hypothesis suggests that one way to mitigate the impact of traumatic stress is to increase metabolic capacity. Pre-exposure to mild or moderate stress may have such a function. In support of this, manipulations like exercise (Greenwood & Fleshner, Citation2008) or treatment with methylene blue (Gonzalez-Lima & Bruchey, Citation2004) increase metabolic capacity and eliminate the helplessness effect.
Stress pre-training also might impact processes that are orthogonal to the immediate causes of impairment. There is considerable interest in neuropeptide Y (NPY) as a potent antagonist of both the hypothalamic–pituitary–adrenocortical axis (HPA) and sympathoadrenomedullary (SAM) axis (Heilig, Citation2004). Stress pre-exposure might upregulate brain and peripheral concentrations of NPY, thereby reducing the overall impact of the traumatic stress session. Other mechanisms could have a similar impact. Repeated exposure to the pre-training stressor could result in habituation to shock and the resulting fear response, in which case the traumatic stress would be perceived as less severe and thereby eliminate the helplessness effect (Drugan et al., Citation1984; Jackson & Minor, Citation1988; Mineka et al., Citation1984; Minor et al., Citation1990 Citation1991). Helplessness is usually observed only when the stressor is exceptionally severe (Minor et al., Citation1994b).
Exposure to traumatic stress can be detrimental to one’s physical and mental health, often resulting in psychological disorders such as post-traumatic stress disorder (PTSD) and major depression (refer Minor et al., Citation2011; Plumb et al., Citation2013 for reviews). These disorders are often accompanied by the inability to effectively cope with subsequent stress. The learned helplessness procedure is an effective tool to study the deleterious effects of traumatic stress as the resulting behavior mimics a number of symptoms of PTSD and major depression, including anhedonia, insomnia, psychomotor retardation, fatigue and anorexia or hyperphagia (Minor et al., Citation2011; Plumb et al., Citation2013). With this procedure, we have been able to show that repeated exposure to mild or moderate stress with interpolated rest builds resilience to traumatic stress and reduces the subsequent symptoms of PTSD and comorbid depression in rats.
Conclusion
The present experiments provide evidence that stress resilience can be accomplished in adulthood without repeated exposure to severe stress (Weiss et al., Citation1976) – mild to moderate stress is sufficient to produce hormesis. Repeated exposure to mild restraint stress or moderate shock stress eliminates both the enhanced fear conditioning and shuttle-escape deficits otherwise produced by traumatic, inescapable shock. There is a tendency for resilience to increase as the number of pre-exposure sessions increases, although the difference failed to achieve statistical significance in two experiments. Importantly, interpolated rest between pre-exposure stress sessions is not only a critical factor when the stressor is mild, but also is critical as the stressor becomes more severe.
Declaration of interest
This research was supported by the Stress and Motivated Behavior Institute (SMBI), the University of California, Los Angeles (UCLA) Academic Senate, and the Office of Naval Research. All authors reported no biomedical financial interests or potential conflicts of interest.
References
- Anisman H. (1978). Neurochemical changes elicited by stress: behavioral correlates [Chapter 3]. In: Anisman H, Bignami G, editors. Psychopharmacology of aversively motivated behavior. 1st ed. New York: Plenum Press. p. 119–71
- Başoğlu M, Mineka S, Paker M, Aker T, Livanou M, Gök S. (1997). Psychological preparedness for trauma as a protective factor in survivors of torture. Psychol Med 27:1421–33
- Bateson P, Barker D, Clutton-Brock T, Deb D, D’Udine B, Foley RA, Gluckman P, et al. (2004). Developmental plasticity and human health. Nature 430:419–21
- Boyce WT, Chesterman E. (1990). Life events, social support, and cardiovascular reactivity in adolescence. J Dev Behav Pediatr 11:105–11
- Calabrese EJ, Bachmann KA, Bailer AJ, Bolger M, Borak J, Cai L, Cedergreen N, et al. (2007). Biological stress response terminology: integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmaco l222:122–8
- Denenberg VH. (1967). Stimulation in infancy, emotional reactivity, and exploratory behavior. In: Glass DC, editor. Neurophysiology and emotion. 1st ed. New York: Rockefeller University Press. p 161–90
- Denenberg VH, Haltmeyer GC. (1967). Test of the monotonicity hypothesis concerning infantile stimulation and emotional reactivity. J Comp Physiol Psychol 63:394–6
- Dess NK, Minor TR, Brewer J. (1989). Suppression of feeding and body weight by inescapable shock: modulation by quinine adulteration, stress reinstatement, and controllability. Physiol Behav 45:975–83
- Drugan RC, Ryan SM, Minor TR, Maier SF. (1984). Librium prevents the analgesia and shuttlebox escape deficit typically observed following inescapable shock. Pharmacol Biochem Behav 21:749–54
- Figueroa-Guzman Y, Mueller C, Vranjkovic O, Wisniewski S, Yang Z, Li SJ, Bohr C, et al. (2011). Oral administration of levo-tetrahydropalmatine attenuates reinstatement of extinguished cocaine seeking by cocaine, stress or drug-associated cues in rats. Drug Alcohol Depend 116:72–9
- Gonzalez-Lima F, Bruchey AK. (2004). Extinction memory improvement by the metabolic enhancer methylene blue. Learn Mem 11:633–40
- Greenwood BN, Fleshner M. (2008). Exercise, learned helplessness, and the stress-resistant brain. Neuromol Med 10:81–98
- Hammack SE, Cooper MA, Lezak KR. (2012). Overlapping neurobiology of learned helplessness and conditioned defeat: implications of PTSD and mood disorders. Neuropharmacology 62:565–75
- Heilig M. (2004). The NPY system in stress, anxiety and depression. Neuropeptides 38:213–24
- Hunt, JM. (1965). Traditional personality theory in the light of recent evidence. Am Sci 53:80–96
- Jackson RL, Alexander JH, Maier SF. (1980). Learned helplessness, inactivity, and associative deficits: effects of inescapable shock on response choice escape learning. J Exp Psychol Anim Behav Process 6:1–20
- Jackson RL, Minor TR. (1988). Effects of signaling inescapable shock on subsequent escape learning: implications for theories of coping and “learned helplessness”. J Exp Psychol Anim Behav Process 14:390–400
- Kant GJ, Pastel RH, Bauman RA, Meininger GR, Maughan KR, Robinson III TN, Wright WL, Covington PS. (1995). Effects of chronic stress on sleep in rats. Physiol Behav 57:359–65
- Khoshaba DM, Maddi SR. (1999). Early experiences in hardiness development. Consult Psychol J Pract Res 51:106–16
- Levine S. (1960). Stimulation in infancy. Sci Am 202:80–6
- Li G, He H. (2009). Hormesis, allostatic buffering capacity and physical activity: a new theoretic framework. Med Hypotheses 72:527–32
- Macrì S, Wuerbel H. (2006). Developmental plasticity of HPA and fear responses in rats: a critical review of the maternal mediation hypothesis. Horm Behav 50:667–80
- Macrì S, Zoratto F, Laviola G. (2011). Early-stress regulates resilience, vulnerability and experimental validity in laboratory rodents through mother-offspring hormonal transfer. Neurosci Biobehav Rev 35:1534–43
- Maier SF. (1990). Role of fear in mediating shuttle escape learning deficit produced by inescapable shock. J Exp Psychol Anim Behav Process 16:137–49
- Maier SF, Albin RW, Testa TJ. (1973). Failure to learn to escape in rats previously exposed to inescapable shock depends on the nature of the escape response. J Comp Physiol Psychol 85:581–92
- McAuley JD, Stewart AL, Webber ES, Cromwell HC, Servatius RJ, Pang KC. (2009). Wistar-Kyoto rats as an animal model of anxiety vulnerability: support for a hypervigilance hypothesis. Behav Brain Res 204:162–8
- McEwen BS, Gianaros PJ. (2011). Stress- and allostasis-induced brain plasticity. Annu Rev Med 62:431–45
- McEwen BS, Stellar E. (1993). Stress and the individual. Mechanisms leading to disease. Arch Intern Med 153:2093–101
- Meaney MJ, Aitken DH, Viau V, Sharma S, Sarrieau A. (1989). Neonatal handling alters adrenocortical negative feedback sensitivity and hippocampal type II glucocorticoid receptor binding in the rat. Neuroendocrinology 50:597–604
- Miller NE. (1976). The role of learning in physiological response to stress [Chapter 3] In: Serban G, editor. Psychopathology of human adaptation. 1st ed. New York: Plenum Press. p. 25–46
- Mineka S, Cook M, Miller S. (1984). Fear conditioned with escapable and inescapable shock: effects of a feedback stimulus. J Exp Psychol Anim Behav Process 10:307–24
- Minor TR. (1990). Conditioned fear and neophobia following inescapable shock. Anim Learn Behav 18:212–26
- Minor TR, Chang WC, Winslow JL. (1994a). Stress and adenosine I: effects of methylxanthine and amphetamine stimulants on learned helplessness in rats. Behav Neurosci 108:254–64
- Minor TR, Dess NK, Ben-David E, Chang W. (1994b). Individual differences in vulnerability to inescapable shock in rats. J Exp Psychol Anim Behav Process 20:402–12
- Minor TR, Dess NK, Overmier JB. (1991). Inverting the traditional view of “learned helplessness” [Chapter 3]. In: Denny MR, editor. Fear, avoidance, and phobias: a fundamental analysis. 1st ed. New Jersey: Lawrence-Erlbaum Associates. p 87–133
- Minor TR, Jackson RL, Maier SF. (1984). Effects of task-irrelevant cues and reinforcement delay on choice-escape learning following inescapable shock: evidence for a deficit in selective attention. J Exp Psychol Anim Behav Process 10:543–56
- Minor TR, LoLordo VM. (1984). Escape deficits following inescapable shock: the role of contextual odor. J Exp Psychol Anim Behav Process 10:168–81
- Minor TR, Plumb TN, Schell CJ, Pham AK. (2011). Brain adenosine signaling in psychological trauma and comorbid depression [Chapter XV]. In: Sher L, Vilens A, editors. Neurobiology of post-traumatic stress disorder. New York: Nova Science Publishers, Inc. p 229–57
- Minor TR, Trauner MA, Lee C, Dess N. (1990). Modeling signal features of escape response: effects of cessation conditioning in “Learned Helplessness” paradigm. J Exp Psychol Anim Behav Process 16:123–36
- Plumb TN, Sterlace SR, Cavanaugh KA, Minor TR. (2013). Stress, brain adenosine signaling, and fatigue-related behavioral processes [Chapter 25]. In: Masino SM, Boison D, editors. Adenosine: a key link between metabolism and central nervous system activity. 1st ed. New York: Springer Publishers. p 535–58
- Radak Z, Chung HY, Koltai E, Tayler AW, Goto S. (2008). Exercise, oxidative stress and hormesis. Ageing Res Rev 7:34–42
- Schulkin J. (2003). Allostasis: a neural behavioral perspective. Horm Behav 43:21–7
- Servatius RJ, Ottenweller JE, Natelson BH. (1995). Delayed startle sensitization distinguishes rats exposed to one or three stress sessions: further evidence toward an animal model of PTSD. Biol Psychiatry 38:539–46
- Shors TJ. (2004). Learning during stressful times. Learn Mem 11:137–44
- Southam CM, Ehrlich J. (1943). Effects of extracts of western red-cedar heartwood on certain wood-decaying fungi in culture. Phytopathology 33:517–24
- Sterling P, Eyer J. (1988). Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of life stress, cognition, and health. 1st ed. New York: J. Wiley & Sons. p 629–49
- Weiss JM, Glaser HI, Pohorecky LA. (1976). Coping behavior and neuro-chemical changes: an alternative explanation for the original “learned helplessness” experiments [Chapter 13]. In: Serban GH, Kling A, editors. Animal models in human psychobiology. 1st ed. New York: Plenum Press. p 141–73
- Weiss JM, Goodman PA, Losito BG, Corrigan S, Charry JM, Bailey WH. (1981). Behavioral depression produced by an uncontrollable stressor: relationship to norepinephrine dopamine, and serotonin levels in various regions of rat brain. Brain Res Rev 3:167–205
- Weiss JM, Simson PC. (1985). Neurochemical mechanisms underlying stress-induced depression. In: Field TM, McCabe PM, Schneiderman N, editors. Stress and coping. 1st ed. New Jersey: Lawrence Erlbaum Associates. p 93–116
- Zacharko RM, Anisman H. (1991). Stressor-induced anhedonia in the mesocorticolimbic system. Neurosci Biobehav Rev 15:391–405

