Abstract
Human papillomavirus (HPV) infection is associated with a wide spectrum of disease that ranges from self-limited skin warts to life-threatening cancers. Since HPV plays a necessary etiological role in cervical cancer, it is logical to use HPV as a marker for early detection of cervical cancer and precancer. Recent advances in technology enable the development of high-throughput HPV assays of different formats, including DNA-based, mRNA-based, high-risk group-specific and type-specific methods. The ultimate goal of these assays is to improve the accuracy and cost-effectiveness of cervical screening programs. HPV testing has several potential advantages compared to cytology-based screening. However, since the cancer to transient infection ratio is always low in the general population, HPV test results are bound to have a low positive predictive value that may subject women to unnecessary follow-up investigations. The wide-spread administration of prophylactic HPV vaccine will substantially decrease the incidence of cancer and precancer. This poses a number of challenges to cytology-based screening, and the role of HPV testing is expected to increase. Finally, apart from technical and cost-effectiveness considerations, one should also keep in mind the psycho-social impact of using sexually-transmitted agents as a marker for cancer screening.
| Abbreviations: | ||
| ASCUS, | = | atypical squamous cells of undetermined significance; |
| ASC-H, | = | atypical squamous cells – cannot exclude high-grade squamous intraepithelial lesion; |
| CIN, | = | cervical intraepithelial neoplasia; |
| EV, | = | epidermodysplasia verruciformis; |
| HC2, | = | hybrid capture assay version 2; |
| HPV, | = | human papillomavirus; |
| HSIL, | = | high-grade squamous intraepithelial lesion; |
| IS, | = | international standard; |
| LEEP, | = | loop electrosurgical excision procedure; |
| LSIL, | = | low-grade squamous intraepithelial lesion; |
| LCR, | = | long control region; |
| MCMP, | = | minichromosome maintenance proteins; |
| ORF, | = | open reading frame; |
| PCR, | = | polymerase chain reaction; |
| Rb, | = | retinoblastoma; |
| TOP2, | = | topoisomerase II; |
| US FDA, | = | United States Food and Drug Administration; |
| WHO, | = | World Health Organization |
Introduction
To date, more than 120 types of human papillomavirus (HPV) have been characterizedCitation1. HPV exclusively infects epithelial cells and is associated with a broad spectrum of clinical manifestations that range from self-limiting lesions to life-threatening diseasesCitation2–13.
With the advances in molecular techniques over the last decade, our understanding of this family of ubiquitous viruses has improved tremendously, a number of previously unrecognized important disease associations have been confirmed, and detection assays for epidemiological study and for confirming diagnosis to assist patient management have been developed. Vaccines targeting two HPV types that show the strongest association with cervical cancer have been developedCitation14. Assessment of vaccine cost-effectiveness and priority becomes an important task for public health policy makers. To this end, HPV detection and typing assays are indispensable in defining the fraction of disease attributed to the types covered by current vaccines.
Disease spectrum
Benign lesions
Non-genital skin infections
With the improvement in the sensitivity of detection assays and coverage for a variety of HPV types, it is now recognized that asymptomatic carriage of HPV on healthy skin is common and can persist over several yearsCitation15. Persistence of HPV infection on non-genital skin does not seem to be related to age, sex, immunosuppressive treatment or history of wartsCitation16,Citation17. Clinical manifestations of these lesions are characteristic. In most situations, diagnosis can be made clinically without HPV testingCitation18–19. Testing for HPV in these lesions is mainly for epidemiological study or other research purposes.
HPV causes benign warts on the skin, which present as flat or firm non-itchy papulesCitation10. Commonly affected areas include hands (verrucae palmares) and feet (verrucae plantares), which are associated typically with HPV 1, 2, and 4. Skin warts, except those growing over press areas, are non-painful. Verrucae planae are flat skin-colour warts found on the face, hands and forearms, and are caused most commonly by HPV 3 and 7. Periungal warts found at the nail fold are often painful. Butcher’s warts are found rarely on the hands of butchers who have repeated trauma that predisposes them to infection with, most commonly, HPV 7; although it is a well-known occupational disease, there is no evidence that the source of infection is animal papillomavirusesCitation20.
Genital infections
Genital tract HPV infection is the most common sexually-transmitted infection. Infections are often subclinical. Clinical lesions (condylomata acuminata) are often multiple and appear as exophytic papillomas, flesh or brown in colour. HPV 6 and 11 are the types most commonly found in visible anogenital warts, but other types, including those typically found in non-genital skin, can also be detected, especially from anogenital areas without visible lesionsCitation3,Citation21–25.
Oral infections
The oral mucosa is also susceptible to HPV infection. Such infection involving the larynx represents a rare but severe diseaseCitation3,Citation7,Citation26–28. Laryngeal papillomatosis has two age-related incidence peaks; those with early childhood onset are acquired vertically from maternal condyloma, whereas the adult onset group is presumably acquired via orogenital contact. In both groups, HPV 6 and 11 are the most commonly found HPV types. The lesions, which develop mainly over vocal cords and trachea, present as hoarseness of voice and stridor. Lesions may extend to lungs, nose and oral cavity. Treatment is difficult as the lesions often recur; thus it is also known as recurrent respiratory papillomatosis. Heck’s disease, another rare condition, presents as multiple papillomas over the lip and buccal mucosaCitation29–31.
Malignant lesions
The hypothesis that HPV plays a role in the development of cervical cancer was proposed in the mid-1970s. Over the last 40 years, a strong body of evidence has accumulated to prove the etiological association between HPV infection and a number of human cancers in addition to cervical cancerCitation11,Citation32,Citation33.
Epidermodysplasia verruciformis
Epidermodysplasia verruciformis (EV), a genetically-inherited chronic skin condition, presents as disseminated flat warts. Recent studies have shown that some EV patients have mutations in the EVER1 and EVER2 genesCitation34–36. Certain HPV types belonging to the beta genus (HPV 5, 8, 9, 12, 14, 15, 17, 19, 25, 36, 38, 47 and 50) are specifically linked to EV. These EV-associated HPV types are also commonly found in the general population. Some immunosuppressed individuals who do have EV may develop lesions caused by EV-associated HPV types. There is no evidence that EV patients are more susceptible to infection or disease manifestations caused by the alpha genus of HPV. About half of the EV patients develop squamous cell carcinoma in sun-exposed areas. Most malignant lesions are associated with HPV 5 and 8, though the pathogenic mechanism of these HPV types is still not yet clearCitation8,Citation37,Citation38.
Non-melanoma skin cancers
In recent years, some evidence suggests that non-melanoma skin cancers including basal cell and squamous cell carcinoma may be linked to cutaneous HPV infection. The suspicion of a viral cause for cutaneous squamous cell carcinoma is based on the observation that its incidence increases dramatically in solid organ transplant recipients receiving long-term immunosuppressive therapy. While sun-exposure is a recognized risk factor, infection with cutaneous HPV (mainly the beta genus) seems to play a role in the development of non-melanoma skin cancers, especially squamous cell carcinoma. HPV types belonging either to beta species 1 or 2 have been detected from squamous cell carcinoma specimens, but unlike anogenital cancers, no predominant HPV types can be identifiedCitation39,Citation40. The etiological association between non-melanoma skin cancers and HPV infection is difficult to prove as the same spectrum of HPV types is prevalent among healthy subjectsCitation9,Citation41. Furthermore, HPV may not be required in maintaining the cancer phenotype and it may therefore escape detection in tumour specimensCitation42.
The mucosal group of HPV is associated with several malignant conditions. Bowenoid papulosis presents as multiple small flat pigmented papules on the external genitalia. Histologically, it is a carcinoma-in-situ, it can evolve to invasive carcinoma, and it is often associated with HPV 16. A subset of intraepithelial neoplasia and carcinoma of the penis, vulva and vagina is also associated with HPV infection, mainly HPV1643.
Cancer of vagina
This uncommon cancer in women has an age-standardized rate of 0.3–0.7 per 100,000 worldwideCitation44. The limited information about the role of HPV infection and occurrence of vaginal cancer is mostly based on analysis of a few HPV types using fixed tissuesCitation45. Epidemiological studies indicate that vaginal cancer resembles cervical cancer, and HPV DNA is detected in a majority of vaginal tumours and their precursor lesions. HPV is detected in 82–100% of vaginal intraepithelial neoplasia grade III, and 64–91% of vaginal cancers; as in cervical cancer, HPV16 is the most prevalent type foundCitation45,Citation46.
Cancer of vulva
The age-standardized incidence rates of vulvar cancer lie between 0.5 and 1.5 per 100,000. The geographical pattern of vulvar cancer is different from cervical cancer and high rates are observed in several European populations (Scotland, Denmark, Spain, Italy), whereas the prevalence in sub-Saharan Africa, Southeast Asia, and Latin America is low. Distinct subtypes, such as the warty and basaloid types, have been recognized, but the majority of tumours are squamous cell carcinoma. Etiologically, because vulvar carcinomas are heterogeneous, the prevalence of HPV infection in invasive vulvar cancer cases variesCitation47. Vulvar cancer with basaloid histopathology in young women is often associated with HPV. HPV 16, 31 and 33 are the most frequently-detected types in this type of vulvar cancer and its precursor lesionsCitation48. On the other hand, vulvar cancer with verrucus subtype and some cases of precancerous lesions of vulvar intraepithelial neoplasia are not associated with HPV infectionCitation49. In general, the HPV-positive and -negative groups of vulvar squamous cell carcinoma share a similar prognosisCitation50.
Cancer of anus
The vast majority of anal cancers is associated with HPV infection. Cancers arising in the anal canal and tumors of the external skin (anal margin) are classified as skin cancers. The canal is lined in its upper part by colorectal-type mucosa, and in its lower third by squamous epithelium, with a specialized transitional zone in between. Therefore, cancers are predominantly squamous cell carcinoma, adenocarcinoma, or basaloid and cloacogenic carcinoma. In most populations, squamous cell carcinoma is twice as common in females as males. However the incidence is particularly high among men who have sex with men and the risk is increased further by infection with human immunodeficiency virus, cigarette smoking, anal intercourse, and more lifetime sexual partnersCitation51–53.
Cancer of penis
Globally, this rare cancer accounts for less than 0.5% of all cancers in men. The concordance of cervical and penile cancer in married couples and the geographical distribution of these cancers suggest that they share a common etiologyCitation54. Serological studies have confirmed the role of HPV 16 and HPV 18 in the etiology of penile cancer, and HPV DNA is detected in 40–50% of such cancersCitation55–57.
Cervical cancer
Among the cancers for which a confirmed or probable etiological link with HPV infection has been established, cervical cancer has the strongest association and accounts for the largest share of disease burdenCitation2,Citation12,Citation58–60. In 2008, there were about 530,000 new cases of cervical cancer, and about half this number (275,000) died of the disease worldwide; the age-standardized incidences of new cases and mortality were 15 and 8 per 100,000, respectivelyCitation61,Citation62. Worldwide, cervical cancer ranked third among cancers in women, just following breast cancer (1.3 million new cases) and colorectal cancer (0.57 million new cases) in 2008. The incidence of cervical cancer varies widely and the developing world accounts for more than 85% of both incidence and mortality. The annual age-standardized incidence rates range from 56 per 100,000 (Guinea) to < 1 per 100,000, depending mainly on the availability of organized cervical screening programs. Overall, the lowest disease burden is recorded from Australia, New Zealand, North America and Western Europe, whereas highest burden is seen in Africa, South-Central Asia and South AmericaCitation62.
Oropharyngeal cancer
An increase in incidence of oropharyngeal squamous cell carcinoma, specifically those originated from the tonsil and tongue base, has been observed in some parts of the worldCitation63,Citation64. A proportion of these lesions are associated with HPV infectionCitation65–68. The prevalence of HPV in these tumours varies geographically and reflects the variation in prevalence of oral HPV infection, which in turn mainly depends on the practice of oral sex. HPV-positive oropharnygeal squamous cell carcinomas differs from HPV-negative ones in several molecular aspects, reflecting that they are distinct entities. The molecular features observed in HPV-positive oropharyngeal cancers are consistent with the notion that HPV plays a role in the development of these tumoursCitation5,Citation6,Citation65,Citation69.
Clinically important basic virology
Viral genome and key proteins
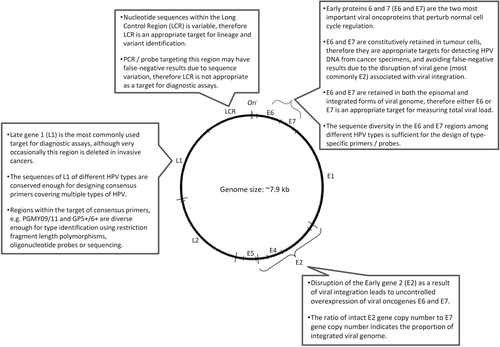
Papillomaviruses have a small double-stranded DNA genome of about 8 kb in length. The key functions and proteins encoded by different regions of the HPV genome that are relevant for designing detection assays are shown in Citation70–72. The viral genome has eight open reading frames (ORF) encoding two structural (late) proteins L1 and L2; these form the viral capsid that is about 55 nm in diameter and protects the viral genome inside. L1 is the major structural protein which is conserved within a given HPV type. The fact that L1 protein is immunogenic and conserved within a given type makes it a prime target as antigens for serological assay as well as prophylactic vaccine developmentCitation73. L1 proteins can reassemble themselves under appropriate in vitro conditions to form virus-like particles which are the main constituent of current prophylactic vaccinesCitation14,Citation53,Citation74–77. L2 is the minor capsid protein which can potentially elicit a broader spectrum of neutralizing antibodies against different types of HPV. The potential of using L2 protein as an additional component in future vaccines is being investigatedCitation78–81. The early proteins E5, E6 and E7 encoded by HPV contribute to tumour progression. The oncogenic activities of E6 and E7 are well-characterized. Both E6 and E7 have numerous cellular targets. E6 proteins encoded by high-risk HPV types primarily bind to the tumour suppressor protein p53, and the binding is mediated by the E6-associated proteins (E6-AP). Overexpression of E6, together with its interactions with other cellular proteins, results in the degradation of p53, anti-apoptosis, chromosomal destabilization, enhancement of foreign DNA integration and activation of telomeraseCitation82–89. The E7 proteins encoded by high-risk HPV types also demonstrate an important role in tumourigenesis. E7 binds to a large number of cellular proteins, most importantly the retinoblastoma protein (Rb) and the Rb-related pocket proteins. Such binding results in inactivation of Rb-related pocket proteins, activation of cyclins, inhibition of cyclin-dependent kinase inhibitors, and enhancement of foreign DNA integration and mutagenesisCitation90–95. The expression of E6 and E7 is tightly controlled via a promoter located at the non-coding Long Control Region (LCR) of the viral genome. Other early proteins encoded by papillomaviruses are E1, E2 and E5. In contrast to E6 and E7, the oncogenic activities of E5 are much less well-defined. Recent studies indicate that E5 plays a role in escape from immune surveillance, upregulation of transcription factors and inhibition of apoptosisCitation96–98. E2 is another important region of the papillomavirus genomeCitation99. E2 proteins form complexes with E1 to initiate viral replication. E2 also regulates the expression of E6 and E7, and can exert suppressive or activating effects depending on the abundance of E2. Disruption of E2 ORF as a result of integration of viral genome into the host genome allows an uncontrolled overexpression of viral oncoproteins E6 and E7, which is a hallmark in cervical cancerCitation100–102.
Concept of HPV type
HPV are classified under the family Papillomaviridae which comprises members infecting humans, non-human primates, birds, and reptilesCitation1,Citation103–105. Unlike many other viruses, the concept of “strain” is not used for papillomaviruses as they cannot be grown by conventional cell culture methods. “Serotype” cannot be applied because of the lack of a robust antibody response following natural infection. Thus, HPV types refer to “genotypes”, which are classified based on sequence similarity. To date, more than 120 HPV types have been well-characterizedCitation1. The L1 ORF is a key region for classification of HPV types (). Technically-speaking, the DNA sequence of the L1 region of an HPV type differs from all other types by more than 10%. When the differences in L1 ORF nucleotide sequences between two isolates are within 2–5%, they are regarded as subtypes; differences below 2% are referred as variants. The findings that sequence divergence of a given HPV type is limited and subtypes are rare indicate that HPV has probably gone through genetic drift that became amplified by founder effects and bottlenecks of evolutionCitation106–111. While the classification of HPV “types” is based on genome sequence, it has strong clinical implicationsCitation112,Citation113. Firstly, the phylogenetic grouping of HPV types reflects tissue tropism observed in clinical infections. HPV types belonging to the alpha genus (also known as supergroup A) mainly infect the mucosal areas, whereas types in the beta genus (also known as supergroup B) mainly infect the cutaneous areas. Secondly, the protective immune response elicited by HPV infection is mainly type-specific. Similarly, the protection induced by the currently available prophylactic vaccines (Gardasil® quadrivalent with HPV 6, 11, 16, 18 from Merck & Co.; Cervarix® bivalent with HPV 16, 18 from GlaxoSmithKline Biologicals) is also mainly type-specific, although some degree of cross-protection, especially for the bivalent vaccine, has been observedCitation114–118. The ability of HPV to induce cancer is also type-related. HPV types can be grouped into high-risk and low-risk based on their association with the development of cervical cancer. The risk associations identified by epidemiological and biochemical studies are similar. There is no dispute in grouping the commonly-found HPV types, but classification of rare types is still not clearCitation119. In view of the current epidemiological, biochemical and phylogenetic data, the mucosal/anogenital HPV types can be grouped as “high-risk” (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59), “probable high-risk” (HPV 68), “possible high-risk”(HPV 26 and 73), and “low- or unknown risk” (HPV 6, 11, 30, 34, 40, 42, 43, 44, 53, 54, 55, 57, 61, 62, 66, 67, 69, 70, 82, 85 and 97)Citation2,Citation105,Citation112,Citation119,Citation120.
Virus detection approaches
Because the replication cycle of papillomaviruses can be completed only in differentiated epithelial cells, isolation of viruses from clinical samples is difficultCitation72. Growth of papillomaviruses has been accomplished only in a specialized primary human keratinocyte-derived cell culture system, organotypic “raft” culture, where epithelial cells are grown on semi-solid agar to allow differentiation of epithelial cells and hence productive replication of papillomavirusesCitation121–123. Even with this technique, only a few types (mainly HPV 11 and 31) have been replicated successfully in laboratory conditions.
HPV is equipped with several immune evasion mechanisms. It multiplies in keratinocytes that have a short-life span, and thus the progeny viruses can be released in a natural way without inducing cell lysis that is seen with other non-enveloped viruses. This avoids triggering of inflammation and immune responses associated with cellular damage. The lack of a viremic phase also minimizes stimulation to the systemic immune system. Furthermore, the virus actively downregulates the synthesis of interferon. As a result, although the immune system plays an important role in clearing infection and it is associated with a strong localized cellular immune response, natural infection with papillomaviruses does not result in a robust antibody responseCitation124–130. For this reason, serological diagnosis has limited clinical and epidemiological value. For instance, only about 50–70% of women with persistent cervical HPV infection mount a detectable antibody response, and most women with transient infection do not have detectable antibodies or have them only for a short timeCitation131–135. Thus, the detection of HPV infection relies mainly on a molecular approach that amplifies the viral genome or mRNA, or detection of viral protein using immunoassays.
DNA-based assays
Because HPV cannot be cultured, all HPV assays currently in use rely on the detection of viral nucleic acids. They can be divided into: 1) target-amplification methods (PCR [polymerace chain reaction] with consensus or type-specific primers, and HPV mRNA amplification), and 2) signal amplification methods (liquid-phase or in situ hybridization). It is necessary to distinguish analytical sensitivity (minimum number of HPV genomes to be present in a sample to generate a positive test result) from clinical sensitivity (proportion of women with disease who test positive) ().
Table 1. Parameters for assessing the clinical performance of HPV tests in cervical cancer screening.
The choice of the HPV test depends on the application (). Assays with high analytical sensitivity are crucial for molecular epidemiological studies and for evaluating vaccine efficacy. HPV typing assays with high analytical sensitivity and specificity are the key in virological surveillance, including the evaluation of vaccination impact on the prevalence of vaccine-covered types, identification of new types, discrimination of types in multiple infection, and monitoring of potential type replacement in the post-vaccine era.
On the other hand, when applied in the clinical situation for cervical cancer screening and post-treatment follow-up, assays with lower analytical sensitivity may produce a better positive predictive valueCitation136–138. It is important that HPV tests are clinically validated under context-specific conditions, especially in the target population.
Hybrid capture HPV DNA assay
Hybrid capture (HC2) is based on liquid phase hybridization using long synthetic RNA probes complementary to the genomic sequences of 13 high-risk types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68) and five low-risk types (6, 11, 42, 43 and 44); these are used to prepare high-risk (B) and low-risk (A) probe cocktails that are used in two separate reactionsCitation139. In practice, often only the high-risk probes are used. DNA present in the biological specimen is hybridized in solution phase with each of the probe cocktails and forms specific HPV DNA-RNA hybrids. These hybrids are then captured by antibodies bound to the wells of microtiter plates that recognize specific RNA-DNA hybrids. After removal of excess antibodies and non-hybridized probes, the immobilized hybrids are detected by a series of reactions that give rise to a luminescent product that is detected by a luminometer. The intensity of emitted light, expressed as relative light units, is proportional to the amount of target DNA present in the specimen, and provides a semiquantitative measure of the viral load. The HC2 is currently available in a 96-well microplate format with automation that is easy to use in clinical settings. An automated robotic platform that allows handling of 96-well microplates has been developed for high-volume testing.
HC2 does not require special facilities to avoid cross-contamination because it does not rely on target amplification to achieve high sensitivity, as do PCR protocols. The recommended cut-off value for test-positive results is 1.0 relative light unit (equivalent to 1 pg HPV DNA per 1 mL of sampling buffer). Several studies have noted that the high-risk probe cocktail in HC2 cross-reacts with HPV types that are not represented in the probe mix. It has been reported that HC2 using the high-risk probe at a 1.0-pg/mL cut-off detected HPV types 53, 66, 67, 73, as well as other undefined types, and raising the cut-off to 10.0 pg/mL did not eliminate the cross reactivity to types 53 and 67Citation140. Cross-reactivity of HC2 high-risk probe to HPV types that have a significant risk for cervical cancer may be considered as beneficial, but cross-reaction with low-risk types causing false-positive results may decrease the specificity and positive predictive valueCitation141.
Consensus, group- or type-specific PCR
Currently, the most important clinical application of HPV detection is to identify women with a higher risk of developing cervical intraepithelial neoplasia or invasive cervical cancer (). HPV DNA detection systems that are designed to catch all the mucosal or anogenital HPV types are referred as “consensus”, those detecting high-risk or low-risk HPV types as a group are referred as “group-specific”, and those identify individual HPV types are referred as “type-specific”. A variety of commercial assays belonging to each category are available ().
Table 2. Commercially available HPV tests.
Target regions
L1: The L1 region is the most frequently-used target for amplifying HPV genome from clinical samples (). L1, on the one hand, is conserved enough for the design of consensus primers to amplify a broad spectrum of HPV types by using a single set of degenerated primers or a cocktail of primersCitation142–149. The commonly-used primer sets are shown in . On the other hand, the L1 region also has sufficient sequence diversity to allow the identification of individual HPV types based on further analysis of the amplified products. Given the definition that L1 sequences of different HPV types should differ by more than 10%, type differentiation is achievable even based on a short fragment, less than 100-bp, of L1. HPV type can be identified by a few approaches. Type-specific restriction fragment length polymorphisms can be generated by using two restriction endonucleases Rsa I and DdeCitation150,Citation151. This method was more popular when sequencing was still expensive and labour intensive. The main disadvantage of restriction fragment length polymorphisms is the ambiguous restriction fragment patterns generated from co-infection with multiple types, especially when three or more types are present in a sample. This approach has gradually been replaced by sequencing because the cost for the latter has fallen substantially in recent years. However, sequencing of PCR product can reveal only the HPV type that predominates in a co-infection. Today, the best approach to identify multiple HPV types simultaneously from a sample is to perform hybridization with multiple HPV type-specific probes immobilized on strips, membranes or array slides ().
Table 3. Commonly used primers targeting the L1 region of HPV genome.
While L1 is regarded as the best target for HPV genome detection, it is absent from a small proportion of invasive cervical cancer samples, probably due to disruption resulting from viral genome integrationCitation152. When such a situation is suspected, the constitutively retained genes, E6 or E7, can be amplified to verify the presence of HPV infection. Furthermore, consensus primer sets may have different analytical sensitivities for different HPV types or variantsCitation147. When adopting assays developed elsewhere, evaluation based on samples collected from the local population is necessary before the assays are used clinically.
E6, E7: The E6 and E7 regions are good alternative targets for amplifying HPV genome from clinical specimens. Firstly, the nucleotide sequence diversity of E6 and E7 between HPV types allows the design of type-specific primers. Secondly, E6 and E7 gene expression is required to maintain the transformed phenotype of infected cellsCitation153–159. Therefore, both E6 and E7 genes are expected to be retained regardless of the status of viral integration. The constitutional presence of E6 and E7 genes makes them an appropriate target for viral load quantificationCitation160,Citation161.
E2: Papillomavirus infection can exist in two forms. The vegetative phase, also known as the replicative or productive phase, is associated with a full episomal form of viral genome. This replicative phase is typically found in the upper layers of epithelium with differentiated keratinocytes, or in benign condyloma where a large amount of virus is releasedCitation32,Citation162–164. In the integrated form, the viral genome is disrupted and therefore the viral replication cycle cannot be completed. It is widely accepted that HPV-mediated cervical carcinogenesis proceeds via the integration of viral genome and disruption of the E2 ORF, thus releasing the suppressive control of E2 on the expression of viral oncogenes E6 and E7. Thus, E2 is often used as a surrogate marker to indicate the status of viral integration. By measuring the ratio between E2 and E6 (or E7) gene copy numbers, one can estimate the proportion of integrated viral genome present in a clinical specimen.
E6/E7 mRNA-based assays
E6 and E7 are oncoproteins involved in carcinogenesis. Persistent expression of E6 and E7 could serve as an indicator of progression from intraepithelial neoplasia to invasive cancerCitation165–167. Detecting the mRNA encoded by E6 or E7 may, therefore, provide a better predictive value for malignant or high-grade lesionsCitation168–171. A few commercial assays based on this approach have been developed recently ()Citation172,Citation173. The PreTect HPV-Proofer (Norchip, Klokkarstua) and NucliSENS Easy Q HPV (BioMerieux, Marcy-l’Étoile) assays are based on the same technology, and are marketed under different brand names in different countries. PreTect Proofer and NucliSENS Easy Q detect E6/E7 mRNA from five high-risk types (HPV 16, 18, 31, 33 and 45) commonly found in high-grade lesions and cancers. The APTIMA HPV Assay (Gen-Probe) provides a broader coverage and targets mRNA of 14 high-risk HPV types. In a review on 11 studies examining these three currently-available commercial assays, the sensitivities for cervical intraepithelial neoplasia grade II and above (CIN [cervical intraepithelial neoplasia] II+) lesions ranged from 41% to 86% for the PreTect Proofer/EasyQ assay and from 90% to 95% for the APTIMA assay, whereas the specificities ranged from 63% to 97% for the PreTect Proofer/Easy Q assay and from 42% to 61% for the APTIMA assayCitation174.
E6/E7 protein-based assays
Similarly, measuring the E6 or E7 proteins may provide a better predictive value than detecting viral DNA aloneCitation175,Citation176. However, E6 and E7 proteins are known to be produced in small amounts in transformed cells. The sensitivity of the E6/E7 protein-based assay is a concern that needs to be addressed. Data on clinical evaluation of the E6/E7 protein-based assay is not yet available.
Building quality control capacity
The need to monitor vaccine effectiveness by effective surveillance programs and the increasing use of HPV assays in the field have stimuated the World Health Organization (WHO) to develop a structured Global Laboratory Network (WHO HPV LabNet). To date, this LabNet includes two Global Reference Laboratories (Sweden and USA) and eight Regional Reference Laboratories (Argentine, Australia, India, Japan, South Africa, Switzerland, Thailand and Tunisia). Work is currently being conducted in the areas of scientific and technical advice, quality assurance, training, and communication (http://www.who.int/biologicals/areas/human_papillomavirus/WHO_HPV_LabNet/en/index.html. Accessed on 9 July 2012). LabNet, with the collaboration of the National Institute of Biological Standards and Control (UK), is establishing international standards (IS) for HPV types, to harmonize HPV nucleic acid amplification technology-based assays. These efforts will be immensely helpful in monitoring the impact of HPV vaccination programs worldwide and in evaluating data on uniform platforms. One of the main aims is to harmonize various HPV detection assays and to minimize inter-laboratory variation by collectively establishing strong and effective quality control and quality assurance programs.
Clinical applications
HPV for primary screening
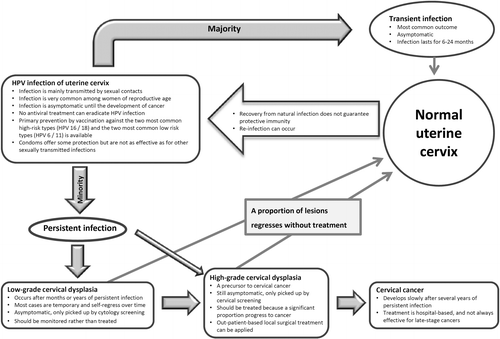
HPV infection progresses slowly to cervical cancer through stages of pathological changes that can be recognized from exfoliated cervical cells (). Detection of lesions at the precancerous stage allows intervention by office-based local surgery. Until recently, cervical screening has been based solely on cytological examination of exfoliated cervical cells. The benefit of regular cervical cytology screening is undisputedCitation177–181. Cervical cancer incidence decreases substantially after the introduction of cervical screening and there are consistent and marked differences in cervical cancer incidence rates between countries with and without organized screening programsCitation61,Citation182. While conventional Pap smear or liquid-based cytology is still the standard of care in many parts of the world, the intrinsic drawbacks of cytology-based screening call for replacement by HPV testing or the addition of adjunct markersCitation183. Compared to molecular markers, cytology is more subjective and requires a stringent quality control and quality assurance program to maintain clinical performance. Cytology is relatively insensitive and is associated with an unavoidable portion of non-specific and self-limiting abnormal results. With the increased availability of high-throughput screening platforms ()Citation184, a large number of large-scale studies have been conducted to investigate the value of using HPV DNA detection (mainly based on hybridization (hybrid capture) or PCR amplification targeting the L1 region) as a primary or supplementary tool for cervical screening.
Advantages
The overall advantages of HPV testing over cytology for screening of cervical cancer are: feasibility for high throughput, greater objectivity in result interpretation, high sensitivity, high negative predictive value, and ability to provide long-term risk stratificationCitation185–192. Furthermore, the performance of HPV assays is less subject to variation across centers. For instance, the reported sensitivity of cytology ranges from 33.8% to 94.0%, whereas that of the HPV assay (HC 2), used in the same series of studies across different continents, varies from 84.9% to 97.6%Citation193–199. However, HPV infections are transient in most women and the prevalence of high-grade intraepithelial neoplasia or cancer among infected women is low. The low specificity and low positive predictive value are the major drawbacks of applying HPV DNA testing in clinical practice. A fine balance has to be established between the sensitivity and specificity of the HPV test to achieve a clinically-useful predictive value ().
Approaches to improve positive predictive value
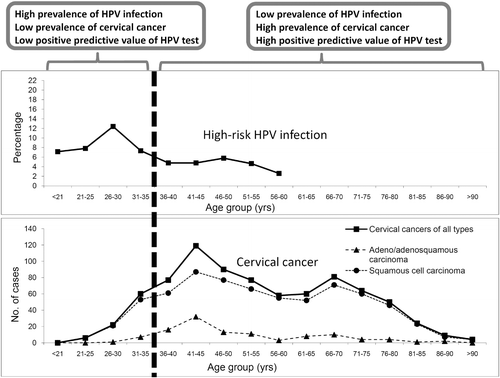
Infection and disease prevalence: Several approaches can be considered to minimize the “background noise” and to improve the positive predictive value. The “background noise” depends on the prevalence of transient HPV infection among the target population for which the HPV test is being applied. Data on the age-specific prevalence of HPV infection and the age-specific incidence of CIN II/III and cervical cancer are essential to derive a cut-off for applying HPV testing ()Citation200. In general, HPV testing is not recommended for women below 30 years of age (or 35 in some countries) for whom transient infection is commonCitation187,Citation201,Citation202.
Figure 3. Improved positive predictive value of HPV test by refining the target population. Figure shows the age-specific prevalence of high-risk HPV infection and age-specific distribution of cervical cancer cases in Hong KongCitation196. Restricting HPV test to women aged ≥ 35 years avoids the age-related infection peak, and covers women with higher incidence of cervical cancer. By setting the testing population to women aged ≥ 35 years (in case of Hong Kong as used in this example) will therefore improve the positive predictive value of HPV testing for cervical cancer.
Super high-risk HPV types: At least 15 HPV types can be linked to cervical cancer with various degrees of risk associationCitation112. Initially, most HPV assays are designed to detect as many high-risk types as possible. In fact, the risk association among HPV types classified under this so-called “high-risk” group varies substantially. In recent years, it has been recognized that covering the HPV types with a small risk may jeopardize the overall positive predictive value of the assay. Thus type-specific assays, especially those targeting a group of HPV types with the highest risk association, have emerged. In this regard, HPV 16 and HPV 18 should be included as they are the two high-risk types most commonly found in cervical cancers across the world. HPV 31, 33 and 45 are the next group to be included, although their ranking showed some degrees of geographical variationCitation203–207. HPV 52 and HPV 58 showed an even more skewed geographical distribution, and the clinical value of including these types into screening assays should be assessed based on the HPV type distribution data derived from the target populationCitation152,Citation204,Citation207–215.
An HPV16/18-specific test is expected to provide the highest positive predictive value. In the most recent guidelines from the United States, HPV16/18 testing is one of the options to triage women found to be HPV-positive but cytology-negative in primary screening using the co-test approachCitation202.
Persistent infection: Since persistent infection is a prerequisite for the development of cervical precancer and cancer, one can improve the positive predictive value by considering HPV testing results performed on specimens collected more than 12 months apart. If HPV is detected in both instances, the chance that it is a persistent rather than transient infection is higher. In this regard, an HPV type-specific test is necessary to differentiate repeated infections with different HPV types from genuine persistent infection with the same HPV type(s). It is only the latter that carries an increased risk of cancer developmentCitation216. However, the limitation of this approach is that a proportion of women may not come back for the second test and may become lost to follow-up.
Reflex follow-up test: Another approach to improve the positive predictive value of HPV testing as a primary screening tool is to carry out a “reflex” follow-up test for HPV-positive samples. The “reflex” approach can prevent an extra visit and unnecessary anxiety while waiting for the follow-up test results. Biomarkers indicating the transforming activity of HPV can potentially serve this purposeCitation102,Citation217,Citation218. The first approach is to detect direct indicators of HPV oncogene expression. At present, this mainly refers to the detection of mRNA encoded by the viral oncogenes, E6 and E7. Among women with normal cytology, atypical squamous cells of undetermined significance (ASCUS) and low-grade squamous intraepithelial lesions (LSIL), E6/E7 mRNA was detected in 30% of the HPV 16, 56% of the HPV 18 and 75% of the HPV 31 DNA-positive women, respectively. The mRNA test therefore potentially has a higher specificity compared to the HPV DNA test, and thus fewer patients would be referred for further testing or close follow-upCitation219. Assays for direct measurement of E6 and E7 proteins from clinical specimens have been described and their clinical performance is being evaluated. The second approach is to detect biomarkers of increased cellular proliferation and chromosomal instability or those upregulated in response to HPV-encoded oncoproteins, including p16INK4a, Ki-67, topoisomerase IIA (TOP2), and minichromosome maintenance proteins (MCMP). Among these, p16INK4a is the most promisingCitation220,Citation221. In many non-HPV-associated tumours, p16INK4a is inactivated by genetic deletion or hypermethylation, which leads to an increase in cyclin-dependent kinase activity and inactivation of RbCitation222. In contrast, in HPV-associated tumours, including cervical intraepithelial neoplasia and invasive cervical cancer, the inactivation of Rb by E7 leads to a marked overexpression of p16INK4a as a result of the lost of negative feedback regulation that depends on Rb activityCitation223–225. It has been shown that, among 425 Pap-negative and HPV-positive women greater than 30 years of age, 25.4% were positive for p16/ki67 dual staining. The dual staining gave a sensitivity of 91.9% for CIN II and 96.4% for CIN III+ after a mean follow-up of 13.8 months (1–27 months)Citation226. The result is encouraging but further studies are needed to support its clinical use.
Viral genome characterization: The patterns of viral integration at different stages of neoplastic progression have been investigated. Diverse, even conflicting, results have been reported. Some studies observed viral integration mainly from specimens of high-grade lesionsCitation227,Citation228, whereas others found that viral integration takes place early during the course of infection and is detected in a substantial proportion of low-grade lesionsCitation229–232. It has been suggested that viral integration is a consequence rather than a cause of chromosomal instabilityCitation233. Based on the available data, the main concern appears to be the lack of specificity. Basically, viral integration can be detected in normal and low-grade lesions, whereas cervical samples from cases of invasive cancer can harbour purely the episomal form of viral genomeCitation234–237.
DNA methylation is an epigenetic event that is linked to cancer development. A number of studies have been conducted to examine the association between the viral DNA methylation pattern and lesion severity. However, as with viral integration, most of the available data suggests that the patterns observed from low-grade and high-grade lesions are too diverse to achieve a clinically-useful predictive valueCitation238–242. Nevertheless, a recent study using a newer approach, pyrosequencing, has produced some promising resultsCitation243. Viral integration and methylation have a strong biological basis, and further studies to explore their clinical application are worthwhile.
Co-test with cytology for primary screening
Co-test refers to the use of both HPV and cytology tests in parallel as first-line screening. The main advantage of co-test is the improvement in sensitivity for CIN II+ lesions; women who are double negative will have an extremely low-risk for CIN II+. The potential gain in cost-effectiveness from the expense of the extra test is the longer interval of safety supported by a double negative result. Data have suggested that the safety interval following an HPV-negative result could be as long as 5–7 yearsCitation244–248. The most recent guidelines from the United Sates recommend HPV and cytology co-testing every 5 years for women aged 30–65 yearsCitation201. However, the management of women with normal cytology but an HPV-positive result is an issue that is not yet completely resolved. The question is what proportion of the HPV-positive, cytology-normal women have transient HPV infection, and how many of them will turn out to be HPV-negative when the co-test is repeated in 1 year. This is fundamentally the same question when HPV testing is applied alone as a primary screening tool, and the answer varies substantially with the analytical sensitivity of the HPV assay and the prevalence of CIN II/III in the target populationCitation249. For patients with a normal Pap smear but an HPV-positive test, their risk of developing abnormal cytology and CIN II+ lesions are significantly higher than those with a double negative result. The increased risk was found to be HPV type-dependent, and the cumulative risk of those infected with HPV 16 reached 26% after a 13-year follow-upCitation250. The United States Food and Drug Administration (US FDA) has approved the use of an HPV16/18 type-specific test in this particular clinical situation for women ≥ 30 years of age in order to identify individuals with a higher risk for developing disease in the future. According to the recent guidelines from the United States, women with HPV-positive and cytology-negative screening results can be either followed with the co-test 12 months later or triaged with the HPV16/18-specific test for referral to colposcopyCitation201.
HPV as a triage for abnormal cytology
The first clinical application of HPV testing was on the triage of patients presented with ASCUS on Pap smear. Patients who were HPV-positive would be referred for colposcopy whilst those who were HPV-negative could be followed by repeating the Pap smear 12 months laterCitation251–254. This is still the most common application of HPV testing, and a large body of evidence is available to support returning women with negative HPV DNA results to a normal screening schedule.
The cost-effectiveness of using HPV testing to triage women with LSIL depends mainly on the context. For instance, it has been shown that about 80% of patients presenting with LSIL were HPV-positive; thus triage of LSIL by HPV testing was not recommendedCitation255. On the other hand, the US FDA has approved the use of HPV testing in post-menopausal women presenting with LSIL since the prevalence rate of HPV is low in this subset of patients. Therefore, data generated from context-specific studies are very important. The main variable is again the prevalence of HPV infection among the population tested. Such an approach may be effective for women well beyond the peak of infection, so that a substantial proportion of women will have an HPV-negative result and can be reassuredCitation256,Citation257.
The underlying risk of having CIN II+ lesions among women with high-grade squamous intraepithelial lesions (HSIL) or atypical squamous cells is high, but HSIL (ASC-H) cannot be excluded, and colposcopy may be indicated for CIN II+ even if the HPV test result is negativeCitation258,Citation259. HPV testing is also not very useful for women with atypical glandular cells because the underlying pathology may reside in the uterus. Whilst a positive HPV test suggests a cervical lesion, a negative test does not rule out such conditions as endometrial hyperplasia or cancer that are not HPV-related, and the risk of these is substantial in post-menopausal womenCitation260.
HPV for post-treatment surveillance
CIN II is often regarded as a threshold for treatment (). The predominant mode is excision of the transformation zone using the loop electrosurgical excision procedure (LEEP). Since about 5 to 10% of patients have persistent or recurrent CIN II+ after LEEP, continual surveillance after treatment is needed. When compared to conventional cytology, HPV testing is more sensitive, carries a higher negative predictive value for recurrent or residual lesions, and is recommended by the American Congress of Obstetricians and Gynecologists for post-treatment follow-upCitation261–263. In a cohort of 917 patients treated by LEEP, 81% were double-negative at 6 months after treatment and their risk of being diagnosed with CIN III+ was low. It has been suggested that patients with both negative cytology and negative HPV DNA can return to the 3-year screening programCitation264.
Future challenges
The currently available prophylactic HPV vaccines are highly effective for the prevention of HPV16/18-related cervical neoplasia and offer some degree of cross-protection for lesions caused by related HPV types. A number of countries have implemented vaccination programs for adolescents and the coverage is expected to increase in the coming yearsCitation265. These countries are also likely to be those running organized cervical screening programsCitation265–268.
In the coming decades, several issues regarding cervical screening will need to be addressedCitation269,Citation270. The decrease in absolute incidence of cervical intraepithelial neoplasia and cervical cancer among those vaccinated will jeopardize the positive predictive value of any screening test. To achieve maximum cost-effectiveness, the screening strategy for those who have received the vaccine before their sexual debut should be different from those who have notCitation271. These two populations will co-exist for a few decades and it will be a challenge to public health professionals to organize two systems in parallel for the same disease. Among those vaccinated, the chance of detecting a genuine abnormal signal (abnormal cytology results representing cervical precancer or cancer) will decrease, whereas the proportion of samples showing “noise” (abnormal cytology results due to inflammation and reactive changes that are self-limiting) will increase. The positive predictive value of minor cytological abnormalities will be even lower because of the reduced prevalence of CIN. As a result, because of its subjective nature, the reading and interpreting cytology results will be even more prone to human error. For these reasons, assays based on objective methods such as the detection of HPV and biomarkers will be advantageous.
Since HPV is a sexually-transmitted infection, using HPV testing for cervical screening may lead to anxiety and concerns about sexual relationships, and may have an emotional impact on the quality of life and a negative impact on mental healthCitation272.
Other practical challenges are the diversion of resources towards vaccination, and the resulting pressure for less frequent screening. At present, the recommended frequency in most countries is not less than once every 2–3 years. To lengthen the screening interval means getting closer to the interval required for advancing from low-grade to high-grade intraepithelial lesions or even to the development of invasive cancer. In other words, there may be just one chance to pick up at-risk women before the full development of invasive cancer. This will require a test with extreme sensitivity that is subject to a low positive predictive value, especially in the vaccinated population with a low incidence of disease. Lengthening of the interval for cytological examination is not advisable because the sensitivity of the test is low and repeated negative cytological tests are required to ensure no underlying precancerous or cancerous lesions. The high sensitivity of HPV testing, on the other hand, is more reassuring and allows lengthening of the screening interval. To overcome the low positive predictive value of highly-sensitive HPV testing in the vaccinated population with a low incidence of disease, a supplementary test for other biomarkers or co-testing with cytology is the way to move forward.
Conclusions
Technically speaking, HPV testing has several advantages over cytology-based screening, particularly in the situation where the incidence of cervical cancer and precancer decreases substantially following the widespread use of vaccination. However, although it is beyond the scope of this review, the psycho-social stigma associated with testing for a sexually-transmitted infection needs to be addressed. It has been shown that, even among well-educated women, most have not heard of HPV and do not know its association with cervical cancerCitation273–276. Public education should go in parallel with the technical development in HPV testing. Moreover, whatever technology is used for screening, it is important to point out that the key for success is to reach a high coverage. Screening for cancer is like looking for a needle in a haystack, which is always challenging.
Declaration of interest
None to declare
References
- Bernard HU, Burk RD, Chen Z, van Doorslaer K, Hausen H, de Villiers EM. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 2010;401:70–79.
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet 2007;370:890–907.
- Syrjänen S. Current concepts on human papillomavirus infections in children. APMIS 2010;118:494–509.
- Partridge JM, Koutsky LA. Genital human papillomavirus infection in men. Lancet Infect Dis 2006;6:21–31.
- Feller L, Wood NH, Khammissa RA, Lemmer J. Human papillomavirus-mediated carcinogenesis and HPV-associated oral and oropharyngeal squamous cell carcinoma. Part 1: human papillomavirus-mediated carcinogenesis. Head Face Med 2010;6:14.
- Feller L, Wood NH, Khammissa RA, Lemmer J. Human papillomavirus-mediated carcinogenesis and HPV-associated oral and oropharyngeal squamous cell carcinoma. Part 2: human papillomavirus associated oral and oropharyngeal squamous cell carcinoma. Head Face Med 2010;6:15.
- Gallagher TQ, Derkay CS. Recurrent respiratory papillomatosis: update 2008. Curr Opin Otolaryngol Head Neck Surg 2008;16:536–542.
- Dubina M, Goldenberg G. Viral-associated nonmelanoma skin cancers: a review. Am J Dermatopathol 2009;31:561–573.
- Feltkamp MC, de Koning MN, Bavinck JN, Ter Schegget J. Betapapillomaviruses: innocent bystanders or causes of skin cancer. J Clin Virol 2008;43:353–360.
- Handisurya A, Schellenbacher C, Kirnbauer R. Diseases caused by human papillomaviruses (HPV). J Dtsch Dermatol Ges 2009;7:453–466; quiz 466, 467.
- Pfister H. Chapter 8: Human papillomavirus and skin cancer. J Natl Cancer Inst Monogr 2003;31:52–56.
- zur Hausen H. Papillomaviruses in the causation of human cancers – a brief historical account. Virology 2009;384:260–265.
- Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev 2003;16:1–17.
- Lowy DR, Schiller JT. Prophylactic human papillomavirus vaccines. J Clin Invest 2006;116:1167–1173.
- Forslund O, Antonsson A, Nordin P, Stenquist B, Hansson BG. A broad range of human papillomavirus types detected with a general PCR method suitable for analysis of cutaneous tumours and normal skin. J Gen Virol 1999;80:2437–2443.
- Hazard K, Karlsson A, Andersson K, Ekberg H, Dillner J, Forslund O. Cutaneous human papillomaviruses persist on healthy skin. J Invest Dermatol 2007;127:116–119.
- Antonsson A, Forslund O, Ekberg H, Sterner G, Hansson BG. The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of these viruses. J Virol 2000;74:11636–11641.
- Mammas IN, Sourvinos G, Spandidos DA. Human papilloma virus (HPV) infection in children and adolescents. Eur J Pediatr 2009;168:267–273.
- Kirnbauer R, Lenz P, Okun MM. Human Papillomavirus. In: Bolognia J, Jorizzo J, Rapini R, ed. Dermatology, Vol. 1. London: Mosby, 2008:1183–1198.
- McLaughlin JS, Shafritz AB. Cutaneous warts. J Hand Surg Am 2011;36:343–344.
- Syrjänen KJ. Annual disease burden due to human papillomavirus (HPV) 6 and 11 infections in Finland. Scand J Infect Dis Suppl 2009;107:3–32.
- Chan PK, Luk AC, Luk TN, Lee KF, Cheung JL, Ho KM, Lo KK. Distribution of human papillomavirus types in anogenital warts of men. J Clin Virol 2009;44:111–114.
- Oon SF, Winter DC. Perianal condylomas, anal squamous intraepithelial neoplasms and screening: a review of the literature. J Med Screen 2010;17:44–49.
- Hsueh PR. Human papillomavirus, genital warts, and vaccines. J Microbiol Immunol Infect 2009;42:101–106.
- Ghaemmaghami F, Nazari Z, Mehrdad N. Female genital warts. Asian Pac J Cancer Prev 2007;8:339–347.
- Larson DA, Derkay CS. Epidemiology of recurrent respiratory papillomatosis. APMIS 2010;118:450–454.
- Bonagura VR, Hatam LJ, Rosenthal DW, de Voti JA, Lam F, Steinberg BM, Abramson AL. Recurrent respiratory papillomatosis: a complex defect in immune responsiveness to human papillomavirus-6 and -11. APMIS 2010;118:455–470.
- Tasca RA, Clarke RW. Recurrent respiratory papillomatosis. Arch Dis Child 2006;91:689–691.
- Feller L, Khammissa RA, Wood NH, Malema V, Meyerov R, Lemmer J. Focal epithelial hyperplasia (Heck disease) related to highly active antiretroviral therapy in an HIV-seropositive child. A report of a case, and a review of the literature. SADJ 2010;65:172–175.
- Feller L, Khammissa RA, Wood NH, Marnewick JC, Meyerov R, Lemmer J. HPV-associated oral warts. SADJ 2011;66:82–85.
- González JV, Gutiérrez RA, Keszler A, Colacino Mdel C, Alonio LV, Teyssie AR, Picconi MA. Human papillomavirus in oral lesions. Medicina (B Aires) 2007;67:363–368.
- zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2002;2:342–350.
- Grulich AE, Jin F, Conway EL, Stein AN, Hocking J. Cancers attributable to human papillomavirus infection. Sex Health 2010;7:244–252.
- Ramoz N, Rueda LA, Bouadjar B, Montoya LS, Orth G, Favre M. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat Genet 2002;32:579–581.
- Orth G. Genetics of epidermodysplasia verruciformis: Insights into host defense against papillomaviruses. Semin Immunol 2006;18:362–374.
- Lazarczyk M, Cassonnet P, Pons C, Jacob Y, Favre M. The EVER proteins as a natural barrier against papillomaviruses: a new insight into the pathogenesis of human papillomavirus infections. Microbiol Mol Biol Rev 2009;73:348–370.
- Sterling JC. Human papillomaviruses and skin cancer. J Clin Virol 2005;32:S67–71.
- Patel T, Morrison LK, Rady P, Tyring S. Epidermodysplasia verruciformis and susceptibility to HPV. Dis Markers 2010;29:199–206.
- Forslund O, Iftner T, Andersson K, Lindelof B, Hradil E, Nordin P, et al; Viraskin Study Group. Cutaneous human papillomaviruses found in sun-exposed skin: Beta-papillomavirus species 2 predominates in squamous cell carcinoma. J Infect Dis 2007;196:876–883.
- Patel AS, Karagas MR, Perry AE, Nelson HH. Exposure profiles and human papillomavirus infection in skin cancer: an analysis of 25 genus beta-types in a population-based study. J Invest Dermatol 2008;128:2888–2893.
- Asgari MM, Kiviat NB, Critchlow CW, Stern JE, Argenyi ZB, Raugi GJ, et al. Detection of human papillomavirus DNA in cutaneous squamous cell carcinoma among immunocompetent individuals. J Invest Dermatol 2008;128:1409–1417.
- Akgül B, Cooke JC, Storey A. HPV-associated skin disease. J Pathol 2006;208:165–175.
- Bleeker MC, Heideman DA, Snijders PJ, Horenblas S, Dillner J, Meijer CJ. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol 2009;27:141–150.
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006;24 (Suppl 3):S3/11–25.
- Muñoz N, Castellsagué X, de González AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006;24 (Suppl 3):S3/1–10.
- Chao A, Chen TC, Hsueh C, Huang CC, Yang JE, Hsueh S, et al. Human papillomavirus in vaginal intraepithelial neoplasia. Int J Cancer 2012;131:E259–E268.
- Hildesheim A, Han CL, Brinton LA, Kurman RJ, Schiller JT. Human papillomavirus type 16 and risk of preinvasive and invasive vulvar cancer: results from a seroepidemiological case-control study. Obstet Gynecol 1997;90:748–754.
- Tumours of the vulva. In: Tavassoli FA, Devilee P, eds. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Lyon: IARC Press; 2003: 313–333.
- Junge J, Poulsen H, Horn T, Hørding U, Lundvall F. Human papillomavirus (HPV) in vulvar dysplasia and carcinoma in situ. APMIS 1995;103:501–510.
- Alonso I, Fusté V, del Pino M, Castillo P, Torné A, Fusté P, et al. Does human papillomavirus infection imply a different prognosis in vulvar squamous cell carcinoma? Gynecol Oncol 2011;122:509–514.
- Daling JR, Madeleine MM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer 2004;101:270–280.
- Dietz CA, Nyberg CR. Genital, oral, and anal human papillomavirus infection in men who have sex with men. J Am Osteopath Assoc 2011;111(3 Suppl 2):S19–S25.
- Palefsky J. Can HPV vaccination help to prevent anal cancer? Lancet Infect Dis 2010;10:815–816.
- Smith PG, Kinlen LJ, White GC, Adelstein AM, Fox AJ. Mortality of wives of men dying with cancer of the penis. Br J Cancer 1980;41:422–428.
- Picconi MA, Eiján AM, Distéfano AL, Pueyo S, Alonio LV, Gorostidi ST, et al. Human papillomavirus (HPV) DNA in penile carcinomas in Argentina: analysis of primary tumors and lymph nodes. J Med Virol 2000;61:65–69.
- Palefsky JM. Human papillomavirus-related disease in men: not just a women’s issue. J Adolesc Health 2010;46(4 Suppl):S12–S19.
- Anic GM, Giuliano AR. Genital HPV infection and related lesions in men. Prev Med 2011;53 (Suppl 1):S36–S41.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69–90.
- WHO. 2008–2013 Action Plan for the Global Strategy for the Prevention and Control of Noncommunicable Diseases. Geneva, Switzerland: World Health Organization 2008;1–42.
- Yang BH, Bray FI, Parkin DM, Sellors JW, Zhang ZF. Cervical cancer as a priority for prevention in different world regions: an evaluation using years of life lost. Int J Cancer 2004;109:418–424.
- Arbyn M, Castellsagué X, de Sanjosé S, Bruni L, Saraiya M, Bray F, Ferlay J. Worldwide burden of cervical cancer in 2008. Ann Oncol 2011;22:2675–2686.
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–2917.
- Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011;29:4294–4301.
- Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol 2008;26:612–619.
- Allen CT, Lewis JS Jr, El-Mofty SK, Haughey BH, Nussenbaum B. Human papillomavirus and oropharynx cancer: biology, detection and clinical implications. Laryngoscope 2010;120:1756–1772.
- Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 2010;11:781–789.
- Syrjänen S, Lodi G, von Bültzingslöwen I, Aliko A, Arduino P, Campisi G, et al. Human papillomaviruses in oral carcinoma and oral potentially malignant disorders: a systematic review. Oral Dis 2011;17 (Suppl 1):58–72.
- Toner M, O’Regan EM. Head and neck squamous cell carcinoma in the young: a spectrum or a distinct group? Part 1. Head Neck Pathol 2009;3:246–248.
- Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer 2011;11:9–22.
- Howley PM, Lowy DR. Papillomavirus. In: Knipe DM, Howley PM, et al., eds: Fields Virology, Vol. 2. 5th ed. Philadelphia: Lippincott Williams and Wilkins, 2007: 2299–2354.
- Longworth MS, Laimins LA. Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol Mol Biol Rev 2004;68:362–372.
- Doorbar J. The papillomavirus life cycle. J Clin Virol 2005;32 (Suppl 1):S7–S15.
- Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA 1992;89:12180–12184.
- Zhou J, Sun XY, Stenzel DJ, Frazer IH. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology 1991;185:251–257.
- Chen J, Ni G, Liu XS. Papillomavirus virus like particle-based therapeutic vaccine against human papillomavirus infection related diseases: immunological problems and future directions. Cell Immunol 2011;269:5–9.
- Villa LL. HPV prophylactic vaccination: The first years and what to expect from now. Cancer Lett 2011;305:106–112.
- Frazer IH. Cervical cancer vaccine development. Sex Health 2010;7:230–234.
- Tumban E, Peabody J, Peabody DS, Chackerian B. A pan-HPV vaccine based on bacteriophage PP7 VLPs displaying broadly cross-neutralizing epitopes from the HPV minor capsid protein, L2. PLoS One 2011;6:e23310.
- Conway MJ, Cruz L, Alam S, Christensen ND, Meyers C. Cross-neutralization potential of native human papillomavirus N-terminal L2 epitopes. PLoS One 2011;6:e16405.
- Caldeira Jdo C, Medford A, Kines RC, Lino CA, Schiller JT, Chackerian B, Peabody DS. Immunogenic display of diverse peptides, including a broadly cross-type neutralizing human papillomavirus L2 epitope, on virus-like particles of the RNA bacteriophage PP7. Vaccine 2010;28:4384–4393.
- Stanley M. Prospects for new human papillomavirus vaccines. Curr Opin Infect Dis 2010;23:70–75.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 1990;248:76–79.
- Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 1993;75:495–505.
- White AE, Livanos EM, Tlsty TD. Differential disruption of genomic integrity and cell cycle regulation in normal human fibroblasts by the HPV oncoproteins. Genes Dev 1994;8:666–677.
- Havre PA, Yuan J, Hedrick L, Cho KR, Glazer PM. p53 inactivation by HPV16 E6 results in increased mutagenesis in human cells. Cancer Res 1995;55:4420–4424.
- Kessis TD, Connolly DC, Hedrick L, Cho KR. Expression of HPV16 E6 or E7 increases integration of foreign DNA. Oncogene 1996;13:427–431.
- Ronco LV, Karpova AY, Vidal M, Howley PM. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev 1998;12:2061–2072.
- Thomas M, Banks L. Inhibition of Bak-induced apoptosis by HPV-18 E6. Oncogene 1998;17:2943–2954.
- Klingelhutz AJ, Foster SA, McDougall JK. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature 1996;380:79–82.
- Reznikoff CA, Yeager TR, Belair CD, Savelieva E, Puthenveettil JA, Stadler WM. Elevated p16 at senescence and loss of p16 at immortalization in human papillomavirus 16 E6, but not E7, transformed human uroepithelial cells. Cancer Res 1996;56:2886–2890.
- Dyson N, Howley PM, Münger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 1989;243:934–937.
- Dyson N, Guida P, Münger K, Harlow E. Homologous sequences in adenovirus E1A and human papillomavirus E7 proteins mediate interaction with the same set of cellular proteins. J Virol 1992;66:6893–6902.
- Arroyo M, Bagchi S, Raychaudhuri P. Association of the human papillomavirus type 16 E7 protein with the S-phase-specific E2F-cyclin A complex. Mol Cell Biol 1993;13:6537–6546.
- Funk JO, Waga S, Harry JB, Espling E, Stillman B, Galloway DA. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev 1997;11:2090–2100.
- Smotkin D, Wettstein FO. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci USA 1986;83:4680–4684.
- Chang JL, Tsao YP, Liu DW, Huang SJ, Lee WH, Chen SL. The expression of HPV-16 E5 protein in squamous neoplastic changes in the uterine cervix. J Biomed Sci. 2001;8:206–213.
- Maufort JP, Shai A, Pitot HC, Lambert PF. A role for HPV16 E5 in cervical carcinogenesis. Cancer Res 2010;70:2924–2931.
- Venuti A, Paolini F, Nasir L, Corteggio A, Roperto S, Campo MS, Borzacchiello G. Papillomavirus E5: the smallest oncoprotein with many functions. Mol Cancer 2011;10:140.
- Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006;110:525–541.
- Romanczuk H, Thierry F, Howley PM. Mutational analysis of cis elements involved in E2 modulation of human papillomavirus type 16 P97 and type 18 P105 promoters. J Virol 1990;64:2849–2859.
- Zheng ZM, Baker CC. Papillomavirus genome structure, expression, and post-transcriptional regulation. Front Biosci 2006;11:2286–2302.
- Snijders PJ, Steenbergen RD, Heideman DA, Meijer CJ. HPV-mediated cervical carcinogenesis: concepts and clinical implications. J Pathol 2006;208:152–164.
- de Villiers EM, Bernard HU, Broker T, Delius H, zur Hausen H. Family Papillomaviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, eds. Virus Taxonomy. Eighth Report of the International Committee on Taxonomy of Viruses. San Diego: Elsevier Academic Press, 2005:239–255.
- Family Papillomaviridae. In: Van Regenmortel MH, Fauquet CM, Bishop DH, Carstens EB, Estes MK, Lemon SM, et al., eds. Virus Taxonomy. Seventh Report of the International Committee for the Taxonomy of Viruses. San Diego: Academic Press, 2000:247–251.
- de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology 2004;324:17–27.
- Calleja-Macias IE, Villa LL, Prado JC, Kalantari M, Allan B, Williamson AL, et al. Worldwide genomic diversity of the high-risk human papillomavirus types 31, 35, 52, and 58, four close relatives of human papillomavirus type 16. J Virol 2005;79:13630–13640.
- Chan PK, Luk AC, Park JS, Smith-McCune KK, Palefsky JM, Konno R, et al. Identification of human papillomavirus type 58 lineages and the distribution worldwide. J Infect Dis 2011;203:1565–1573.
- Chen Z, Schiffman M, Herrero R, Desalle R, Anastos K, Segondy M, et al. Evolution and taxonomic classification of human papillomavirus 16 (HPV16)-related variant genomes: HPV31, HPV33, HPV35, HPV52, HPV58 and HPV67. PLoS One 2011;6:e20183.
- Chen Z, DeSalle R, Schiffman M, Herrero R, Burk RD. Evolutionary dynamics of variant genomes of human papillomavirus types 18, 45, and 97. J Virol 2009;83:1443–1455.
- Prado JC, Calleja-Macias IE, Bernard HU, Kalantari M, Macay SA, Allan B, et al. Worldwide genomic diversity of the human papillomaviruses-53, 56, and 66, a group of high-risk HPVs unrelated to HPV-16 and HPV-18. Virology 2005;340:95–104.
- Raiol T, Wyant PS, de Amorim RM, Cerqueira DM, Milanezi NG, Brígido Mde M, et al. Genetic variability and phylogeny of the high-risk HPV-31, -33, -35, -52, and -58 in central Brazil. J Med Virol 2009;81:685–692.
- Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al.; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003;348:518–527.
- Schiffman M, Herrero R, Desalle R, Hildesheim A, Wacholder S, Rodriguez AC, et al. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology 2005;337:76–84.
- Herrero R. Human papillomavirus (HPV) vaccines: limited cross-protection against additional HPV types. J Infect Dis 2009;199:919–922.
- Wheeler CM, Castellsagué X, Garland SM, Szarewski A, Paavonen J, Naud P, et al.; for the HPV PATRICIA Study Group. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012;13:100–110.
- Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsagué X, et al; for the HPV PATRICIA Study Group. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012;13:100–110.
- Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis 2009;199:926–935.
- Wheeler CM, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Perez G, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16–26 years. J Infect Dis 2009;199:936–944.
- Schiffman M, Clifford G, Buonaguro FM. Classification of weakly carcinogenic human papillomavirus types: addressing the limits of epidemiology at the borderline. Infect Agent Cancer 2009;4:8.
- Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F; WHO International Agency for Research on Cancery. Carcinogenicity of human papillomaviruses. Lancet Oncol 2005;6:204.
- Chow LT, Broker TR. In vitro experimental systems for HPV: epithelial raft cultures for investigations of viral reproduction and pathogenesis and for genetic analyses of viral proteins and regulatory sequences. Clin Dermatol 1997;15:217–227.
- Dollard SC, Wilson JL, Demeter LM, Bonnez W, Reichman RC, Broker TR, Chow LT. Production of human papillomavirus and modulation of the infectious program in epithelial raft cultures. OFF. Genes Dev 1992;6:1131–1142.
- Meyers C, Frattini MG, Hudson JB, Laimins LA. Biosynthesis of human papillomavirus from a continuous cell line upon epithelial differentiation. Science 1992;257:971–973.
- Stanley M. Immune responses to human papillomavirus. Vaccine 2006;24 (Suppl 1):S16–S22.
- Frazer IH. Interaction of human papillomaviruses with the host immune system: a well evolved relationship. Virology 2009;384:410–414.
- Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol 2004;4:211–222.
- Le Bon A, Tough DF. Links between innate and adaptive immunity via type I interferon. Curr Opin Immunol 2002;14:432–436.
- Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol 2005;23:307–336.
- Chang YE, Laimins LA. Microarray analysis identifies interferon-inducible genes and Stat-1 as major transcriptional targets of human papillomavirus type 31. J Virol 2000;74:4174–4182.
- Nees M, Geoghegan JM, Hyman T, Frank S, Miller L, Woodworth CD. Papillomavirus type 16 oncogenes downregulate expression of interferon-responsive genes and upregulate proliferation-associated and NF-kappaB-responsive genes in cervical keratinocytes. J Virol 2001;75:4283–4296.
- Kobayashi A, Greenblatt RM, Anastos K, Minkoff H, Massad LS, Young M, et al. Functional attributes of mucosal immunity in cervical intraepithelial neoplasia and effects of HIV infection. Cancer Res 2004;64:6766–6774.
- Kirnbauer R, Hubbert NL, Wheeler CM, Becker TM, Lowy DR, Schiller JT. A virus-like particle enzyme-linked immunosorbent assay detects serum antibodies in a majority of women infected with human papillomavirus type 16. J Natl Cancer Inst 1994;86:494–499.
- Wikström A, van Doornum GJ, Quint WG, Schiller JT, Dillner J. Identification of human papillomavirus seroconversions. J Gen Virol 1995;76:529–539.
- Carter JJ, Wipf GC, Hagensee ME, McKnight B, Habel LA, Lee SK, et al. Use of human papillomavirus type 6 capsids to detect antibodies in people with genital warts. J Infect Dis 1995;172:11–18.
- Carter JJ, Koutsky LA, Hughes JP, Lee SK, Kuypers J, Kiviat N, Galloway DA. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis 2000;181:1911–1919.
- Heideman DA, Hesselink AT, Berkhof J, van Kemenade F, Melchers WJ, Daalmeijer NF, et al. Clinical validation of the Cobas 4800 HPV test for cervical screening purposes. J Clin Microbiol 2011;49:3983–3985.
- Meijer CJ, Berkhof J, Castle PE, Hesselink AT, Franco EL, Ronco G, et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int J Cancer 2009;124:516–520.
- Meijer CJ, Berkhof H, Heideman DA, Hesselink AT, Snijders PJ. Validation of high-risk HPV tests for primary cervical screening. J Clin Virol 2009;46(Suppl 3):S1–S4.
- Lörincz A. Hybrid capture method for detection of human papillomavirus DNA in clinical specimens: a tool for clinical management of equivocal Pap smears and for population screening. J Obstet Gynaecol Res 1996;22:629–36.
- Peyton CL, Schiffman M, Lorincz AT, Hunt WC, Mielzynska I, Bratti C, et al. Comparison of PCR- and hybrid capture-based human papillomavirus detection systems using multiple cervical specimen collection strategies. Clin Microbiol 1998;36:3248–3254.
- Castle PE, Schiffman M, Burk RD, Wacholder S, Hildesheim A, Herrero R, et al. Restricted cross-reactivity of hybrid capture 2 with nononcogenic human papillomavirus types. Cancer Epidemiol Biomarkers Prev 2002;11:1394–1399.
- Ting Y, Manos M. Detection and typing of genital human papillomaviruses. In: Innis M, Gelfand D, Sninsky J, White T, eds. PCR Protocols: A Guide to Methods and Applications. San Diego: Academic Press, 1990:356–366.
- Gravitt PE, Peyton CL, Alessi TQ, Wheeler CM, Coutlée F, Hildesheim A, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol 2000;38:357–361.
- Estrade C, Menoud PA, Nardelli-Haefliger D, Sahli R. Validation of a low-cost human papillomavirus genotyping assay based on PGMY PCR and reverse blotting hybridization with reusable membranes. J Clin Microbiol 2011;49:3474–3481.
- de Roda Husman AM, Walboomers JM, van den Brule AJ, Meijer CJ, Snijders PJ. The use of general primers GP5 and GP6 elongated at their 3′ ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol 1995;76:1057–1062.
- Jacobs MV, Snijders PJ, van den Brule AJ, Helmerhorst TJ, Meijer CJ, Walboomers JM. A general primer GP5+/GP6(+)-mediated PCR-enzyme immunoassay method for rapid detection of 14 high-risk and 6 low-risk human papillomavirus genotypes in cervical scrapings. J Clin Microbiol 1997;35:791–795.
- Chan PK, Cheung TH, Tam AO, Lo KW, Yim SF, Yu MM, et al. Biases in human papillomavirus genotype prevalence assessment associated with commonly used consensus primers. Int J Cancer 2006;118:243–245.
- Kleter B, van Doorn LJ, ter Schegget J, Schrauwen L, van Krimpen K, Burger M, et al. Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am J Pathol 1998;153:1731–1739.
- Jeney C, Takács T, Sebe A, Schaff Z. Detection and typing of 46 genital human papillomaviruses by the L1F/L1R primer system based multiplex PCR and hybridization. J Virol Methods 2007;140:32–42.
- Bernard HU, Chan SY, Manos MM, Ong CK, Villa LL, Delius H, et al. Identification and assessment of known and novel human papillomaviruses by polymerase chain reaction amplification, restriction fragment length polymorphisms, nucleotide sequence, and phylogenetic algorithms. J Infect Dis 1994;170:1077–1085.
- Chan PK, Li WH, Chan MY, Ma WL, Cheung JL, Cheng AF. High prevalence of human papillomavirus type 58 in Chinese women with cervical cancer and precancerous lesions. J Med Virol 1999;59:232–238.
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189:12–19.
- Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer 2010;10:550–560.
- McLaughlin-Drubin ME, Münger K. Oncogenic activities of human papillomaviruses. Virus Res 2009;143:195–208.
- Liu Y, Chen JJ, Gao Q, Dalal S, Hong Y, Mansur CP, et al. Multiple functions of human papillomavirus type 16 E6 contribute to the immortalization of mammary epithelial cells. J Virol 1999;73:7297–7307.
- Pim D, Banks L. Interaction of viral oncoproteins with cellular target molecules: infection with high-risk vs low-risk human papillomaviruses. APMIS 2010;118:471–493.
- Ghittoni R, Accardi R, Hasan U, Gheit T, Sylla B, Tommasino M. The biological properties of E6 and E7 oncoproteins from human papillomaviruses. Virus Genes 2010;40:1–13.
- Ganguly N, Parihar SP. Human papillomavirus E6 and E7 oncoproteins as risk factors for tumorigenesis. J Biosci 2009;34:113–123.
- Howie HL, Katzenellenbogen RA, Galloway DA. Papillomavirus E6 proteins. Virology 2009;384:324–334.
- Gnanamony M, Peedicayil A, Subhashini J, Ram TS, Rajasekar A, Gravitt P, Abraham P. Detection and quantitation of HPV 16 and 18 in plasma of Indian women with cervical cancer. Gynecol Oncol 2010;116:447–451.
- Flores-Munguia R, Siegel E, Klimecki WT, Giuliano AR. Performance assessment of eight high-throughput PCR assays for viral load quantitation of oncogenic HPV types. J Mol Diagn 2004;6:115–124.
- Chow LT, Broker TR, Steinberg BM. The natural history of human papillomavirus infections of the mucosal epithelia. APMIS 2010;118:422–449.
- Cheng S, Schmidt-Grimminger DC, Murant T, Broker TR, Chow LT. Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev 1995;9:2335–2349.
- Chow LT, Broker TR. Mechanisms and regulation of papillomavirus DNA replication. In: Campo MS, ed. Recent Advances in Papillomavirus Research. Norwich, UK: Caister Academic Press, 2006:53–71.
- Sotlar K, Selinka HC, Menton M, Kandolf R, Bültmann B. Detection of human papillomavirus type 16 E6/E7 oncogene transcripts in dysplastic and nondysplastic cervical scrapes by nested RT-PCR. Gynecol Oncol 1998;69:114–121.
- Sotlar K, Stubner A, Diemer D, Menton S, Menton M, Dietz K, et al. Detection of high-risk human papillomavirus E6 and E7 oncogene transcripts in cervical scrapes by nested RT-polymerase chain reaction. J Med Virol 2004;74:107–116.
- Lie AK, Risberg B, Borge B, Sandstad B, Delabie J, Rimala R, et al. DNA- versus RNA-based methods for human papillomavirus detection in cervical neoplasia. Gynecol Oncol 2005;97:908–915.
- Andersson S, Hansson B, Norman I, Gaberi V, Mints M, Hjerpe A, et al. Expression of E6/E7 mRNA from ‘high risk’ human papillomavirus in relation to CIN grade, viral load and p16INK4a. Int J Oncol 2006;29:705–711.
- Ratnam S, Coutlee F, Fontaine D, Bentley J, Escott N, Ghatage P, et al. Clinical performance of the PreTect HPV-Proofer E6/E7 mRNA assay in comparison with that of the Hybrid Capture 2 test for identification of women at risk of cervical cancer. J Clin Microbiol 2010;48:2779–2785.
- Castle PE, Dockter J, Giachetti C, Garcia FA, McCormick MK, Mitchell AL, et al. A cross-sectional study of a prototype carcinogenic human papillomavirus E6/E7 messenger RNA assay for detection of cervical precancer and cancer. Clin Cancer Res 2007;13:2599–2605.
- Cattani P, Zannoni GF, Ricci C, D’Onghia S, Trivellizzi IN, Di Franco A, et al. Clinical performance of human papillomavirus E6 and E7 mRNA testing for high-grade lesions of the cervix. J Clin Microbiol 2009;47:3895–3901.
- Dockter J, Schroder A, Hill C, Guzenski L, Monsonego J, Giachetti C. Clinical performance of the APTIMA HPV Assay for the detection of high-risk HPV and high-grade cervical lesions. J Clin Virol 2009;45 (Suppl 1):S55–S61.
- Halfon P, Benmoura D, Agostini A, Khiri H, Martineau A, Penaranda G, Blanc B. Relevance of HPV mRNA detection in a population of ASCUS plus women using the NucliSENS EasyQ HPV assay. J Clin Virol 2010;47:177–181.
- Burger EA, Kornør H, Klemp M, Lauvrak V, Kristiansen IS. HPV mRNA tests for the detection of cervical intraepithelial neoplasia: a systematic review. Gynecol Oncol 2011;120:430–438.
- Dreier K, Scheiden R, Lener B, Ehehalt D, Pircher H, Müller-Holzner E, et al. Subcellular localization of the human papillomavirus 16 E7 oncoprotein in CaSki cells and its detection in cervical adenocarcinoma and adenocarcinoma in situ. Virology 2011;409:54–68.
- Ressler S, Scheiden R, Dreier K, Laich A, Müller-Holzner E, Pircher H, et al. High-risk human papillomavirus E7 oncoprotein detection in cervical squamous cell carcinoma. Clin Cancer Res 2007;13:7067–7072.
- Franco EL, Duarte-Franco E, Rohan TE. Evidence-based policy recommendations on cancer screening and prevention. Cancer Detect Prev 2002;26:350–361.
- Sasieni P, Adams J. Effect of screening on cervical cancer mortality in England and Wales: analysis of trends with an age period cohort model. BMJ 1999;318:1244–1245.
- van der Aa MA, Pukkala E, Coebergh JW, Anttila A, Siesling S. Mass screening programmes and trends in cervical cancer in Finland and the Netherlands. Int J Cancer 2008;122:1854–1858.
- Bulkmans NW, Rozendaal L, Voorhorst FJ, Snijders PJ, Meijer CJ. Long-term protective effect of high-risk human papillomavirus testing in population-based cervical screening. Br J Cancer 2005;92:1800–1802.
- Sasieni PD, Cuzick J, Lynch-Farmery E. Estimating the efficacy of screening by auditing smear histories of women with and without cervical cancer. The National Co-ordinating Network for Cervical Screening Working Group. Br J Cancer 1996;73:1001–1005.
- WHO/ICO Information Centre on HPV and Cervical Cancer. HPV and cervical cancer in the 2007 report. Vaccine 2007;25:C1–C230.
- Cuzick J, Clavel C, Petry KU, Meijer CJ, Hoyer H, Ratnam S, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer 2006;119:1095–1101.
- Poljak M, Kocjan BJ. Commercially available assays for multiplex detection of alpha human papillomaviruses. Expert Rev Anti Infect Ther 2010;8:1139–1162.
- Naucler P, Ryd W, Törnberg S, Strand A, Wadell G, Elfgren K, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007;357:1589–1597.
- Bulkmans NW, Berkhof J, Rozendaal L, van Kemenade FJ, Boeke AJ, Bulk S, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007;370:1764–1772.
- Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, et al.; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol 2010;11:249–257.
- Cuzick J, Arbyn M, Sankaranarayanan R, Tsu V, Ronco G, Mayrand MH, et al. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine 2008;26 (Suppl 10):K29–K41.
- Koliopoulos G, Arbyn M, Martin-Hirsch P, Kyrgiou M, Prendiville W, Paraskevaidis E. Diagnostic accuracy of human papillomavirus testing in primary cervical screening: a systematic review and meta-analysis of non-randomized studies. Gynecol Oncol 2007;104:232–246.
- Cuschieri KS, Cubie HA. The role of human papillomavirus testing in cervical screening. J Clin Virol 2005;32 (Suppl 1):S34–S42.
- Wright TC Jr. HPV DNA testing for cervical cancer screening. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet 2006;95 (Suppl 1):S239–S246.
- Dehn D, Torkko KC, Shroyer KR. Human papillomavirus testing and molecular markers of cervical dysplasia and carcinoma. Cancer 2007;111:1–14.
- Bigras G, de Marval F. The probability for a Pap test to be abnormal is directly proportional to HPV viral load: results from a Swiss study comparing HPV testing and liquid-based cytology to detect cervical cancer precursors in 13,842 women. Br J Cancer 2005;93:575–581.
- Ronco G, Segnan N, Giorgi-Rossi P, Zappa M, Casadei GP, Carozzi F, et al.; New Technologies for Cervical Cancer Working Group. Human papillomavirus testing and liquid-based cytology: results at recruitment from the new technologies for cervical cancer randomized controlled trial. J Natl Cancer Inst 2006;98:765–774.
- Belinson J, Qiao YL, Pretorius R, Zhang WH, Elson P, Li L, et al. Shanxi Province Cervical Cancer Screening Study: a cross-sectional comparative trial of multiple techniques to detect cervical neoplasia. Gynecol Oncol 2001;83:439–444.
- Herrero R, Hildesheim A, Bratti C, Sherman ME, Hutchinson M, Morales J, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst 2000;92:464–474.
- Cuzick J, Szarewski A, Cubie H, Hulman G, Kitchener H, Luesley D, et al. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet 2003;362:1871–1876.
- Petry KU, Menton S, Menton M, van Loenen-Frosch F, de Carvalho Gomes H, Holz B, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer 2003;88:1570–1577.
- Wright TC Jr, Denny L, Kuhn L, Pollack A, Lorincz A. HPV DNA testing of self-collected vaginal samples compared with cytologic screening to detect cervical cancer. JAMA 2000;283:81–86.
- Chan PK, Chang AR, Yu MY, Li WH, Chan MY, Yeung AC, Cheung TH, et al. Age distribution of human papillomavirus infection and cervical neoplasia reflects caveats of cervical screening policies. Int J Cancer 2010;126:297–301.
- Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, Gordillo-Tobar A, Levin C, Mahé C, Wright TC; Alliance for Cervical Cancer Prevention Cost Working Group. Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med 2005;353:2158–2168.
- Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, et al. ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147–172.
- de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al.; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010;11:1048–1056.
- Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer 2011;128:927–935.
- Insinga RP, Liaw KL, Johnson LG, Madeleine MM. A systematic review of the prevalence and attribution of human papillomavirus types among cervical, vaginal, and vulvar precancers and cancers in the United States. Cancer Epidemiol Biomarkers Prev 2008;17:1611–1622.
- Clifford GM, Smith JS, Plummer M, Muñoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer 2003;88:63–73.
- Chan PK, Cheung TH, Li WH, Yu MY, Chan MY, Yim SF, et al. Attribution of human papillomavirus types to cervical intraepithelial neoplasia and invasive cancers in Southern China. Int J Cancer 2012;131:692–705.
- Yip YC, Ngai KL, Vong HT, Tzang LC, Ji S, Yang M, Chan PK. Prevalence and genotype distribution of cervical human papillomavirus infection in Macao. J Med Virol 2010;82:1724–1729.
- Hlaing T, Yip YC, Ngai KL, Vong HT, Wong SI, Ho WC, et al. Distribution of human papillomavirus genotypes among cervical intraepithelial neoplasia and invasive cancers in Macao. J Med Virol 2010;82:1600–1605.
- Chan PK, Ho WC, Yu MY, Pong WM, Chan AC, Chan AK, Cheung et al. Distribution of human papillomavirus types in cervical cancers in Hong Kong: current situation and changes over the last decades. Int J Cancer 2009;125:1671–1677.
- Chan PK, Lam CW, Cheung TH, Li WW, Lo KW, Chan MY, et al. Association of human papillomavirus type 58 variant with the risk of cervical cancer. J Natl Cancer Inst 2002;94:1249–1253.
- Huang S, Afonina I, Miller BA, Beckmann AM. Human papillomavirus types 52 and 58 are prevalent in cervical cancers from Chinese women. Int J Cancer 1997;70:408–411.
- Wu EQ, Zhang GN, Yu XH, Ren Y, Fan Y, Wu YG, et al. Evaluation of high-risk human papillomaviruses type distribution in cervical cancer in Sichuan province of China. BMC Cancer 2008;8:202.
- Ding DC, Hsu HC, Huang RL, Lai HC, Lin CY, Yu MH, Chu TY. Type-specific distribution of HPV along the full spectrum of cervical carcinogenesis in Taiwan: an indication of viral oncogenic potential. Eur J Obstet Gynecol Reprod Biol 2008;140:245–251.
- Asato T, Maehama T, Nagai Y, Kanazawa K, Uezato H, Kariya K. A large case-control study of cervical cancer risk associated with human papillomavirus infection in Japan, by nucleotide sequencing-based genotyping. J Infect Dis 2004;189:1829–1832.
- Castle PE, Rodríguez AC, Burk RD, Herrero R, Wacholder S, Alfaro M, et al.; Proyecto Epidemiológico Guanacaste (PEG) Group. Short term persistence of human papillomavirus and risk of cervical precancer and cancer: population based cohort study. BMJ 2009;339:b2569.
- Wentzensen N, von Knebel Doeberitz M. Biomarkers in cervical cancer screening. Dis Markers 2007;23:315–330.
- Cuschieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev 2008;17:2536–2545.
- Molden T, Kraus I, Karlsen F, Skomedal H, Nygård JF, Hagmar B. Comparison of human papillomavirus messenger RNA and DNA detection: a cross-sectional study of 4,136 women >30 years of age with a 2-year follow-up of high-grade squamous intraepithelial lesion. Cancer Epidemiol Biomarkers Prev 2005;14:367–372.
- Gupta N, Srinivasan R, Rajwanshi A. Functional biomarkers in cervical precancer: an overview. Diagn Cytopathol 2010;38:618–623.
- Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, Gillio-Tos A, De Marco L, et al.; New Technologies for Cervival Cancer Screening (NTCC) Working Group. Use of p16-INK4A overexpression to increase the specificity of human papillomavirus testing: a nested substudy of the NTCC randomised controlled trial. Lancet Oncol 2008;9:937–945.
- Klaes R, Friedrich T, Spitkovsky D, Ridder R, Rudy W, Petry U, et al. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer 2001;92:276–284.
- Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T. Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol 1998;153:1741–1748.
- Keating JT, Cviko A, Riethdorf S, Riethdorf L, Quade BJ, Sun D, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol 2001;25:884–891.
- Nakao Y, Yang X, Yokoyama M, Ferenczy A, Tang SC, Pater MM, Pater A. Induction of p16 during immortalization by HPV 16 and 18 and not during malignant transformation. Br J Cancer 1997;75:1410–1416.
- Petry KU, Schmidt D, Scherbring S, Luyten A, Reinecke-Lüthge A, Bergeron C, et al. Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 dual-stained cytology. Gynecol Oncol 2011;121:505–509.
- Hudelist G, Manavi M, Pischinger KI, Watkins-Riedel T, Singer CF, Kubista E, Czerwenka KF. Physical state and expression of HPV DNA in benign and dysplastic cervical tissue: different levels of viral integration are correlated with lesion grade. Gynecol Oncol 2004;92:873–880.
- Wentzensen N, Vinokurova S, von Knebel Doeberitz M. Systematic review of genomic integration sites of human papillomavirus genomes in epithelial dysplasia and invasive cancer of the female lower genital tract. Cancer Res 2004;64:3878–3884.
- Kulmala SM, Syrjänen SM, Gyllensten UB, Shabalova IP, Petrovichev N, Tosi P, et al. Early integration of high copy HPV16 detectable in women with normal and low grade cervical cytology and histology. J Clin Pathol 2006;59:513–517.
- Gallo G, Bibbo M, Bagella L, Zamparelli A, Sanseverino F, Giovagnoli MR, et al. Study of viral integration of HPV-16 in young patients with LSIL. J Clin Pathol 2003;56:532–536.
- Cheung JL, Cheung TH, Tang JW, Chan PK. Increase of integration events and infection loads of human papillomavirus type 52 with lesion severity from low-grade cervical lesion to invasive cancer. J Clin Microbiol 2008;46:1356–1362.
- Cheung JL, Cheung TH, Ng CW, Yu MY, Wong MC, Siu SS, et al. Analysis of human papillomavirus type 18 load and integration status from low-grade cervical lesion to invasive cervical cancer. J Clin Microbiol 2009;47:287–293.
- Vinokurova S, Wentzensen N, Kraus I, Klaes R, Driesch C, Melsheimer P, et al. Type-dependent integration frequency of human papillomavirus genomes in cervical lesions. Cancer Res. 2008;68:307–313.
- Arias-Pulido H, Peyton CL, Joste NE, Vargas H, Wheeler CM. Human papillomavirus type 16 integration in cervical carcinoma in situ and in invasive cervical cancer. J Clin Microbiol 2006;44:1755–1762.
- Ho CM, Chien TY, Huang SH, Lee BH, Chang SF. Integrated human papillomavirus types 52 and 58 are infrequently found in cervical cancer, and high viral loads predict risk of cervical cancer. Gynecol Oncol 2006;102:54–60.
- Cheung JL, Lo KW, Cheung TH, Tang JW, Chan PK. Viral load, E2 gene disruption status, and lineage of human papillomavirus type 16 infection in cervical neoplasia. J Infect Dis 2006;194:1706–1712.
- Chan PK, Cheung JL, Cheung TH, Lo KW, Yim SF, Siu SS, Tang JW. Profile of viral load, integration, and E2 gene disruption of HPV58 in normal cervix and cervical neoplasia. J Infect Dis 2007;196:868–875.
- Bhattacharjee B, Sengupta S. CpG methylation of HPV 16 LCR at E2 binding site proximal to P97 is associated with cervical cancer in presence of intact E2. Virology 2006;354:280–285.
- Badal V, Chuang LS, Tan EH, Badal S, Villa LL, Wheeler CM, et al. CpG methylation of human papillomavirus type 16 DNA in cervical cancer cell lines and in clinical specimens: genomic hypomethylation correlates with carcinogenic progression. J Virol 2003;77:6227–6234.
- Ding DC, Chiang MH, Lai HC, Hsiung CA, Hsieh CY, Chu TY. Methylation of the long control region of HPV16 is related to the severity of cervical neoplasia. Eur J Obstet Gynecol Reprod Biol 2009;147:215–220.
- Kalantari M, Calleja-Macias IE, Tewari D, Hagmar B, Lie K, Barrera-Saldana HA, et al. Conserved methylation patterns of human papillomavirus type 16 DNA in asymptomatic infection and cervical neoplasia. J Virol 2004;78:12762–12772.
- Kim K, Garner-Hamrick PA, Fisher C, Lee D, Lambert PF. Methylation patterns of papillomavirus DNA, its influence on E2 function, and implications in viral infection. J Virol 2003;77:12450–12459.
- Mirabello L, Sun C, Ghosh A, Rodriguez AC, Schiffman M, Wentzensen N, et al. Methylation of human papillomavirus type 16 genome and risk of cervical precancer in a costarican population. J Natl Cancer Inst. 2012;104:556–565.
- Sherman ME, Lorincz AT, Scott DR, Wacholder S, Castle PE, Glass AG, et al. Baseline cytology, human papillomavirus testing, and risk for cervical neoplasia: a 10-year cohort analysis. J Natl Cancer Inst 2003;95:46–52.
- Kjaer S, Høgdall E, Frederiksen K, Munk C, van den Brule A, Svare E, et al. The absolute risk of cervical abnormalities in high-risk human papillomavirus-positive, cytologically normal women over a 10-year period. Cancer Res 2006;66:10630–10636.
- Dillner J, Rebolj M, Birembaut P, Petry KU, Szarewski A, Munk C, et al.; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008;337:a1754.
- Wright TC Jr, Schiffman M, Solomon D, Cox JT, Garcia F, Goldie S, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol 2004;103:304–309.
- Cervical cytology screening. ACOG Practice Bulletin no. 109. Obstet Gynecol 2009;114:1409–20.
- Kinney W, Stoler MH, Castle PE. Special commentary: patient safety and the next generation of HPV DNA tests. Am J Clin Pathol 2010;134:193–199.
- Kjær SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010;102:1478–1488.
- Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D; 2006 ASCCP-Sponsored Consensus Conference. 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis 2007;11:201–222.
- Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. ASCUS-LSIL Triage Study (ALTS) Group. Am J Obstet Gynecol 2003;188:1383–1392.
- Arbyn M, Buntinx F, Van Ranst M, Paraskevaidis E, Martin-Hirsch P, Dillner J. Virologic versus cytologic triage of women with equivocal Pap smears: a meta-analysis of the accuracy to detect high-grade intraepithelial neoplasia. J Natl Cancer Inst 2004;96:280–293.
- Castle PE, Sideri M, Jeronimo J, Solomon D, Schiffman M. Risk assessment to guide the prevention of cervical cancer. Am J Obstet Gynecol 2007;197:356.e1–6.
- Arbyn M, Sasieni P, Meijer CJ, Clavel C, Koliopoulos G, Dillner J. Chapter 9: Clinical applications of HPV testing: a summary of meta-analyses. Vaccine 2006;24:S3/78–89.
- Ronco G, Cuzick J, Segnan N, Brezzi S, Carozzi F, Folicaldi S, et al.; NTCC working group. HPV triage for low grade (L-SIL) cytology is appropriate for women over 35 in mass cervical cancer screening using liquid based cytology. Eur J Cancer 2007;43:476–480.
- Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intraepithelial lesions: baseline data from a randomized trial. The Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS) Group. J Natl Cancer Inst 2000;92:397–402.
- Castle PE, Fetterman B, Thomas Cox J, Shaber R, Poitras N, Lorey T, Kinney W. The age-specific relationships of abnormal cytology and human papillomavirus DNA results to the risk of cervical precancer and cancer. Obstet Gynecol 2010;116:76–84.
- Sherman ME, Castle PE, Solomon D. Cervical cytology of atypical squamous cells-cannot exclude high-grade squamous intraepithelial lesion (ASC-H): characteristics and histologic outcomes. Cancer 2006;108:298–305.
- Castle PE, Fetterman B, Poitras N, Lorey T, Shaber R, Kinney W. Relationship of atypical glandular cell cytology, age, and human papillomavirus detection to cervical and endometrial cancer risks. Obstet Gynecol 2010;115:243–248.
- Paraskevaidis E, Arbyn M, Sotiriadis A, Diakomanolis E, Martin-Hirsch P, Koliopoulos G, et al. The role of HPV DNA testing in the follow-up period after treatment for CIN: a systematic review of the literature. Cancer Treat Rev 2004;30:205–211.
- Arbyn M, Paraskevaidis E, Martin-Hirsch P, Prendiville W, Dillner J. Clinical utility of HPV-DNA detection: triage of minor cervical lesions, follow-up of women treated for high-grade CIN: an update of pooled evidence. Gynecol Oncol 2005;99(3 Suppl 1):S7–S11.
- Jones J, Saleem A, Rai N, Shylasree TS, Ashman S, Gregory K, et al. Human papillomavirus genotype testing combined with cytology as a ‘test of cure’ post treatment: The importance of a persistent viral infection. J Clin Virol 2011;52:88–92.
- Kitchener HC, Walker PG, Nelson L, Hadwin R, Patnick J, Anthony GB, et al. HPV testing as an adjunct to cytology in the follow up of women treated for cervical intraepithelial neoplasia. BJOG 2008;115:1001–1007.
- Dorleans F, Giambi C, Dematte L, Cotter S, Stefanoff P, Mereckiene J, et al.; VENICE 2 project gatekeepers group. The current state of introduction of human papillomavirus vaccination into national immunisation schedules in Europe: first results of the VENICE2 2010 survey. Euro Surveill 2010;15:19730.
- European Medicines Agency. Cervarix human papillomavirus vaccine [types 16, 18] (recombinant, adjuvanted, adsorbed). Available at: http://www.emea.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000721/human_med_000694.jsp&mid=WC0b01ac058001d124. Accessed on 9 July 2012.
- European Medicines Agency. Gardasil human papillomavirus vaccine [types 6, 11, 16, 18] (recombinant, adsorbed). Available at: http://www.emea.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000703/human_med_000805.jsp&mid=WC0b01ac058001d124. Accessed on 9 July 2012.
- Brotherton JM, Deeks SL, Campbell-Lloyd S, Misrachi A, Passaris I, Peterson K, et al. Interim estimates of human papillomavirus vaccination coverage in the school-based program in Australia. Commun Dis Intell 2008;32:457–61.
- Franco EL, Cuzick J. Cervical cancer screening following prophylactic human papillomavirus vaccination. Vaccine 2008;26:A16–23.
- Franceschi S, Denny L, Irwin KL, Jeronimo J, Lopalco PL, Monsonego J, et al. Eurogin 2010 roadmap on cervical cancer prevention. Int J Cancer 2011;128:2765–2774.
- Mayrand MH, Franco EL. Integrating novel primary- and secondary-prevention strategies: the next challenge for cervical cancer control. Future Oncol 2010;6:1725–1733.
- Drolet M, Brisson M, Maunsell E, Franco EL, Coutlée F, Ferenczy A, et al. The psychosocial impact of an abnormal cervical smear result. Psychooncology 2011 Jun 21. doi: 10.1002/pon.2003.
- Wardle J, Waller J, Brunswick N, Jarvis MJ. Awareness of risk factors for cancer among British adults. Public Health 2001;115:173–174.
- Waller J, McCaffery K, Forrest S, Szarewski A, Cadman L, Wardle J. Awareness of human papillomavirus among women attending a well woman clinic. Sex Transm Infect 2003;79:320–322.
- Dell DL, Chen H, Ahmad F, Stewart DE. Knowledge about human papillomavirus among adolescents. Obstet Gynecol 2000;96:653–656.
- Anhang R, Goodman A, Goldie SJ. HPV communication: review of existing research and recommendations for patient education. CA Cancer J Clin 2004;54:248–259.