Abstract
Sulfoxaflor, a molecule that targets sap-feeding insects, was assessed for carcinogenic potential in groups of 50 Fischer rats fed with diets containing 0, 25, 100, 500 (males), or 750 (females) ppm sulfoxaflor for 2 years according to OECD 453. Sulfoxaflor did not alter the number of rats with Leydig cell tumors (LCTs: 88% of controls and 90–92% in treated groups). The size of LCT was increased at 100 and 500 ppm. The spontaneous incidence of LCT in Fischer rat is 75–100% compared with less than 0.01% in humans. These fundamental interspecies differences in spontaneous incidence of LCT are the result of quantitative and qualitative differences in Leydig cell response to hormonal stimuli. There are nine known modes of actions (MoA) for LCT induction. Analysis sulfoxaflor data suggested a hormone-based dopamine enhancement MoA causing the LCT effect through: 1) increased neuronal dopamine release via specific dopaminergic neuron-based nicotinic acetylcholine receptor (nAChR) agonism, leading to 2) decreased serum prolactin (Prl) levels, 3) downregulation of luteinizing hormone receptor (LHR) gene expression in Leydig cells, 4) transient decreases in serum testosterone, 5) increased serum LH levels, and 6) promotion of LCTs. The analysis suggested that sulfoxaflor promoted LCTs through a subtle stimulation of dopamine release. The MoA for LCT promotion in the carcinogenicity study is considered to have no relevance to humans due to qualitative and quantitative differences between rat and human Leydig cells. Therefore, the Fischer 344 rat LCT promotion associated with lifetime administration of high-dose levels of sulfoxaflor would not pose a cancer hazard to humans.
Introduction
Sulfoxaflor (XDE-208, X11422208, XR-208, [1-(6-trifluormethylpyridin-3-yl)ethyl)](methyl)-oxido-l4-sulfanylidenecyanamide) is an active substance with insecticidal properties mediated through its agonism at the highly abundant insect nicotinic acetylcholine receptor (nAChR). The mammalian toxicological studies required for the registration of a new plant protection product (PPP) such as this are extensive and include acute oral/dermal toxicity, skin and eye irritation, skin sensitization, genetic toxicity, systemic toxicity, developmental and reproductive toxicity, neurotoxicity, and carcinogenicity. In particular, carcinogenicity testing of the new PPP is considered critical to assess the potential effects of a compound that might have been chronically over lifetime exposure.
In recent years, there has been a widespread drive to have more relevant testing strategies (e.g., International Life Sciences Institute/Health and Environmental Sciences Institute— Agricultural Chemical Safety Assessment Technical Committee [ILSI/HESI-ACSA] and new EU Directives [1107/2009 EC]). However, in contrast to the initiatives aimed at reducing unnecessary toxicity testing, it is also becoming more common for active substances to be tested above the “standard” toxicity testing requirements and incorporated higher-tier investigative studies such as mode-of-action (MoA) work to enable registration of the new PPPs. This increase in higher-tier and customized study testing is driven by a general trend to obtain mechanistic understanding of the underlying hazard characteristics of molecules to inform risk management decisions. These additional MoA data can be useful to support or refute the human relevance of a toxicity finding, which may lead to cessation of further development of a new active substance or to further research to elucidate the MoA. The MoA/HRF was developed by the International Programme on Chemical Safety (IPCS) of the World Health Organization (WHO) (CitationBoobis et al. 2006, Citation2008, CitationSonich-Mullin et al. 2001) and ILSI (CitationMeek et al. 2003, Seed et al. Citation2005), and can be used as a template upon which to elucidate the human relevance of effects observed in animals. As the MoA/HRF approach is becoming an accepted component of PPP human health assessment, this paper, along with the companion papers (CitationTerry et al. 2014, CitationEllis-Hutchings et al. 2014, CitationLeBaron et al. 2014), discusses the application of the MoA/HRF approach to a recently registered active substance, sulfoxaflor.
To assess carcinogenicity potential of sulfoxaflor, groups of 50 F344 Du/Crl rats were fed with diets formulated with 0, 25, 100, 500 (males only), or 750 (females only) ppm sulfoxaflor for 2 years according to OECD test guideline 453. While sulfoxaflor did not alter the number of male rats with Leydig cell tumors (LCTs: 88% of controls and 90–92% in treated groups), it did cause an increased LCT size at 100 and 500 ppm as well as a significant increase in the incidence of bilateral LCT at 500 ppm (88%) when compared to controls (64%). In addition to LCT promotion, there was a marginal increase in preputial gland tumor incidence in the 500-ppm dose group, which was considered secondary to the androgen perturbations associated with the promotion of LCT. Moreover, rat preputial gland findings have doubtful relevance to humans as this gland is not found in humans, and there were no tumorigenic effects in any other specialized sebaceous glands in rats or mice, including clitoral gland in female or preputial gland in male mice. Taken together, this associated observation was considered secondary to the LCT MoA and will not be discussed further in this MoA/HRF.
In order to understand the basis for the sulfoxaflor-induced increase in LCT size, several MoA studies were conducted. The analysis of the relevant toxicity and MoA studies of sulfoxaflor herein provides the context to fully evaluate the proposed MoA for LCT. This analysis is based on the specific mechanistic data generated following exposure to sulfoxaflor and indicates that the LCT promotion seen in the rat chronic/carcinogenicity study was through subtle, but chronic, enhancement of dopamine release, and subsequent inhibition of prolactin (Prl) release from the pituitary gland, ultimately leading to a dopamine agonism/enhancement LCT MoA in a uniquely susceptible animal model, the Fischer 344 rat. This MoA is considered to have no relevance to humans due to qualitative and quantitative differences between human and the Fischer rat Leydig cells. In addition to providing data to support or refute specific LCT MoA, the observation of hormone level alterations in a LCT MoA study clearly supports a risk assessment for a non-genotoxic and threshold (i.e., nonlinear) MoA LCT incidence across species.
The toxicology relating to Leydig cell tumorigenesis in rats and its human relevance have been reviewed extensively (CitationCook et al. 1999, CitationClegg et al. 1997, CitationPrentice and Miekle 1995). LCTs initially appear as hyperplasia of interstitial cells that can grow with age to the diameter of a single normal seminiferous tubule, at which point they are classified as adenomas per guidance from the National Toxicology Program (NTP; CitationBoorman et al. 1987, Citation1990).
The high background incidence of LCTs in Fischer 344 rats has been well known for decades with spontaneous adenomas commonly present at 12 months and their incidenceww increasing to 75–100% by 24 months (CitationBoorman et al. 1990). In contrast, the CD rat has a background incidence of 1–5% at 24 months, while CD-1 mouse incidences are even lower at < 1–2.5% (CitationCook et al. 1999). With regard to human relevance, estimates of human LCTs are orders of magnitude lower with incidences ranging 0.00004–0.01% (CitationCook et al. 1999, CitationMati et al. 2002). summarizes these species and strain differences.
Table 1. Species/strain background incidence of Leydig cell tumors.
Molecular basis of the difference in species/strain incidence of LCTs
Given the strong similarities in the hypothalamic-pituitary-gonadal (HPG) axis among rats (Fischer and CD), mice, and humans, the stark difference in prevalence of LCT, especially between F344 rats and humans, suggested that these interspecies differences are due to quantitative differences in Leydig cell response to stimuli via luteinizing hormone and GnRH receptors (GnRHR) as well as due to qualitative differences such as the presence of prolactin receptors (PrlR) and GnRHR on the rat Leydig cell, but not on that of the humans. In addition, much of the testosterone (T) in human serum is bound to steroid-binding protein, whereas in rodents T circulates as free hormone and is more easily conjugated and metabolized (CitationHammond 2011). This difference makes rodents more susceptible to alterations in T levels than humans.
In both rodents and humans, LH stimulates Leydig cells to produce T; however, rat Leydig cells have 20,000 LH receptors (LHRs) compared with only 1,500 LHR in human Leydig cells (CitationHuhtaniemi 1983). This > 10-fold higher number of LHR in the rat confers a far greater sensitivity to slight changes in LH levels, compared with the relatively unresponsive human Leydig cell. It is due to the large number of “spare” receptors in the rat that LHR occupancy of only 1% is sufficient to elicit a signal transduction cascade response, which confers the greater sensitivity to slight changes in LH levels of rats (CitationKatzung 1995).
In addition, rat, but not human, Leydig cells have GnRHR (CitationClayton and Huhtaniemi 1982) and PrlR on their surface (CitationCook et al. 1999). Therefore, stimulation of rat Leydig cells through these receptors is a rodent-specific mechanism by which LCT induction can also occur. For GnRHR, this position is supported by the fact that GnRH agonists such as buserelin can induce LCTs in rats through the pituitary gland and direct activation at the Leydig cell, but at high doses can suppress T via inhibition of LH release through negative feedback at the level of the pituitary gland (CitationDonabauer et al. 1987, CitationNegro-Vilar and Valenca 1988). For PrlR involvement in LCTs, dopamine agonists, such as muselergine, reduce Prl release by the anterior pituitary gland, which results in a decreased binding to PrlR on Leydig cells (CitationPrentice and Miekle 1995). This decreased PrlR stimulation results in downregulation of LHRs and, therefore, lower T levels, which feeds back to induce LH release from the pituitary leading to Leydig cell stimulation and hyperplasia (CitationPrentice et al. 1992).
Human relevance of rodent LCTs
As summarized here and reviewed extensively elsewhere (CitationCook et al. 1999, CitationMati et al. 2002), LCTs in rats can be induced through alteration at the HPG axis resulting in excessive stimulation of Leydig cells, with Fischer 344 rats having almost 100% prevalence of this tumor type by 24 months of age. Research into differences between rat and human Leydig cells supports this epidemiological data: rat Leydig cells are more responsive to T homeostasis perturbations due to a higher number of LHRs and the presence of Prl and GnRHR on the cell surface.
Taken together, others have previously determined that “…. human Leydig cells are quantitatively less sensitive than rat Leydig cells in their proliferative response to LH, and hence in their sensitivity to chemically induced LCTs. It can be concluded that no observable effect levels for the induction of LCTs in rodent bioassays provide an adequate margin of safety for protection of human health and that the data support a nonlinear mode of action (i.e., threshold response).” Finally these authors conclude that “…. the data suggest that nongenotoxic compounds that induce LCTs in rats most likely have low relevance to humans under most exposure conditions because humans are quantitatively less sensitive than rats.” (CitationCook et al. 1999).
Modes of Action for rodent Leydig cell tumors
It is generally accepted in the literature that there are nine known MoA for LCT induction in rats (CitationCook et al. 1999), which we have placed into three categories of human relevance (i.e., relevant, low relevance, and no relevance). Since the original Cook publication, additional studies have been performed to provide details on these different mechanisms, which will be referenced in the alternative MoA analysis section; however, taken together all known MoAs fall into these nine areas, which are the following:
Relevant to humans
1. Mutagenicity
Low relevance to humans
2. Androgen receptor (AR) antagonism
3. Estrogen receptor agonism/antagonism
4. 5-Alpha-reductase inhibition
5. Aromatase inhibition
6. Reduced T biosynthesis
7. Increased T metabolism
No relevance to humans
8. GnRH (LHRH) agonism
9. Dopamine agonism/enhancement
Detailed explanations of these MoAs are described elsewhere (CitationCook et al. 1999), but apart from MoA #1 (mutagenicity), all operate via a hormonally mediated MoA that eventually results in a common key event of a sustained increase in circulating LH levels, thereby causing trophic stimulation of Leydig cells leading to hypertrophy/hyperplasia and ultimately LCTs. Therefore, it was important to identify early key events in this process to conclusively demonstrate the specific MoA for sulfoxaflor.
The proposed MoA for sulfoxaflor promotion of Fischer rat LCT is MoA #9, which will be discussed in more detail here. An assessment of potential alternative MoAs for sulfoxaflor is presented later in this document.
Key events for MoA #9 (dopamine agonism/enhancement)
Within the dopamine agonism/enhancement MoA, the catecholamine neurotransmitter dopamine (also known as Prl inhibitory factor) is released from the hypothalamus and travels via the hypothalamic–hypophyseal portal system to the anterior pituitary gland where it directly inhibits release of Prl hormone into systemic circulation (CitationCasarett et al. 2007). Higher serum Prl levels causes downregulation of LHRs within the rat Leydig cells (CitationPrentice et al. 1992). Decreased LHR gene expression results in slight decreases in T production, which feeds back to the hypothalamus and pituitary gland to cause a compensatory increase in circulating LH to maintain T at physiologic concentrations (CitationCook et al. 1999). As with all hormone-based, threshold mechanisms of rodent Leydig cell tumorigenesis, the compensatory increase in LH levels leads to increased Leydig cell proliferation and tumors.
As a number of the rodent LCT MoAs have common hallmarks of changes in LH and T levels, it is important to pay attention particularly to the components of each MoA that would help clearly distinguish the early key events. In the case of dopamine agonism/enhancement MoA, the unique key events are an increase in dopamine within the hypothalamic–hypophyseal portal system, decrease in circulating serum Prl levels, and a decrease in LHR gene expression within the testis. Unfortunately, measuring neurotransmitter levels within the portal system between the hypothalamus to the anterior pituitary is an extremely difficult procedure, but this experiment was performed for sulfoxaflor through microdialysis implantation. In addition to direct data, inhibition of Prl secretion is primarily dependent on dopamine signaling; therefore, a decrease in circulating serum Prl levels, which is easily measured, is an appropriate indirect measure of neuronal dopamine enhancement and also a unique identifying feature of the dopamine agonism/enhancement MoA. Therefore, release of dopamine in addition to secondary indicators of causality such as lower serum Prl and LHR gene expression within the testis is critical to distinguish this particular MoA. Other measurements, such as increased LH and decreased T levels, while directly related to a hormonally mediated MoA, are common to many other LCT MoAs. The key events for the assessment of the dopamine agonism/enhancement MoA are described in detail below, listed in , and presented diagrammatically in .
Figure 1. Key events for dopamine agonism/enhancement MoA superimposed upon a diagram of the HPG axis. In short, sulfoxaflor induces (KE#1) an increase in dopamine release via nAChR agonism, (KE#2) leading to decreased serum Prl levels, (KE#3) downregulation of LHR gene expression in Leydig cells within the testis, (KE#4) a transient decrease in T levels, which result in feedback stimulation for (KE#5) increase serum LH levels, which over the course of the 2-year carcinogenicity study result in (KE#6) promotion of Leydig cell tumor growth.
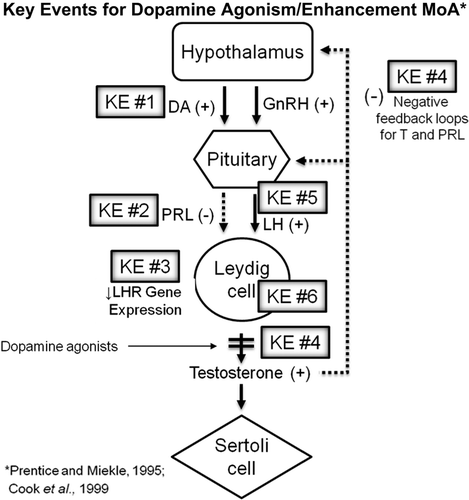
Table 2. Key events for dopamine agonism/enhancement MoA.
Sulfoxaflor rodent Leydig cell tumor postulated MoA
The relevant experimental data for evaluation of the sulfoxaflor-induced rodent LCT MoA and human relevance include guideline short-term/sub-chronic studies in the rat (28 days and 90 days old), the two-generation reproductive toxicity study in rats, oncogenicity studies in the rat and mouse, as well as specific in vivo and in vitro Leydig cell MoA studies. Salient data from these studies will be presented in more detail during the evaluation of the MoA. During the MoA analysis, it is important to note that the apical endpoint findings are an increase in LCT size in Fischer 344 rats given 100 or 500 ppm sulfoxaflor for 2 years. The extremely high background incidence of LCT in control Fischer rats at this age (historical range, 75–100%; 88% for controls in the sulfoxaflor study) is indicative of the unique biology of this strain of rat (CitationCook et al. 1999). Therefore, for hormone-based MoAs, one would expect only subtle changes in young animals during shorter durations of exposure as the apical endpoint of increased LCT size results from a combination of the testis biology in a senescent Fischer rat and promotion of this normal biological process by sulfoxaflor exposure.
The hypothesized key events for the sulfoxaflor-induced rodent LCTs are listed in , and the data that support these key events are described in subsequent sections in this document.
Key Event #1: Increased dopamine release via nAChR agonism
Dopamine is a catecholamine neurotransmitter associated with reward centers of the brain. Primary types of dopaminergic neurons in the adult rat brain exist within the following:
Dorsal (nigrostriatal) pathway originating in the substantia nigra and terminating in the caudate-putamen
Ventral (mesolimbic) pathway originating in the ventral tegmental area and terminating in the nucleus accumbens
Neuroendocrine pathway originating in the arcuate nucleus and terminating in the median eminence (CitationGianoulakis 1998).
This third type of dopaminergic neuron pathway is relevant for the LCT dopamine agonism/enhancement MoA as it is the pathway responsible for dopamine release at the median eminence into the hypothalamic–hypophyseal portal veins to inhibit Prl release in the anterior pituitary (CitationGianoulakis 1998, CitationCasarett et al. 2007).
Central nAChRs, such as α4β2 and α4α6β2 nAChRs, play a key regulatory role in dopamine release from dopaminergic neurons in the brain (CitationMaskos 2010). Microinjection of cholinergic agonists in the substantia nigra pars compacta, a brain region containing dopminergic neurons, dose dependently increased dopamine efflux (CitationBlaha and Winn 1993). Partial agonists to the α4β2 nAChR have been used as smoking cessation drugs (e.g., Tabex or cytisine) by causing release of dopamine in smaller portions to compensate for nicotine withdrawal (CitationCassels et al. 2005).
Sulfoxaflor is a known nAChRs partial agonist in insects, which was hypothesized to increase dopaminergic neurotransmission in the tuberoinfundibular system, resulting in increased dopamine release into the hypothalamic portal circulation and inhibition Prl release by the pituitary. The connection has been established with pharmaceutical dopamine agonists (CitationPrentice et al. 1992). It is plausible that the LCT promotion seen in the rat chronic/carcinogenicity study was through subtle, but prolonged, agonism at the central nAChRs within the median eminence causing release of dopamine and inhibition of Prl release from the pituitary gland.
Therefore, the release of dopamine via central nAChR agonism by sulfoxaflor was tested using microdialysis experiments that measured dopamine outflow into the mediobasal hypothalamus following exposure to sulfoxaflor. In these experiments, Crl/CD(SD) rats were administered sulfoxaflor directly into the mediobasal hypothalamus using a microdialysis probe. Since the concentration of the analytes crossing the semi-permeable membrane of the probe is ∼10-fold lower than the concentration in the perfusion fluid, sulfoxaflor was reverse-dialyzed at concentrations of 400 μM or 2 mM to replicate plasma concentrations of 40 or 200 μM. These concentrations of sulfoxaflor administered through acute microdialysis resulted in a statistically significant 15 or 26% increase in dopamine release, respectively (). Due to the fact that the test system was developed using CD rat, this strain had to be utilized for these studies as opposed to F344 rats. In addition, although administered concentrations to the brain were in the high range of what would be expected in the systemic circulation following 500-ppm exposure to sulfoxaflor (∼50 μM at 500 ppm versus 40 or 200 μM in microdialysis experiments), these acute exposure data support the inherent ability of sulfoxaflor to induce dopamine release, which is the first key event to support this LCT MoA.
Table 3. Mean extracellular dopamine concentration evoked by sulfoxaflor or potassium ions.
Key Event #2: Decreased serum Prl levels
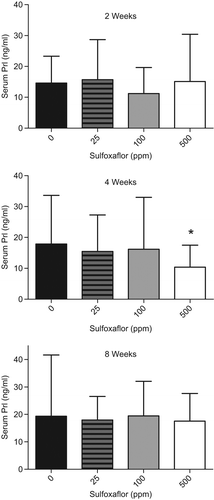
In direct response to dopamine release from the hypothalamus to the anterior pituitary gland (Key Event #1), Prl secretion to the systemic circulation is inhibited. In order to generate data for this key event as well as Key Events #2–5, a 90-day LCT MoA study was conducted in young adult F344 rats as well as Crl:CD(SD) rats (for completeness). As the rat cancer bioassay was conducted in F344 rats, the data from the LCT MoA study presented in this manuscript will be from the F344 rat 90-day exposure. The levels of serum Prl were measured in the LCT MoA study at 2, 4, and 8 weeks of exposure to 0, 25, 100, or 500 ppm sulfoxaflor in Fischer rats. There was no effect of sulfoxaflor treatment on serum Prl levels after 2 weeks of treatment; however, there was a 1.7-fold decrease in serum Prl at 4 weeks in the 500-ppm group with a concomitant 2-fold increase in serum LH levels (see Key Event #5), as shown in . The effect on Prl levels was not observed at the 8-week timepoint, which suggests compensation of the HPG axis by this timepoint. Note that the increase in baseline Prl levels from 2–8 weeks in controls as seen in this study is typical for young male rats (CitationPrentice et al. 1992). It is important to note that the decrease in serum Prl from this 90-day LCT MoA study was not apparent at 100 ppm, which was the mid-dose level, and associated with promotion of LCTs, in the carcinogenicity study. Terminal blood samples were also collected from this LCT MoA study; however, because Prl is a stress related hormone, levels across all groups were induced in response to carbon dioxide-euthanasia-associated stress: being 3- to 5-fold higher than in-life bleeds and the data are not shown here.
Figure 2. A 90-day Fischer rat Leydig cell tumor MoA study was conducted with groups of 15 rats given 0, 25, 100, or 500 ppm of sulfoxaflor. Data to support Key Event #2 were derived from measurements of serum Prl levels at 2-, 4-, or 8-week intervals in this LCT MoA study. There was a subtle, but statistically significant (*p < 0.05) decrease in serum Prl at 4 weeks in rats administered with diet containing 500 ppm of sulfoxaflor.
While subtle and transient, the Prl hormone data provide support for the dopamine agonism/enhancement MoA with the key signature of a decrease in Prl levels. This would only be observed with the dopamine agonism/enhancement MoA and would not be associated with the other possible mechanisms leading to LCTs. Furthermore, the decrease in Prl levels was associated with a compensatory increase in LH levels (), which in turn acted as the primary trophic stimulus over the 2-year Fischer rat carcinogenicity study leading to LCT promotion. The additional concordance of a slight increase in T with increased LH levels at 4 weeks supports that this LH increase is a biologically meaningful effect in Fischer rats (see Key Event #5). Due to the persistent compensatory nature of the HPG axis, coupled with the fact that chronic sulfoxaflor exposure for 2 years was required for increased LCT size (and increased bilateral, incidence) in Fischer rats, it is not surprising that the changes observed in the hormone data from this short-term MoA study are temporal in nature. In general for hormone-based MoAs, one would expect only subtle changes in young animals during shorter durations of exposure as the apical endpoint of increased LCT size results from a combination of the testis biology in a senescent Fischer rat and promotion of this normal biological process by sulfoxaflor exposure. This interpretation is supported by the fact that conclusive sulfoxaflor Leydig cell effects in the guideline toxicity studies occurred only at the 2-year timepoint. In addition, female Fischer rats had no apparent increase or decrease in mammary tumor incidence, further supporting the subtle nature of these Prl changes.
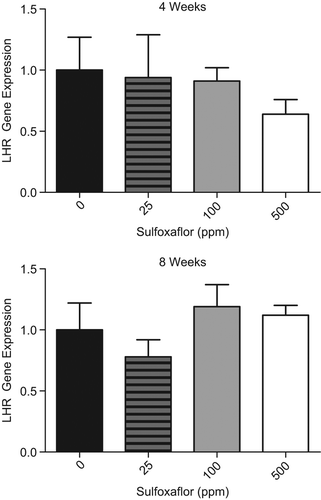
Figure 3. In the 90-day Fischer rat LCT MoA study, whole testis homogenates were prepared from animals at 4- and 8-week interim necropsies for gene expression analysis. Consistent with Key Event #3, there was a decrease in LHR gene expression at 4 weeks of 500-ppm sulfoxaflor exposure. The timing of this observation was concomitant with decreased serum Prl levels (Key Event #2).
Key Event #3: Downregulation of LHR gene expression in Leydig cells
In Key Event #3 of the dopamine agonism/enhancement MoA, lower serum Prl levels (Key Event #2) in rats would lead to downregulation of LHR gene expression (CitationWilliams et al. 2007, CitationPrentice et al. 1992). Lower LHR expression would lead to a transient dip in T production, leading to HPG-axis feedback stimulation and ultimately to increased LH release. Therefore if the dopamine agonism/enhancement MoA were operant, LHR gene expression would be decreased consistent with decreased circulating Prl hormone and increased LH. In order to evaluate this hypothesis, whole testis homogenates from the previously mentioned Fischer rat 90-day LCT MoA study, which was referenced in Key Event #2, were generated for gene expression analysis. Real-time PCR was performed on 4- and 8-week isolated Fischer rat testis mRNA for the LHR and PrlR genes in order to determine whether there was molecular concordance to the hormone data.
Consistent with the dopamine agonism/enhancement MoA and the decreased Prl levels in the 4-week Fischer rat hormone data in Key Event #2, there was a ∼1.6-fold dose-dependent decrease in LHR gene expression at the 4-week, but not 8-week, timepoint (). In addition, there was a decrease in PrlR gene expression at the 4-week, but not 8-week, timepoint (data not shown). While not statistically significant, the magnitude of gene expression changes is consistent with the dynamic range of these genes in vivo and likely represents a biologically meaningful effect based on alterations in hormone levels. This conclusion is supported by a publication where administration of exogenous Prl to rats for 4-weeks resulted in a ∼2-fold increase in LHR gene expression (Williams et al. 2007). Consistent with the data from Key Event #2, the 100-ppm group from this 90-day LCT MoA study did not demonstrate an alteration in LHR gene expression.
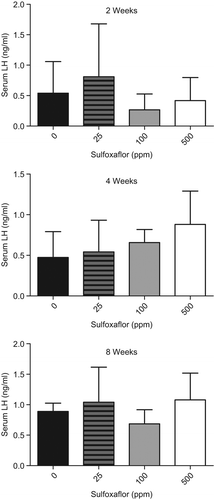
Figure 4. Serum levels of LH were measured at 2, 4, and 8 weeks in the 90-day Fischer rat LCT MoA study described in and under Key Event #2. The serum LH data show an increase in circulating LH levels at the 4-week timepoint in the 500-ppm group. These data support Key Event #4 and are consistent with the dose level and timepoint with Key Events #2 and #3. Similar to the previous data, the 2-week and 8-week timepoint did not demonstrate an increase or decrease in the measured parameter.
Consistent with the decrease in serum Prl observed after 4 weeks of treatment with 500-ppm of sulfoxaflor, there was a biologically significant decrease in LHR gene expression at this dose level and timepoint, based upon the magnitude of response (∼2-fold) that would occur with direct Prl administration to rats (Williams et al. 2007). Also consistent with the Prl hormone data were no differences from control of any other treatment group for LHR gene expression.
Key Event #4: Transient decrease in serum T levels
Downregulation of the LHR in Key Event #3 leads to a transient decrease in serum T levels in Key Event #4 (CitationCook et al. 1999). In LCT MoA experiments with the dopaminergic pharmaceutical agent mesulergine, serum T levels were similar to those of controls at 2 weeks of treatment, slightly lower than those of controls at 4 weeks, returned to baseline by 10 weeks, and were elevated at 13 weeks (CitationPrentice et al. 1992). Within the previously mentioned sulfoxaflor-treated Fischer rat 90-day LCT MoA study (data used for Key Events #2, 3, and 5), there were no measured decreases in serum T levels at the 2-, 4-, or 8-week timepoint shown in . However, in an OECD 416 guideline two-generation reproductive toxicity study in Crl/CD(SD) rats, there was a treatment-related delay in balanopreputial separation (BPS) for male offspring in the high-dose group of 400 ppm sulfoxaflor, but not at the mid-dose of 100 ppm (). Once again, the dose–response relationship data demonstrate consistency for the final apical endpoint at the higher-dose levels, but not at 100 ppm. These BPS data from the two-generation study are important support for Key Event #4 as the process of BPS as a pubertal onset marker in male rats is dependent on androgen levels, as T injection to castrated rats is sufficient to induce BPS (CitationKorenbrot 1977). Therefore, in order for sulfoxaflor to induce a delay in BPS within the two-generation reproductive toxicity study, there had to be a decrease in T levels (for at least some duration) during postnatal development. Further support for this statement is the fact that dopamine agonists such as bromocriptine induce a delay in male rat BPS (CitationMarty et al. 2001).
Table 4. Sulfoxaflor: Fischer rat serum T levels (ng/g).
Table 5. Sulfoxaflor: Crl:CD (SD) rat Balanopreputial separation.
This decrease in T levels leading to a delay in BPS must have been a transient event as there were no effects on accessory sex gland weight, histopathology, or any other anti- androgenic finding in the adult males within the two-generation reproductive toxicity study that had a delay in BPS. While many anti-androgenic molecules can cause a delay in BPS, these direct acting anti-androgens also cause a shortening in anogenital distance (AGD) at birth (CitationWolf et al. 2000). Interestingly, there was no effect on AGD within the two-generation study on sulfoxaflor, which is also consistent with a dopamine agonist/enhancer MoA as maternal Prl levels during gestation are sufficient to abrogate any Prl decrease effect in perinatal male rats (CitationBen-Jonathan and Hnasko 2001).
Key Event #5: Increased serum LH levels
Common to most hormone-based LCT MoAs is an increase in serum LH acting as the causative agent for providing trophic stimulus of Leydig cells towards hyperplasia and eventually adenomas (CitationCook et al. 1999). The dopamine agonism/enhancement MoA is no exception to an eventual increase in LH (Key Event #5) leading to LCTs. With respect to sulfoxaflor, there was a dose-dependent increase in serum LH levels at the 4-week timepoint in Fischer rats (), consistent with timing of decreased Prl level, which was observed in the 90-day LCT MoA study (data used for Key Events #2–5). As with the previous data from this study, there were changes at the 500-ppm group that are consistent with the MoA, but these were not observed at 100 ppm. This consistency with the early key events in young adult Fischer rats is inconsistent with the increase in testis weight observed at 100 ppm in the carcinogenicity study. It is important to note though that the increase in bilateral incidence of LCT in the carcinogenicity study was only observed at 500 ppm, while a subtle increase in overall tumor size was only observed at 100 ppm.
There was no effect of treatment on Fischer rat hormone levels at the 2- or 8-week timepoints; however, at 4 weeks, there was an ∼1.9-fold dose-dependent increase in LH levels concomitant with a ∼1.7-fold dose-dependent decrease in Prl levels.
Due to the persistent compensatory nature of the HPG axis, coupled with the fact that chronic (i.e., 2 years) sulfoxaflor exposure was required for increased LCT size in Fischer rats, it is not surprising that the changes observed in the hormone data are transient in nature. This is supported by the fact that conclusive Leydig cell hyperplastic effects in the guideline toxicity studies occurred only at the 2-year timepoint.
Key Event #6: Promotion of Leydig cell tumors
A rat chronic/carcinogenicity study (OECD 453) has 60 male and 60 female rats per dose level (control plus three treated groups) administered test material for 1 year (chronic portion: 10/sex/group) or 2 years (carcinogenicity portion: 50/sex/group). In the sulfoxaflor rat chronic/carcinogenicity study, Fischer 344 rats per group were given 0, 25, 100, or 500 ppm of sulfoxaflor for 24 months. Toxicokinetic data in rats demonstrate that at these dose levels, sulfoxaflor has nearly complete absorption, is not metabolized, and plasma concentrations of the molecule are proportional to administered doses. In the carcinogenicity study, there was a treatment-related increase in paired testis weight at 100 and 500 ppm that was due to an increased size of LCT in these animals (). Histopathological results confirmed that there was no increase in the overall incidence of LCT across the groups with 88, 92, 90, and 92% of male rats with these tumors at 0, 25, 100, and 500 ppm, respectively. However, there was a significant increased incidence of animals with bilateral LCT at 500 ppm, which supported the fact that the testis weight increases were secondary to the growth of underlying Leydig cell hyperplasia into adenomas.
Table 6. Sulfoxaflor: Two-year Fischer rat testes weights.
At the 1-year chronic timepoint in the rat chronic/carcinogenicity study (10/sex/group), there were 0, 1, 3, and 3 LCT at the 0-, 25-, 100-, and 500-ppm dose groups, which was deemed unrelated to treatment because this was within the historical control range (0–3 LCT at 1 year) and a lack of a dose-response between 100 and 500 ppm at this timepoint. Hence, the hormone-mediated nature of the sulfoxaflor-induced LCT required 2 years of persistent treatment, indicating the subtle treatment-related alteration upon a background of an aging rat undergoing age-related hormonal senescence.
Summary of sulfoxaflor Leydig cell tumor MoA
The proposed MoA for sulfoxaflor-induced Fischer 344 rat LCT promotion is through dopamine enhancement potentially mediated by agonism of the molecule on neuroendocrine dopaminergic nAChRs within the median eminence in the rat. The relevant endpoints for this MoA are summarized on . This analysis is based on the mechanistic and standard, repeat-dose toxicity studies in rats administered with sulfoxaflor.
Table 7. Sulfoxaflor: Temporality and dose response for MoA key events related to male F344 rat Leydig cell tumors.
With respect to dose–response relationship, due to the subtle nature of the effects, no precursor key events were observed at 100 ppm, but only at 500 ppm. A dose–response relationship for these apical endpoint effects existed with increased testis size and increased incidence of bilateral tumors at 500 ppm. Due to the high background incidence of these tumors in Fischer rats, the lack of a response for precursor key events with the MoA analysis at the 100-ppm dose level is not surprising.
Summary of Key Event #1: Increased dopamine release via nAChR agonism
The release of dopamine in response to sulfoxaflor exposure within the hypothalamus was tested directly using microdialysis experiments at concentrations in the brain targeting serum level at, and above, the 500-ppm dose level. It is plausible that the LCT promotion seen in the rat chronic/carcinogenicity study was through subtle, but prolonged, agonism at the central nAChRs within the median eminence causing release of dopamine and inhibition of Prl release from the pituitary gland.
Summary of Key Event #2: Decreased serum Prl levels
In direct response to Key Event #1 of dopamine release from the hypothalamus to the anterior pituitary gland, Prl secretion to the systemic circulation is inhibited. There was no effect of sulfoxaflor treatment on serum Prl levels after 2 weeks of treatment; however, there was a 1.7-fold decrease in serum Prl at 4 weeks in the 500-ppm group with a concomitant 2-fold increase in serum LH levels (see Key Event #5).
Summary of Key Event #3: Downregulation of LHR gene expression in Leydig cells
Consistent with the dopamine agonism/enhancement MoA and the decreased Prl levels in the 4-week Fischer rat hormone data in Key Event #2, there was a dose-dependent decrease in LHR and PrlR gene expression at the 4-week, but not at 8-week, timepoint. While not robust, the magnitude of gene expression changes is consistent with the dynamic range of these genes in vivo and likely represents a biologically meaningful effect based on alterations in hormone levels.
Summary of Key Event #4: Decreased serum T levels
Downregulation of the LHR in Key Event #3 leads to a transient decrease in serum T levels in Key Event #4 (CitationCook et al. 1999). Within the two-generation reproductive toxicity study, there was a treatment-related delay in balanopreputial separation BPS) for male offspring in the high-dose group of 400 ppm sulfoxaflor. The process of BPS as a pubertal onset marker in a male rat is dependent on androgen levels; therefore, in order for sulfoxaflor to induce a delay in BPS, there had to be a decrease in T levels during postnatal development.
Summary of Key Event #5: Increased serum LH levels
Common to most hormone-based LCT MoAs is an increase in serum LH acting as the causative agent for providing trophic stimulus of Leydig cells towards hyperplasia and eventually adenomas. Sulfoxaflor induced an increase in serum LH levels at the 4-week timepoint in Fischer rats, consistent with timing of decreased Prl level observed in the LCT MoA study.
Summary of Key Event #6: Promotion of Leydig cell tumors
In a rat chronic/carcinogenicity study, there was a treatment-related increase in testis weight at 100 and 500 ppm that was determined through histpathological examination to be due to an increased size of LCT in these animals. In addition, there was a statistically identified increased incidence in bilateral LCT incidence at 500 ppm (88% vs. 64% in controls), but not at 100 ppm.
Before these findings were observed at the 2-year timepoint of the rat chronic/carcinogenicity study, the only related effect was limited a slight 2.4-day delay in BPS at 400 ppm in the two-generation study in Crl/CD (SD) rats. There were no other effects on other reproduction-related (i.e., androgen-mediated) endpoints, suggesting a subtle, transient alteration in T levels (data not shown). The endpoints that were within normal limits included the following:
testes, epididymides, accessory glands in Fischer rats, CD rats or CD-1 mice (CD-1 mice dose levels 20X rat LOEL and 80X rat NOEL; data not shown);
sperm parameters (counts, motility, morphology);
reproduction – fertility, mating indices, time to mating;
development, including in the developmental neurotoxicity study;
markers of androgenic/anti-androgenic effects; and
male AGD
Strength, consistency, and specificity of association of effects with key events
The biological processes resulting in rat LCTs have been reviewed extensively (CitationCook et al. 1999, CitationClegg et al. 1997, CitationPrentice and Miekle 1995). LCTs initially appear as hyperplasia of interstitial cells that can grow with age to the diameter of a single normal seminiferous tubule, at which point they are classified as adenomas per guidance from the National Toxicology Program (NTP; CitationBoorman et al. 1987, Citation1990).
Results from the LCT MoA revealed a dose-dependent increase in LH concentrations concomitant with a dose- dependent decrease in Prl levels for Fischer rats at the 4-week timepoint. There was no effect of treatment on Prl, LH, or T at all other timepoints. Consistent with the dopamine agonism/enhancement MoA, and the decreased Prl levels in the 4-week Fischer rat hormone data, was a dose-dependent decrease in LHR gene expression at the 4-week, but not at 8-week, timepoint. While not statistically significant, the magnitude of gene expression changes is consistent with the dynamic range of these genes in vivo and likely represents a biologically significant effect based on alterations in hormone levels.
Consistency is difficult to ascertain when evaluating hormone data due to inherent variability, feedback compensation by the HPG axis, and the very long latency for the apical endpoint effect of Leydig cell hyperplasia and tumors. With respect to dose–response relationship, due to the subtle nature of the effects, no precursor key events were observed at 100 ppm, but only at 500 ppm. A dose–response relationship for these effects existed (i.e., 500 ppm showed a greater effect across all key events than 100 ppm); however, as sulfoxaflor merely increased the magnitude (i.e., size) of LCTs due to the high background levels of these tumors in Fischer rats, the lack of a response at the lower 100- ppm dose level is considered consistent with the very subtle effects seen at the end of the 2-year study.
The specificity of the data for the dopamine agonism/enhancement MoA is the decrease in circulating serum Prl levels and decreased LHR gene expression. These findings would only be observed with the dopamine agonism/enhancement MoA and is not associated with the other eight possible MoAs leading to LCT (see alternative MoA analysis below). Furthermore, the decrease in serum Prl was associated with a compensatory increase in serum LH, which in turn could act as the primary trophic stimulus over a 2-year Fischer rat oncogenicity study leading to LCT promotion. Due to the persistent compensatory nature of the HPG axis, coupled with the fact that chronic (i.e., 2 years) sulfoxaflor exposure was required for increased LCT size in Fischer rats, it is not surprising that the changes observed in the hormone data are temporal in nature. In fact, conclusive Leydig cell hyperplastic effects in the guideline toxicity studies occurred only at the 2-year timepoint.
Biological plausibility and coherence
Dietary administration of sulfoxaflor to Fischer rats results in the early key events (decrease in serum Prl and LHR gene expression) that lead to an increase in serum LH levels. The MoA demonstrated for sulfoxaflor is consistent with well-known MoA for dopamine agonists/enhancers and is consistent with current understanding of hormone-based Leydig cell tumorigenesis. The data for sulfoxaflor are entirely consistent with a non-genotoxic, threshold, MoA.
Assessment of postulated sulfoxaflor Fischer rat LCT MoA
The data for sulfoxaflor support a subtle, but chronic, enhancement of dopamine release, and subsequent inhibition of Prl release from the pituitary gland, ultimately leading to a dopamine agonism/enhancement LCT MoA in a uniquely susceptible animal model, the Fischer 344 rat. The MoA demonstrated for sulfoxaflor is consistent with the literature and with current understanding of rodent LCTs. As mentioned previously, the data for sulfoxaflor are consistent with this non-genotoxic MoA of the Leydig cell. In vitro and in vivo studies show that sulfoxaflor does not have a genotoxic MoA (see below).
The data for sulfoxaflor are judged with a moderate degree of confidence to adequately explain the increase in size of Fischer rat LCTs following chronic dietary administration of sulfoxaflor, and judged with a very high degree of confidence to support a hormonally mediated, threshold-based, nonlinear MoA.
Consideration of alternative MoA
It is generally accepted in the peer-reviewed literature that there are nine known modes of action for LCT induction in rats, which fall into three categories of human relevance (i.e., relevant, low relevance, and no relevance; Cook et al.1999). These are the following:
Relevant to humans
1. Mutagenicity
Low relevance to humans
2. AR antagonism
3. Estrogen receptor agonism/antagonism
4. 5-Alpha-reductase inhibition
5. Aromatase inhibition
6. Reduced T biosynthesis
7. Increased T metabolism
No relevance to humans
8. GnRH (LHRH) agonism
9. Dopamine agonism/enhancement
In the process of conducting and evaluating experiments aimed at testing the proposed MoA for sulfoxaflor promotion of LCTs in Fischer 344 rats, it was possible to rule out a number of alternative MoAs. Each of these alternative MoAs will be considered in turn and direct and/or indirect data generated with sulfoxaflor will be discussed. Wherever possible, sulfoxaflor will be compared to prototypical compounds which are known to cause LCT or LC hyperplasia through these alternative MoAs.
Mutagenicity – not plausible
Mutagenic agents either initiate LCs and then LH would promote the development of the tumor, or act via an unidentified hormonal mechanism (that may or may not be related to their mutagenic or clastogenic activity). An example of a mutagenic compound that causes LCTs is cadmium. Sulfoxaflor was clearly negative in the battery of in vitro and in vivo genotoxicity assays for mutagenicity and clastogenicity (). These included the bacterial reverse mutation (Ames) test, in vitro mammalian chromosome aberration (RLCAT) test, the in vitro mammalian cell gene mutation (CHO/HGPRT) test, and the mammalian erythrocyte micronucleus (MNT) test. In addition, if the Leydig cell effects in Fischer rats were caused by a genotoxicity MoA, it would be expected to have an earlier onset. From the 2-year rat study, when considering LCT incidence in rats from all treatment groups that were moribund or found dead prior to test day 500, there was no evidence of earlier onset of LCT.
Table 8. Sulfoxaflor: Summary of genotoxicity studies.
Based on the weight of evidence, considering both direct data which shows sulfoxaflor is non-genotoxic, and indirect data generated from the toxicology package that indicates no earlier onset of LCTs, a mutagenicity MoA is not a plausible alternative MoA for the Leydig cell effects seen in Fischer rats after 2 years of treatment with sulfoxaflor.
AR antagonism – not plausible
AR antagonists compete with T and DHT for binding to the AR. This competition reduces the androgenic signal to the hypothalamus and adenohypophysis, resulting in an increase in LH secretion with a concomitant elevation of T secretion, resulting in the development of LCTs (Cook et al. 1993). In order to assess the potential for sulfoxaflor interactions with AR, promoter/reporter transactivation assays with the MDA-kb2 cell line was utilized per previously described methods (CitationWilson et al. 2002). Positive controls for agonism (dihydrotestosterone [DHT]) and antagonism (nilutamide, NIL) were used in the assay to demonstrate positive and negative responses for agonist and antagonist potential (). Utilization of sulfoxaflor in this system revealed no effect on agonism or antagonism ().
Figure 5. Assessment of AR agonism and antagonism potential of sulfoxaflor was assessed using the MDA-kb2 cell line. These assays utilized an agonist positive control (DHT), an antagonist positive control (nilutamide), and the test article (sulfoxaflor). The left column presents agonism experiments (DHT, nilutamide, or sulfoxaflor in the presence or absence of nilutamide), while the right column presents antagonism experiments (DHT, nilutamide, or sulfoxaflor in the presence of 1 nM or 1 μM DHT). The agonist data demonstrate a positive response to DHT, which was abrogated in the presence of nilutamide, and a lack of response for nilutamide alone or sulfoxaflor ± nilutamide. The antagonist data demonstrate a lack of antagonism for DHT against itself, the ability of nilutamide to antagonize DHT in a dose–responsive manner at both 1 nM and 1 μM DHT, and the lack of a response for sulfoxaflor with DHT. Taken together, these data support a lack of AR agonism or antagonism potential by sulfoxaflor.
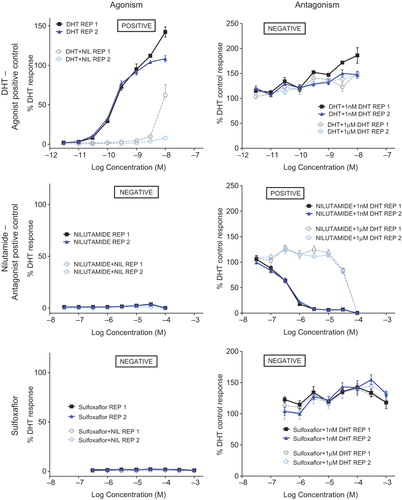
In addition to the in vitro AR agonism/antagonism data, the lack of a treatment-related increase in LCTs at 1 year of treatment with sulfoxaflor is in contrast to the prototypical AR antagonists such as vinclozolin and flutamide. Vinclozolin and flutamide also have a fingerprint of effect that includes reduced AGD, male reproductive malformation (such as hypospadias) and reduced accessory sex gland weights in reproductive toxicity studies (CitationCook et al. 1999). Sulfoxaflor did not cause any consistent androgen-associated effects in the toxicology package that would indicate an AR antagonist MoA. The study most sensitive to these types of endpoints is the guideline OECD 416 two-generation reproductive toxicity study. This study showed no treatment-related effects on AGD, no effects on testis or accessory sex gland (i.e., prostate, seminal vesicle, and epididymis) weight or histopathology, no evidence of malformations (e.g., hypospadias or ectopic testes), and no effects on mating, fertility, time to mating, or gestation length.
Based on the weight of evidence, considering both direct data which shows sulfoxaflor is negative for AR transactivation for agonism and antagonism, and indirect data generated from the toxicology package that indicates no AR antagonist MoA, an AR antagonist MoA is not a plausible alternative MoA for the Leydig cell effects seen in Fischer rats after 2 years of treatment with sulfoxaflor.
Estrogen receptor agonism/antagonism – not plausible
Estrogen receptor agonists/antagonists result in changes in estradiol levels which ultimately cause an increase in LH levels resulting in the development of LCTs. In order to assess the ability of sulfoxaflor to interaction with ER, transactivation assays with the T47D-KBluc cell line were conducted per previously described methods (CitationWilson et al. 2004). Positive control data for ER agonism (estradiol, E2) and antagonism (ICI-182,780) were utilized in the test system (). Utilization of sulfoxaflor in this test system revealed no agonism or antagonism (). Interestingly these types of compounds induce LCTs almost exclusively in the mouse rather than in the rat (CitationCook et al. 1999). Sulfoxaflor does not induce LCTs in the mouse: despite the fact that dose levels in the mouse carcinogenicity study were more than an order of magnitude higher than in the rat carcinogenicity study, and there were no effects on reproductive organs, including the testes, in that study (Thomas et al. 2010). Prototypical estrogen receptor agonist/antagonists, such as diethylstilbestrol, cause effects on vaginal patency, estrus cyclicity, female reproductive tract histopathologic and organ weight effects (CitationCook et al. 1999). There were no effects on female reproductive indices, organ weights, reproductive histopathology, vaginal patency, or estrus cyclicity in any sulfoxaflor rodent study including the two-generation reproductive toxicity study. In addition, there was no effect on mammary tumor incidence in the rat or mouse carcinogenicity studies.
Figure 6. Assessment of ER agonism and antagonism potential of sulfoxaflor was assessed using the T47D-KBluc cell line. These assays utilized an agonist positive control (E2), an antagonist positive control (ICI), and the test article (sulfoxaflor). The left column presents agonism experiments (E2, ICI, or sulfoxaflor in the presence or absence of ICI), while the right column presents antagonism experiments (E2, ICI, or sulfoxaflor in the presence of 0.1 nM or 100 nM E2). The agonist data demonstrate a positive response for E2, which was abrogated in the presence of ICI, and a lack of response for ICI alone or sulfoxaflor ± ICI. The antagonist data demonstrate a lack of antagonism for E2 against itself, the ability of nilutamide to antagonize E2 in a dose–responsive manner at 0.1 nM E2, and the lack of a response for sulfoxaflor with E2. There was a steep drop off for transactivation at only the highest concentration of sulfoxaflor in the antagonism experiment that occurred at both 0.1 and 100 nM E2 in the same magnitude, which was characteristic of an artifact of the test system (e.g., precipitation) as it occurred at only a single highest concentration of sulfoxaflor and was similar between 0.1- and 100-nM antagonist groups. Taken together, these data support a lack of ER agonism or antagonism potential by sulfoxaflor.
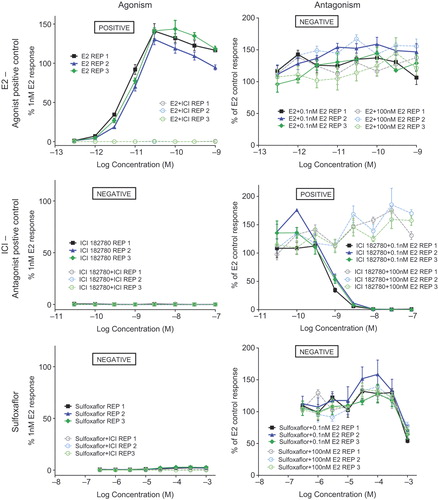
Based on the weight of evidence, considering both direct data which shows sulfoxaflor is negative for ER binding and ER transactivation for agonism and antagonism, and indirect data generated from the toxicology package that indicates no ER antagonist MoA, an ER agonist/antagonist MoA is not a plausible alternative MoA for the Leydig cell effects seen in Fischer rats after 2 years of treatment with sulfoxaflor.
5-Alpha reductase inhibition – not plausible
5-Alpha reductase inhibitors result in decreased conversion of T to DHT. This reduces the net androgenic signal received by the hypothalamus and pituitary, thereby causing a compensatory increase in LH levels, resulting in the development of LCTs (CitationCook et al. 1999). The prostate is differentially sensitive to effects on DHT: for example, DHT has 5-fold greater affinity for AR than T (CitationFeldman and Feldman 2001). Because of this, the prostate would be the most sensitive organ to be affected compared to other accessory sex glands. 5-Alpha inhibitors can reduce prostate 20–30% although T can remain normal (CitationSteers 2001). No effect of sulfoxaflor on prostate weight in any in vivo study has been observed (data not shown).
Interestingly, 5α-reductase inhibitors induce LCTs in mice and LC hyperplasia in rats (CitationCook et al. 1999). Sulfoxaflor does not induce LCTs in the mouse: despite the fact that dose levels in the mouse oncogenicity study were more than an order of magnitude higher than in the rat oncogenicity study, and there were no effects on reproductive organs, including the testes, in that study. In addition, in the previously mentioned 90-day Fischer rat LCT MoA study (see Key Events #2–4), gene expression of whole testis homogenates after 4 and 8 weeks of treatment demonstrated no effect on 5-alpha-reductase levels in the testes of sulfoxaflor-treated rats ().
Figure 7. Quantitative RT-PCR was performed on whole testis homogenates at the 4- and 8-week interim necropsy of the previously described 90-day Fischer rat LCT MoA study for evaluation of gene expression in the steroidogenic pathway. Genes analyzed included StAR (steroidogenic acute regulatory protein), Cyp11a1 (P450side chain cleavage), Cyp17a1 (17alpha-hydroxylase), HSD3b (3-beta hydroxysteroid dehydrogenase), and SDR5a1 (5-alpha reductase). There was no effect of treatment on any of these genes, suggesting that reduced T biosynthesis was not the operant MoA.
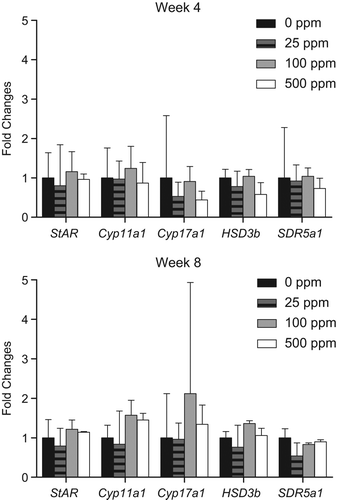
The prototypical 5-alpha-reductase inhibitor is finesteride, which causes reduced AGD, hypospadias, and reductions in accessory sex gland organ weights (CitationCook et al. 1999). There was no indication of reduced AGD or effects on reproductive organ weights in the guideline OECD 416 two-generation reproductive toxicity study.
Based on the weight of evidence, considering both direct data which show that sulfoxaflor has no effect on testes 5-alpha reductase gene expression and indirect data generated from the toxicology package that indicates no prostate effect, a 5-alpha reductase inhibition MoA is not a plausible alternative MoA for the Leydig cell effects seen in Fischer rats after 2 years of treatment with sulfoxaflor.
Aromatase inhibition — not plausible
Inhibition of this enzyme would result in decreased conversion of androstenedione (ASDN) to estrone, and T to estradiol. This would result in an increase in LH levels leading to the development of LCTs (CitationCook et al. 1999). An in vitro aromatase assay was conducted to assess this endpoint based upon the aromatase assay used in the US EPA endocrine disruptor screening program. In brief, radiolabeled [3H]ASDN was incubated with aromatase enzyme and NADPH to produce estrone and 3H2O. Co-incubation with increasing concentrations of 4-OH ASDN served as a positive control to decrease the total aromatase activity (). Using this assay with sulfoxaflor revealed no effect on aromatase inhibition (). Aromatase inhibitors, such as anastrozole, cause effects on mating and fertility indices as well as on female reproductive organ weights and histopathology (CitationCook et al. 1999, CitationTurner et al. 2000). There were no effects on mating, sperm parameters (counts, motility, and morphology), or fertility indices in the guideline OECD 416 two-generation reproductive toxicity study with sulfoxaflor.
Figure 8. Aromatase inhibition potential of sulfoxaflor was evaluated by measuring the conversion of an androgen to an estrogen using recombinant microsomes. Radioactive substrate (3H-ASDN) and NADPH were added to microsomes containing aromatase (CYP19) and reductase complex. Maximal aromatase activity is determined by measuring 3H20 release during this enzymatic converstion of ASDN to estrone and quantitated as a direct measurement of aromatase activity per unit reaction time. In the top graph, 4-OH ASDN was titrated into the reaction as a positive control for aromatase inhibition by competing for this reaction. In the bottom graph, competitive inhibition of aromatase activity with sulfoxaflor revealed no effect, which indicates that sulfoxaflor is not an aromatase inhibitor.
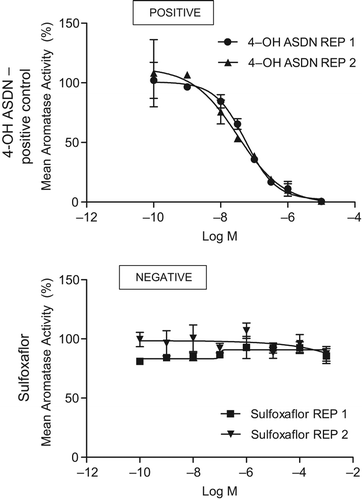
Based on the weight of evidence, considering both direct data which show that sulfoxaflor has no effect on aromatase activity, and indirect data generated from the two-generation reproduction study, an aromatase inhibition MoA is not a plausible alternative MoA for the Leydig cell effects seen in Fischer rats after 2 years of treatment with sulfoxaflor.
Reduced T biosynthesis — not plausible
Inhibition of T biosynthesis would result in lower T and estradiol levels, and increased LH levels, resulting in the development of LCTs (CitationCook et al. 1999). Direct data are provided for sulfoxaflor from the LCT MoA study where Fischer and Crl/CD(SD) rats were treated with 0, 25, 100, or 500 ppm of sulfoxaflor for up to 8 weeks. Gene expression analysis of testes mRNA from this study was conducted on a suite of steroidogenic enzymes to evaluate this potential alternate MoA. There was no dose-dependent effect of treatment on any measured gene in the steroidogenic pathway including steroidogenic acute regulatory protein (StAR), Cyp11a1 (P450 side chain cleavage), Cyp17a1 (17alpha-hydroxylase), 3-beta hydroxysteroid dehydrogenase (HSD3b), or SDR5a1 (5-alpha reductase; data not shown). If reduced T biosynthesis were the operant MoA, one or more of these genes would be affected. Furthermore, the hormone panel data would have shown a sustained decrease in circulating levels of T, which was not observed in the LCT MoA study. Taken together, these data, as well as a lack of female reproductive effects, refute decreased steroidogenesis (MoA #6) as the operant MoA.
Examples of T biosynthesis inhibitors include lansoprazole and calcium channel blockers, which lead to effects on mating and fertility indices as well as on reproductive organ weight and histopathology. While there was an increase in serum cholesterol (the starting material for steroidogenesis) associated with sulfoxaflor administration and a slight delay in preputial separation, there was no effect on female reproductive parameters, which would have been expected with this MoA as androgens are the precursors to estrogens. No effects on mating, fertility, or reproductive organs were observed in the sulfoxaflor guideline OECD 416 two-generation reproductive toxicity study.
Considering both direct data that show that sulfoxaflor has no effect on gene expression involved in the steroidogenic pathway and that there is no transient decrease in circulating levels of T, and indirect data generated from the two-generation reproduction study, a reduced T synthesis MoA is not a plausible alternative MoA. Based on the weight of evidence, the Leydig cell effects seen in Fischer rats after 2 years of treatment with sulfoxaflor are not related to decreased T biosynthesis.
Increased T metabolism – not plausible
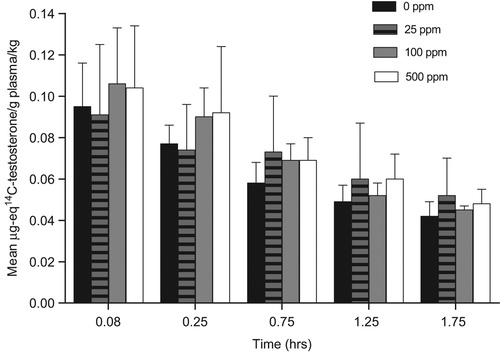
Increased biliary elimination of T would cause lower T levels, and increased LH levels, resulting in the development of LCTs. Based on known nuclear receptor-mediated liver effects with sulfoxaflor administration, this MoA was assessed and direct data are provided for sulfoxaflor from the satellite animals in the rat 90-day LCT MoA study described in Key Events #2–4. In this portion of the study, rats were given 0, 25, 100, or 500 ppm of sulfoxaflor for 2 weeks and then underwent bile cannulation surgery. Rats then received an intravenous administration of 14C-T via the jugular vein. Individual bile samples were collected at pre-determined time intervals of 0–30, 30–60, 60–90, and 90–120 min. Samples were then analyzed from 14C-T-derived radioactivity. Support for MoA #7 would be visualized by a dose-dependent increase in the amount of T-derived radioactivity eliminated in the bile. Although there was a subtle trend at certain timepoints for increased bile radioactivity, there were no statistically significant (alpha = 0.05) or treatment-related differences in the mean 14C-T-derived radioactivity excreted in the bile across all dose groups, per time intervals, for Fischer rats (). Bile flow was similar for the respective dose groups and time intervals (), but most importantly there was no apparent increase in total bile-derived radioactivity () that would be required for this MoA to be induced by sulfoxaflor. These data refute MoA #7 (biliary elimination of T) as the operant MoA.
Figure 9. As part of the 90-day LCT MoA study, satellite Fischer rats underwent bile cannulation at the 2-week timepoint for assessment of the potential for increased T metabolism/biliary elimination induced by sulfoxaflor. Following intravenous injection of 14C-T via the jugular vein, bile samples were collected at 0–0.5, 0.5–1.0, 1.0–1.5, and 1.5–2.0 h and assessed for 14C-T-derived radioactivity. Although there was an apparent dose-related increase in the first 30 minutes, this did not result in a concomitant increase in bile flow () or decrease in plasma 14C-T-derived radioactivity (). Taken together, the data do not support increased T metabolism/biliary elimination as the operant MoA.
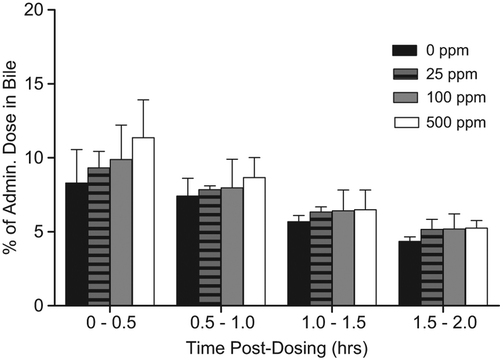
Figure 10. As part of the 90-day LCT MoA study, satellite Fischer rats underwent bile cannulation at the 2-week timepoint for assessment of the potential for increased T metabolism/biliary elimination induced by sulfoxaflor (see ). There was no alteration in bile flow associated with exposure to sulfoxaflor.
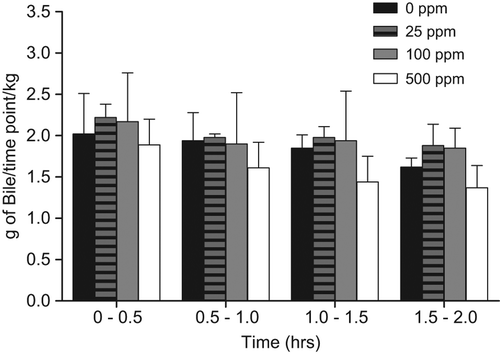
Figure 11. As part of the 90-day LCT MoA study, satellite Fischer rats underwent bile cannulation at the 2-week timepoint for assessment of the potential for increased T metabolism/biliary elimination induced by sulfoxaflor (see ). If this MoA were operant, there should be a decrease in plasma levels of 14C-T-derived radioactivity associated with increased biliary elimination () and bile flow (). There was no alteration of 14C in plasma that was attributable to treatment with sulfoxaflor. Taken together (), the data do not support increased T metabolism/biliary elimination as the operant MoA.
Considering both direct data show that sulfoxaflor has no effect on biliary elimination of T and that there is no transient decrease in T and indirect data generated from the two- generation reproduction study, an increase in biliary excretion of T elimination MoA is not a plausible alternative MoA. Based on the weight of evidence, the Leydig cell effects seen in Fischer rats after 2 years of treatment with sulfoxaflor are not related to increased T metabolism and biliary elimination.
GnRH (LHRH) agonism – not plausible
A prototypical GnRH agonist, such as buerelin, would cause both reduced accessory sex gland weights (due to negative feedback HPG-axis compensation) and histopathologic effects in the pituitary gland, as this is the primary site of functional GnRHR expression. As mentioned previously, there were no effects on accessory sex gland weights in the sulfoxaflor guideline OECD 416 two-generation reproductive toxicity study as well as no treatment-related effects on the pituitary gland in any rat toxicity study including the rat 2-year oncogenicity study.
Based on the weight of evidence, considering indirect data showing no effect on the pituitary gland, a GnRH agonism MoA is not a plausible alternative MoA for the Leydig cell effects seen in Fischer rats after 2 years of treatment with sulfoxaflor.
Conclusion on consideration of alternative MoAs
A summary evaluation for the considered alternative MoAs is presented in . Following consideration of the presented alternative MoAs, it is concluded that there is sufficient evidence to exclude the alternative MoAs for sulfoxaflor promotion of LCTs in Fischer 344 rats.
Table 9. Summary evaluation for other possible MoAs.
Sulfoxaflor rodent Leydig cell tumor HRF
Question 1. Is the weight of evidence sufficient to establish the MoA in animals?
The answer is yes. The MoA for sulfoxaflor-induced Fischer rat LCTs is compatible with that described for dopamine agonists/enhancer-induced tumors (CitationCook et al. 1999). The available data for sulfoxaflor presented in this MoA/HRF provide evidence supporting The dopamine agonism/enhancement MoA in the form of decreased circulating Prl levels, with increased LH, along with decreased testis LHR gene expression. This MoA could operate through sulfoxaflor-mediated enhancement of dopamine release, potentially through agonism of central nAChRs, which play a key regulatory role in dopamine release from dopaminergic neurons in the brain (CitationMaskos 2010). As mentioned previously, sulfoxaflor is an agonist to the fetal rat muscle nAChR and the insect nAChR is the target of the insecticidal mechanism for sulfoxaflor. Based on these data, it is plausible that the LCT promotion seen in the rat chronic/carcinogenicity study was through subtle, but chronic, enhancement of dopamine release, and subsequent inhibition of Prl release from the pituitary gland, ultimately leading to a dopamine agonism/enhancement LCT MoA in a uniquely susceptible animal model, the Fischer 344 rat. In addition, other possible MoAs were examined and evaluated to be unlikely based on analysis of the relevant data for sulfoxaflor.
Question 2. Can human relevance of the MoA be reasonably excluded based on fundamental qualitative differences in key events between experimental animals and humans?
The answer is yes. As previously discussed, this MoA/HRF was designed to evaluate the MoA for the increased size of Fischer rat LCT observed in the sulfoxaflor 2-year rat oncogenicity study at 100 and 500 ppm, and increased incidence of bilateral LCT at 500 ppm. The effect in question is subtle in nature and the background incidence of Fischer rat LCT is 75–100% in 2-year studies compared to orders of magnitude lower ranges of 0.00004–0.01% for humans (CitationCook et al. 1999 and CitationMati et al. 2002). These interspecies differences in background incidence are well understood, and result from quantitative and qualitative differences of Leydig cell response to hormonal stimuli. Rat Leydig cells contain > 10-fold more LHRs than humans, which confers greater sensitivity to slight changes in LH levels (CitationHuhtaniemi 1983, CitationKatzung 1995). In addition to this quantitative difference, rat, but not human, Leydig cells have both PrlR and GnRHR on their surface (CitationClayton and Huhtaniemi 1982, CitationCook et al. 1999). Stimulation of rat Leydig cells through both PrlR and GnRHR are a rat-specific mechanism by which LCT formation can occur. For PrlR involvement in LCT, dopamine agonists (e.g., muselergine) reduce Prl release by the anterior pituitary gland. This results in decreased binding of Prl to PrlR on Leydig cells, leading to downregulation of the LHR and transient reductions in T production, which feeds back to induce LH release from the pituitary leading to Leydig cell stimulation and hyperplasia over time (CitationPrentice and Miekle 1995, CitationPrentice et al. 1992).
A concordance analysis of the key events for a dopamine agonism/ehancement MoA is presented in .
Table 10. Concordance of key events for a dopamine agonism/enhancement LCT MoA in rodents and humans.
Question 3. Can human relevance of the MoA be reasonably excluded based on quantitative differences in either kinetic or dynamic factors between experimental animals and humans?
As human relevance of the experimental animal MoA can be reasonably excluded on the basis of qualitative differences in key events (Question 2); a quantitative assessment of kinetic or dynamic factors is not necessary. However, as described in the background section of this document, there are also significant differences in the background incidence of LCT across species and strains, with Fischer 344 rats being the most and humans being the least sensitive. The biological basis for these differences in susceptibilities is described in detail within the background section of this report and includes both qualitative and quantitative differences in the underlying biology between rat and human Leydig cells.
Conclusions
Statement of confidence in the evaluation
This MoA and HRF evaluation for sulfoxaflor-induced LCTs in Fischer rats follows the guideline established for this process (CitationSonich-Mullin et al. 2001, CitationCohen et al. 2003, CitationMeek et al. 2003, CitationUS EPA 2005, CitationBoobis et al. 2007). The extensive toxicological database for sulfoxaflor, including several focused in vitro and in vivo MoA experiments, provides the necessary data to evaluate the MoA for sulfoxaflor-induced rodent LCTs. Analysis of these data revealed a proposed hormone-based dopamine enhancement MoA through the following key events: 1) increased neuronal dopamine release via nAChR agonism, leading to 2) decreased serum Prl levels, 3) downregulation of luteinizing hormone (LH) receptor gene expression in Leydig cells, 4) transient decreases in serum T, 5) increased serum LH levels, and 6) promotion of Leydig cell tumorigenesis. The subtle nature of the supportive data for this MoA is not surprising given the latency and subtle nature of the effects in question. The two findings that anchor the analysis to the dopamine enhancement MoA are the decreased serum Prl levels and concomitant decrease in LHR gene expression. These findings are unique to the key event progression of this particular MoA.
The conclusion from this evaluation is that the LCT promotion observed in the carcinogenicity study was through a subtle, but chronic, dopamine enhancement MoA in a uniquely susceptible animal model, the Fischer 344 rat. The data for sulfoxaflor are judged with a moderate degree of confidence to adequately explain the promotion of Fischer rat LCTs following chronic dietary administration of sulfoxaflor, and judged with a very high degree of confidence to support a hormonally mediated, threshold-based, nonlinear MoA.
Other possible MoAs for Leydig cell tumorigenesis as described by Cook (1999) have been evaluated with respect to sulfoxaflor. This in-depth analysis of alternative MoAs revealed direct and/or indirect data to refute the eight other known possible MoAs to develop rodent LCTs. Importantly, very strong in vitro and in vivo data exist to refute a genotoxic mechanism. Taken together, all other MoAs have been dismissed for sulfoxaflor-induced LCT because they lack plausibility and coherence with the significant data from the mechanistic and guideline toxicity studies on sulfoxaflor.
Identification of data gaps
Due to the subtle nature and long latency for the effects in question, in combination with feedback compensation by the HPG axis, it is not surprising that the hormone and associated key events are transient during short-term studies. The apical endpoint data also support this point as the 12-month portion of the chronic/carcinogenicity study with sulfoxaflor did not reveal a treatment-related effect on LCT incidence, which would support a subtle promotion of the underlying LCT growth between 1 and 2 years as opposed to an initiation effect. Therefore, these are not considered data gaps as it is more a function of the underlying biology. However, there are two data gaps identified during the analysis of this MoA, which are 1) lack of direct data for Key Event #4, and 2) incomplete demonstration of key events at the 100-ppm dose level.
Key Event #4 within this MoA is a transient decrease in serum T levels. Under the conditions of the LCT MoA study, there were no measurable decreases in serum T; however, as described within the analysis of Key Event #4, the delay in BPS from the two-generation reproductive toxicity study supports a transient decrease in T. While these data are supportive and provide strong indirect evidence on a T effect, there are no hormone measurement data that show a decrease in serum levels of T.
Secondly, while there are data supporting the MoA at 500 ppm, no precursor key events were observed at 100 ppm. A dose–response relationship for these apical endpoint effects existed with increased testis size and increased incidence of bilateral tumors at 500 ppm. Due to the high background incidence of these tumors in Fischer rats, the lack of precursor key events for this subtle, hormone-based MoA at the lower 100-ppm dose level is not surprising, especially given the transient and compensatory nature of hormone regulation in the HPG axis.
Regulatory response
The regulatory assessment of the increased promotion of LCT in sulfoxaflor-treated Fischer 344 rats has been informative as to the level of concern of such an effect in this particular strain of rat. The Citation2011 Joint FAO/WHO Meeting on Pesticide Residues (JMPR) concluded that although the proposed MoA was not completely demonstrated, the increased incidence of bilateral LCT is of low relevance to humans as there are large qualitative and quantitative differences between rats and humans regarding Leydig cell responses to hormonal stimuli. In addition, the effects only occurred at high doses, did not occur in mice and would be anticipated to exhibit a threshold. As a consequence, JMPR considered these changes not relevant to the human dietary risk assessment of sulfoxaflor. Indeed, such is the high background incidence of this tumour type in Fischer 344 male rats that the CitationUS EPA (2012) considered that the animal model was not sensitive enough to detect a treatment-related effect on this endpoint, and the apparent increase in LCT was concluded as a non-treatment related effect. In Europe, the effect was considered treatment related but was not considered relevant to humans: “Based on mechanistic data, the Committee agreed with the dossier submitter not to classify this substance for carcinogenicity [or reproductive toxicity]” (CitationECHA 2013).
Implications for risk assessment
Sulfoxaflor causes promotion of LCTs in a Fischer rat carcinogenicity study. This MoA/HRF document provides support for a dopamine enhancement MoA working through the PrlR on rat Leydig cells, which is considered not relevant to humans due to qualitative differences between the species in the expression of PrlR on rat, but not on human, Leydig cells. In addition to qualitative differences, the effect in question is subtle in nature, and on a background incidence of Fischer rat LCT is 75–100% in 2-year studies compared to ranges of 0.00004–0.01% for humans.
Taken together, the promotion of Fischer rat LCT observed in the oncogenicity study has a MoA that is hormonally mediated and threshold-based, and would be considered to have no relevance to humans due to qualitative and quantitative differences between rat and human Leydig cells. In addition, the Fischer rat LCTs associated with administration of high-dose level of sulfoxaflor would not pose a cancer hazard to humans, and NOAELs based on this finding should not be used to drive reference doses (e.g., chronic reference dose [cRfD] or acceptable daily intake [ADI]). In any case, if they were considered relevant to humans, a margin of exposure risk assessment (e.g., nonlinear, thresholded) would be protective of human health. For example, the potential chronic human exposure to sulfoxaflor via low levels of crop residues in the diet is estimated to be in the range of 0.0049–0.028 mg/kg bw/day (worst-case). This corresponds to 10–56% of the sulfoxaflor cRfD (0.05 mg/kg bw/day). In addition, potential human exposure is 750–4300 times lower than the dose level at which these effects occurred in animals.
Acknowledgements
The authors thank all of the pathology, biotransformation, mammalian toxicology, and cellular and molecular toxicology study personnel for their hard work on generating the data to support this manuscript. In particular, thanks to Carol Zablotny, Julie Passage, H. Lynn Kan, Steven Hansen, Amy Clark, Dr. Alene McCoy, Alix Booms, and Dr. Bhaskar Gollapudi.
Declaration of interest
The authors are employed by The Dow Chemical Company, the developer and producer of sulfoxaflor. The authors have sole responsibility for the writing and content of the paper.
References
- Ben-Jonathan N, Hnasko R. (2001). Dopamine as a prolactin (PRL) inhibitor. Endocr Rev, 22, 724–63.
- Boobis AR, Cohen SM, Dellarco V, McGregor D, Meek ME, Vickers C, et al. (2006). IPCS framework for analyzing the relevance of a cancer mode of action for humans. Crit Rev Toxicol, 36, 781–92.
- Boobis AR, Cohen SM, Dellarco V, McGregor D, Meek ME, Vickers C, et al. (2007). IPCS Framework for analysing the relevance of a cancer mode of action for humans. Harmonization Project Document No. 4, World Health Organization, Geneva. pp. 10–29.
- Boobis AR, Cohen SM, Dellarco V, McGregor D, Meek ME, Vickers C, et al. (2006). IPCS framework for analyzing the relevance of a cancer mode of action for humans. Crit Rev Toxicol, 36, 781–92.
- Boobis AR, Doe JE, Heinrich-Hirsch B, Meek ME, Munn S, Ruchirawat M, et al. (2008). IPCS framework for analyzing the relevance of a noncancer mode of action for humans. Crit Rev Toxicol, 38, 87–96.
- Blaha CD, Winn P. (1993). Modulation of dopamine efflux in the striatum following cholinergic stimulation of the substantia nigra in intact and pedunculopontine tegmented nucleus-lesioned rats. J Neurosci, 13, 1035–44.
- Boorman GA, Chapin RE, Mitsumori K. (1990). Testis and Epididymis. In: Boorman GA, Eustis SL, Elwell MR, Montgomery CA, MacKenzie WF, Eds. Pathology of the Fischer Rat: Reference and Atlas. San Diego: Academic Press, pp. 405–18.
- Boorman GA, Hamlin MH, Eustis SL. (1987). Focal interstitial cell hyperplasia testes, rat. In: Jones TC, Mohr U, Hunt RD, Eds. Genital System, Monographs on Pathology of Laboratory Animals Sponsored by the International Life Sciences Institute. Berlin, Heidelberg: Springer Verlag, pp. 201–204.
- Casarett LJ, Doull J, Klaassen CD. (2007). Casarett & Doull's Toxicology: The Basic Science of Poisons. New York: McGraw-Hill Professional.
- Cassels BK, Bermúdez I, Dajas F, Abin-Carriquiry JA, Wonnacott S. (2005). From ligand design to therapeutic efficacy: the challenge for nicotinic receptor research. Drug Discov Today, 10, 1657–65.
- Clayton RN, Huhtaniemi IT. (1982). Absence of gonadotropin-releasing hormone receptors in human gonadal tissue. Nature, 299, 56–9.
- Clegg ED, Cook JC, Chapin RE, Foster PMD, Daston GP. (1997). Leydig cell hyperplasia and adenoma formation: mechanisms and relevance to humans. Reprod Toxicol, 11, 101–121.
- Cohen SM, Meek ME, Klaunig JE, Patton DE, Fenner-Crisp PA. (2003). The human relevance of information on carcinogenic modes of action: Overview. Crit Rev Toxicol, 33, 581–9.
- Cook JC, Klinefelter GR, Hardisty JF, Sharpe RM, Foster PM. (1999). Rodent Leydig cell tumorigenesis: a review of the physiology, pathology, mechanisms, and relevance to humans. Crit Rev Toxicol, 29, 169–261.
- Coulson M, Gibson GG, Plant N, Hammond T, Graham M. (2003). Lansoprazole increases testosterone metabolism and clearance in male Sprague-Dawley rats: implications for Leydig cell carcinogenesis. Toxicol Appl Pharmacol, 192, 154–63.
- Donaubauer HH, Kramer M, Kreig K, Mayer D, Von Rechenberg W, Sandow J, Schutz E. (1987). Investigations of the carcinogenicity of the LH-RH analogue buserelin (HOE 766) in rats using the subcutaneous route of administration. Fund Appl Toxicol, 9, 738–52.
- ECHA. (2013). Risk Assessment Committee Conclusion (http://www.echa.europa.eu/view-article/-/journal_content/title/rac-concludes-on-nine-scientific-opinions-for-clh). Accessed on March 23, 2014.
- Ellis-Hutchings RG, Rasoulpour RJ, Terry C, Carney EW, Billington R. (2014). Human relevance framework evaluation of a novel rat developmental toxicity mode of action induced by sulfoxaflor. Crit Rev Toxicol, 44, 45–62.
- Feldman BJ, Feldman D. (2001). The development of androgen-independent prostate cancer. Nat Rev Cancer, 1, 34–45.
- Gianoulakis C. (1998). Alcohol-seeking behavior: the roles of the hypothalamic-pituitary-adrenal axis and the endogenous opioid system. Alcohol Health Res World, 22, 202–10.
- Hammond GL. (2011). Diverse roles for sex hormone-binding globulin in reproduction. Biol Reprod, 85, 431–41.
- Huhtaniemi IT. (1983). Gonadotrophin receptors: correlates with normal and pathological functions of the human ovary and testis.In: Clinical Endocrinology and Metabolism: Receptors in Health and Disease. Philadelphia : Clayton WB Saunders. pp. 117–132.
- JMPR (Joint FAO/WHO Meeting on Pesticide Residues). (2011). Sulfoxaflor Monograph. Geneva, 20–29 September 2011.
- Katzung BG. (1995). Introduction. In: Katzung BG, Eds. Basic and Clinical Pharmacology. Appleton and Lang: Connecticut. pp. 117–132.
- Kerr JB, Sharpe RM. (1986). Effects and interactions of LH and LHRH agonist on testicular morphology and function in hypophysectomized rats. J Reprod Fertil, 76, 175–92.
- Korenbrot CC, Huhtaniemi IT, Weiner RI. (1977). Preputial separation as an external sign of pubertal development in the male rat. Biol Reprod, 17, 298–303.
- Kondarewicz A, Kolasa A, Zawiślak B, Baranowska-Bosiacka I, Marchlewicz M, et al. (2011). Testis morphology in rats chronically treated with letrozole, an aromatase inhibitor. Folia Histochem Cytobiol, 49, 677–84.
- LeBaron MJ, Bhaskar Gollapudi B, Terry C, Billington R, Rasoulpour RJ. (2014). Human relevance framework for rodent liver tumors induced by the insecticide sulfoxaflor. Crit Rev Toxicol, 44, 15–24.
- Leslie GB, Noakes DN, Pollitt FD, Roe FJ, Walker TF. (1981). A two-year study with cimetidine in the rat: assessment for chronic toxicity and carcinogenicity. Toxicol Appl Pharm, 61, 119–37.
- Marty MS, Crissman JW, Carney EW. (2001). Evaluation of the male pubertal assay's ability to detect thyroid inhibitors and dopaminergic agents. Toxicol Sci, 60, 63–76.
- Maskos U. (2010). Role of endogenous acetylcholine in the control of the dopaminergic system via nicotinic receptors. J Neurochem, 114, 641–646. doi:10.1111/J.1471-4159.2010.06798.x (epub).
- Mati W, Lam G, Dahl C, Anderson T, Balslev E (2002). Leydig cell tumour – a rare testicular tumour. Int Urol Nephrol, 33, 103–6.
- Meek ME, Bucher JR, Cohen SM, Dellarco V, Hill RN, Lehman-McKeeman LD, et al. (2003). A framework for human relevance analysis of information on carcinogenic modes of action. Crit Rev Toxicol, 33, 591–653.
- Negro-Vilar A, Valenca MM. (1988). Male neuroendocrinology and endocrine evaluation. In: Lamb JC IV, Foster PMD, Eds. Physiology and Toxicology of Male Reproduction. London: Academic Press Inc, pp. 124–7.
- Newbold RR, Bullock BC, McLachlan JA. (1987). Testicular tumors in mice exposed in utero to diethylstilbestrol. J Urol, 138, 1446–50.
- Prahalada S, Majka JA, Soper KA, Nett TM, Bagdon WJ, Peter CP, et al. (1994). Leydig cell hyperplasia and adenomas in mice treated with finasteride, a 5-alpha reductase inhibitor: a possible mechanism. Fund Appl Toxicol, 22, 211–19.
- Prentice DE, Miekle AW. (1995). A review of drug-induced leydig cell hyperplasia and neoplasia in the rat and some comparisons with man. Hum Exp Toxicol, 14, 562–72.
- Prentice DE, Siegel RA, Donatsch P, Qureshi S, Ettlin RA. (1992). Mesulergine induced Leydig cell tumours, a syndrome involving the pituitary-testicular axis of the rat. Arch. Toxicol, 15, 197–204.
- Rouquie D, Friry-Santini C, Schorsch F, Tinwell H, Bars R. (2009). Standard and molecular NOAELs for rat testicular toxicity induced by flutamide. Toxicol Sci, 109, 59–65.
- Seed J, Carney EW, Corley RA, Crofton KM, DeSesso JM, Foster PM, et al. 2005. Overview: Using mode of action and life stage information to evaluate the human relevance of animal toxicity data. Crit Rev Toxicol, 35, 664–72.
- Sonich-Mullin C, Fielder R, Wiltse J, Baetcke K, Dempsey J, Fenner-Crisp P, et al. (2001). IPCS Conceptual framework for evaluating a mode of action for chemical carcinogenesis. Regul Toxicol Pharmacol, 34, 146–52.
- Steers WD. (2001). 5alpha-reductase activity in the prostate. Urololgy, 58, 17–24.
- Terry C, Rasoulpour RJ, Saghir S, Marty S, Bhaskar Gollapudi B, Billington R. (2014). Application of a novel integrated toxicity testing strategy incorporating “3R” principles of animal research to evaluate the safety of a new agrochemical, sulfoxaflor. Crit Rev Toxicol, 44, 1–14.
- Turner KJ, Morley M, Atanassova N, Swanston ID, Sharpe RM. (2000). Effect of chronic administration of an aromatase inhibitor to adult male rats on pituitary and testicular function and fertility. J Endocrinol, 164, 225–38.
- US EPA. (2005). Guidelines for Carcinogen Risk Assessment. EPA/630/P-03/001B.
- US EPA. (2012). Human Health Risk Review and Carcinogenicity Assessment Review Committee (CARC) Memo (published for public commenting January 2013, DR D2-382604).
- Waalkes MP, Anver M, Diwan BA. (1999). Carcinogenic effects of cadmium in the noble (NBL/Cr) rat: induction of pituitary, testicular, and injection site tumors and intraepithelial proliferative lesions of the dorsolateral prostate. Toxicol Sci, 52, 154–61.
- Williams VL, DeGuzman A, Dang H, Kawaminami M, Ho TW, Carter DG, Walker AM. (2007). Common and specific effects of the two major forms of prolactin in the rat testis. Am J Physiol Endocrinol Metab, 293, E1795–E803.
- Wilson VS, Bobseine K, Lambright CR, Gray LE Jr. (2002). A novel cell line, MDA-kb2, that stably expresses an androgen and glucocorticoid-responsive reporter for the detection of hormone receptor agonists and antagonists. Toxicol Sci, 66, 69–81.
- Wilson VS, Bobseine K, Gray LE Jr. (2004). Development and Characterization of a cell line that stably expresses an estrogen-responsive luciferase reporter for the detection of estrogen receptor agonist and antagonists. Toxicol Sci, 81, 69–77.
- Wolf CJ, LeBlanc GA, Ostby JS, Gray LE Jr. (2000). Characterization of the period of sensitivity of fetal male sexual development to vinclozolin. Toxicol Sci, 55, 152–61.

