Abstract
The main purpose of this work was to develop and evaluate a self-emulsifying drug delivery system (SEDDS) of piperine to enhance its solubility and bioavailability. The formulation was optimized by solubility test and ternary phase diagrams. Then physiochemical properties and in vitro release of SEDDS were characterized. In vivo pharmacokinetics study and in situ single-pass intestinal perfusion were performed to investigate the effects of SEDDS on the bioavailability and intestinal absorption of piperine. The optimized formulation was composed of ethyl oleate, Tween 80 and Transcutol P (3:5.5:1.5, w/w), with the level of the piperine reached 2.5% (w/w). The in vitro dissolution rates of piperine SEDDS were significantly higher than the self-prepared capsules. In vivo pharmacokinetic study showed Cmax1, Cmax2 and area under the curve of piperine after oral administration of SEDDS in rats were 3.8-, 7.2- and 5.2-fold higher than the self-prepared capsules, respectively, and the relative bioavailability of SEDDS was 625.74%. The in situ intestinal absorption study revealed that the effective permeability and the effective absorption rate values of piperine for SEDDS were significantly improved comparing to solutions (p < 0.01). So SEDDS formulation could improve the oral bioavailability and intestinal absorption of piperine effectively.
Introduction
Piperine is a major component alkaloid/amide present in the fruit of black pepper (Piper nigrum) and long pepper (Piper longum). The piperine is used not only for the dietaries as the spices, but also as a useful drug with great potential for medicine. The piperine is one of the most well-known components because of its high medicinal properties (Bhardwaj et al., Citation2002; Gao & Morozowich, Citation2006). In Indian medicine, the piperine and its derivatives were always used for treating epilepsy, headache and so on (Pattanaik et al., Citation2006). As increasingly popular modern-day uses of piperine, it has been reported to inhibit oxidative stress, anti-fungal, anti-inflammatory activity and anti-tumor (Sunila & Kuttan, Citation2004; Bezerra et al., Citation2008; Ee et al., Citation2010). A new study showed that the piperine could enhance the bioavailability of a number of therapeutic drugs recently (Srinivasan, Citation2007). Previous studies have indicated that piperine can increase the absorption of curcumin not only by inhibiting of hepatic and intestinal glucuronidation, but also enhancing the extent of intestinal absorption, then enhance the serum concentration and bioavailability of it in rats and men with no adverse effects (Shoba et al., Citation1998; Suresh & Srinivasan, Citation2007, Citation2010). Meanwhile, piperine can inhibit the P-glycoprotein (P-gp) which is abundantly expressed in cancer cell that impact anti-tumor drugs therapeutic outcome and leads to the multidrug resistance (Han et al., Citation2008; Yoncheva et al., Citation2012; Alzoubi et al., Citation2013). More recently, Singh (Singh et al., Citation2013) provided the direct proof that piperine could affect P-gp function and expression in cell cultures in a manner. Therefore, it is important for piperine as an agent to overcome multidrug resistance. The piperine was researched more about by the intravenous administration, such as the oil-in-water emulsion of piperine, piperine lipid nanoparticle, positively charged stearylamine lipid nanosphere and pegylated lipid nanosphere (Veerareddy et al., Citation2004; Veerareddy & Vobalaboina, Citation2008). Pharmacokinetics of piperine in lipid nanospheres showed a biexponential decline with significantly high AUC than piperine solution, and it meant that some new formulations could change the bioavailability of piperine in vivo. However, piperine has low solubility in water and high lipid/water partition coefficient of 179. 33 (lgp = 2.25) (Wu et al., Citation2012). The low solubility in water and poor dissolution may be the rate-determining step in the absorption processes for piperine. So, a great deal of attentions should focus on the new formulations to improve the solubility and dissolution of piperine.
Recently, self-emulsifying drug delivery system (SEDDS) is a class of emulsion that receives particular attention as a means of enhancing oral bioavailability of hydrophobic drugs with poor aqueous solubility (Neslihan Gursoy & Benita, Citation2004). The self-emulsifying formulation is dispersed in the gastrointestinal (GI) tract to form the spontaneous of microemulsions with a droplet diameter usually within the range of 10–100 nm, thus a large interfacial surface area is provided for pancreatic lipase to promote the rapid release of the drugs which is the limiting step in the absorption from GI tract (Pouton, Citation1985). Many surfactants have been reported to improve the lipophilic drugs oral bioavailability by inhibiting human intestinal efflux transporters (Rege et al., Citation2002; Tang et al., Citation2013). In brief, SEDDS is one formulation technique which could be prepared as physically stable formulation to enhance the bioavailability of the hydrophobic drugs, especially for poorly water soluble traditional Chinese medicines (Zhang et al., Citation2008). In our earlier studies, Tang et al. (Citation2008) and Shao et al. (Citation2010) prepared the new self-emulsifying formulation of Ginkgo Biloba extracts and Wurenchun (alcohol extract from Fructus Schisandrae Chinensis) for improving the bioavailability of the main active components, respectively. However, it seems that the relationship between the SEDDS formulations and the mechanisms for enhancing the lipophilic drugs oral bioavailability was not clear currently. Thus, the investigation of drug absorption in the intestine is extremely important, and it will increase the understanding of the mechanisms of drug across the intestinal epithelium. In situ single pass intestinal perfusion (SPIP) is usually used to investigate the intestinal absorption behavior of drugs.
The main objective of the study was to develop an optimized formulation of piperine SEDDS in order to improve the solubility, the dissolution rate, the bioavailability after oral administration compared with the self-prepared piperine capsules. In the study, the SEDDS formulation of piperine was developed by solubility tests and pseudo-ternary phase diagram, meanwhile the particle size analysis and drug release were used to characterize the optimized formulation. Finally, the relative bioavailability of the piperine SEDDS formulation was evaluated compared with the self-prepared capsules in rats, and in situ SPIP was also conducted for understanding the absorption mechanism.
Materials and methods
Materials
Piperine (purity 98%) was obtained from Shanxi Huike Botanical development, Co, Ltd. (Xi’an, Shannxi, China). Standard substance of piperine was supplied by the National Institute for Food and Drug Control (Beijing, China). Ethyl oleate and oleic acid were purchased from Sinopharm, Shanghai Chemical Co. (Shanghai, China). Soybean oil (food grade), Tween 80 and PEG 400 were obtained from Tianjin Kemi’o Chemical Development Center (Tianjin, China). 1, 2-propylene glycol and hydrochloric acid were obtained from Tianjin Tianda Chemical Plant (Tianjin, China). Cremophor (RH40, and ELP) was kindly provided by BASF Co, Ltd. (Ludwigshafen, Germany). Transcutol P was gifted by Gattefoss Corp (Saint-Priest, France). Sodium dodecyl sulfate was purchased from Sigma Chemical Co. (St. Louise, MO), heparin was purchased from Wanbang Pharm. Co, Ltd. (Xuzhou, Jiangsu, China) and diazepam was purchased from Jinyao Pharm. Co, Ltd. (Tianjin, China). All other chemicals and solvents were reagent grade and used without further purification.
Solubility studies
Solubility studies were conducted by adding an excess amount of piperine (approximately 10 mg) to closed glass vials containing 1 ml of the vehicle (). Then, the samples were vortexed in a shaking water bath for 3 days at 25 °C to stimulate for equilibrium. After all, the resulting samples were centrifuged at 5000 rpm for 10 minutes to separate the undissolved piperine. After dilution of the supernatant using methanol, the quantity of piperine presented in the solution was determined by HPLC system (Waters Corp, Milford, MA) consisting of Millenninum 32 software, 486 UV spectrometer (Waters Corp) and 510 pump. Column was Diamnsil™ C18 column (200 mm × 4.6 mm, 5 µm; Dikma, Beijing, China). Mobile phase, a mixture of methanol and water (77:23, v/v) was eluted at a flow rate of 1.0 ml/min. The mobile phase effluents were monitored at 343 nm. The inter- and intra-day variance of the method was within the acceptable range of less than 2%. The curve was found to be linear in the concentration range of 0.5–50.0 µg/ml (r = 0.9999). All solubility studies were repeated thrice.
Table 1. Blank formulations compatibility test (vehicle compositions for screening SEDDS formulations, with the proportion of oil 30%, surfactant 50%, cosurfactant 20%).
Construction of pseudo-ternary phase diagram
The formulations were prepared in the mixture of excipients in an isothermal water bath. A series of self-emulsifying systems were prepared with different concentrations of oil, surfactant and cosurfactant. All components ratio were regarded as being 100%. The mixture (0.2 ml) was added into 100 mL of water in a glass beaker at 37 °C under gentle agitation (Shao et al., Citation2010). The system with clear appearance or slightly opaque was considered to be a fine emulsion (Kim et al., Citation2011). A pseudo-ternary phase diagram was built to identify the good self-emulsifying region. All studies were repeated thrice. Finally the effect of piperine on the self-emulsifying performance of SEDDS should be investigated, and 2.5% piperine (w/v) was added to the boundary formulations of the self-emulsifying domain of the ternary phase diagrams to observe the emulsifying performance. The formulation with varying concentrations of ethyl oleate (from 25% to 70%), Tween 80 (from 25% to 70%), Transcutol P (from 5% to 25%) and piperine (25 mg) at 30 °C in an glass beaker to obtain a fine blend of mixture at a liquid state.
Preparation of self-emulsifying formulation and particle size determination
The piperine dosage in self-emulsifying formulation was determined according to the earlier report (Wang et al, Citation2010). The oil, surfactant and cosurfactant were weighted by a glass beaker and mixed by vortexing. A certain amount of piperine was added to the mixed liquor and heated at 40 °C with a low speed to make it homogeneous. In the optimal prescription, the fill volume of a size 0 capsule containing 12.5 mg piperine was used for the next experiments at ambient temperature.
The particle size and the distribution of the emulsion were determined by the Zetasizer Nano ZS90 (Malvern Instruments Inc, Westborough, MA). SEDDS formulation (0.2 ml) was diluted into 100 ml purified water with a gentle stirring for the particle size analyzer.
In vitro dissolution study
Dissolution profiles of the SEDDS formulation and the control piperine formulation were obtained using a VK 7000 dissolution testing station (Hanson Research Corporation, Chatsworth, CA) according to the second method of Chinese Pharmacopoeia dissolution procedure (paddle method) and operating at a rotation speed of 100 rpm. The control piperine capsules were self-prepared using the same piperine material (25 mg). SEDDS capsules or the self-prepared capsules were placed into the 900 mL hydrochloric acid at 37 ± 0.5 °C. In order to satisfy the sink conditions, 0.1% sodium dodecyl sulfate was added in the medium. Each sample (5 ml) was withdrawn at 5, 15, 30, 45 and 60 min with replacement by an equal volume of fresh medium media to maintain a constant volume of the dissolution medium. Samples were filtered using a 0.45 μm microfiber filter and then 10 μl sample was injected into the HPLC system. The quantification method of piperine by HPLC was described above. Calibration curves were linear over the range of 0.5–50.0 µg/ml for the piperine (r = 0.9999).
In vivo oral absorption study
Experimental protocols were approved by the Animal Care Committee of Harbin Medical University. Twelve male Sprague-Dawley rats weighting 180–220 g were obtained from Experimental Animal Center of the Second Affiliated Hospital of Harbin Medical University (Harbin, Heilongjiang, China). The rats were randomly divided into two groups. All animals were fasted for 24 h but allowed free access to water before drug administration. Piperine SEDDS or self-prepared capsules were given to six rats by intragastric administration at a dose of two capsules containing 50 mg piperine. After dosing, blood samples (0.3 ml) were collected into the heparinized tubes at predetermined time intervals from rats in each group. And then the plasma samples were immediately separated by centrifugation at 10 000 rpm for 10 min, and the supernatant was transferred to the labeled tubes and stored at −80 °C until analysis. The plasma drug concentration was assayed with the HPLC method.
For 100 μl of plasma samples, 10 μl of internal standard (diazepam, 150 μg/ml in methanol) was added and vortex mixed. Then the plasma was mixed with 0.3 ml absolute methanol in the 2 ml centrifuge tube for assay and centrifuged at 10 000 rpm for 2 min to precipitate the proteins. Finally, an aliquot of 10 μl the supernatant was injected into the HPLC system. The concentration of piperine in rat plasma was determined by HPLC as described above, except using the methanol and water (68:32, v/v) mobile phase at a flow rate of 1.0 ml/min and detection wavelength was set a 320 nm. The calibration curve was linear over the range 0.25–20 μg/ml in plasma (r = 0.9997) with the lower limit of quantification (LLOQ) of 0.1 μg/ml. Meanwhile the method showed well reproducibility with intra-day and inter-day precision, as well as accuracy for piperine. The extraction recovery was 90.15% for piperine and 73.40% for internal standard, respectively.
Pharmacokinetic data analysis
The maximum plasma concentration (Cmax), and the time to reach maximum plasma concentration (Tmax) were compiled from the blood data. The elimination rate constant (Ke) was counted by means of the least-squares regression technique according to the data for the last three or four points of the plasma concentration-time curve and the half-life (t1/2) of the drug was got by 0.693/Ke. The area under the drug concentration time curve was calculated from 0 to 60 h (AUC0→60 h) using the liner trapezoidal method. The relative bioavailability (BA) of SEDDS (test formulation) to the self-prepared capsules (reference formulation) was figured up with the following equation:
Statistical analyses used an unpaired Student’s t-test. The data were presented as mean with standard error (SE) for the each group. The p < 0.05 and p < 0.01 were considered as marginal significant and dramatic significant, respectively.
In situ intestinal absorption studies
The SPIP technique was applied as previously reported (Luo et al., Citation2013; Samiei et al., Citation2013). The male Sprague-Dawley rats were fasted for 24 h but allowed free access to water. According to the method of Huo et al, three intestine segments of duodenum, jejunum and ileum were tested for the intestinal absorption studies (Huo et al., 2011). Firstly, the rats were anaesthetized using an intraperitoneal injection of sodium pentobarbital to get each of the small intestinal segments about 10 cm and cannulated at both ends with plastic tubing. During the experiment a heating lamp was used and the exposed intestines were covered with gauze moistened by frequent applications of warm saline to maintain body temperature. The intestinal segment was washed with phosphate buffer saline maintained at 37 °C for approximately 30 min until the outlet solution was visually clear. Then the intestinal segment was infused by the perfusion fluids which removed by idling the DHL-A peristaltic pump (Shanghai huixin Precision Pump, Shanghai, China) at a rate of 0.25 ml/min for 30 min for balance. Phosphate buffered solution (PBS) was used in the SPIP and it comprised 7.78 mM D-glucose. Each perfusion experiment lasted for 60 min, and samples were collected at an interval of 10 min in pre-weighed glass tubes. The samples were centrifuged at 10 000 rpm for 5 min, the upper layers were filtered through a membrane filter and 10 μl samples were injected into the column for the HPLC assay. The quantification method of piperine by HPLC was also described as before. Calibration curves were linear over the range of 0.10–10.00 µg/ml for the piperine (r = 0.9997). At the end of experiment, the length and perimeter of the perfused intestinal segments were accurately measured.
In the SPIP studies, in order to study whether the absorption of piperine has dose-dependent. The low, middle and high concentration (1, 5 and 10 μg/ml) of piperine in SEDDS solution and control piperine perfusion solution were pumped in the best absorption of intestine segment respectively. All the perfusion solution was maintained at 37 °C. For the absorption segment test, the concentration of 5 μg/ml for SEDDS or control piperine solution were pumped the intestine segment which was taken about 10 cm from duodenum, jejunum and ileum, respectively. For further study, Transcutol P and Tween 80 were selected to investigate the effect of excipients on absorption process. The concentration of piperine solution on was 5 μg/ml, and the quantity of the accessories was added by its proportion in the SEDDS formulation.
Analysis of perfusion date
Intestinal permeability coefficient (Peff) of the drug and the effective absorption rate (Ka) were determined according the following equations:
where Qin is the measured flow rate (ml/min) of entering intestinal perfusate; Qout is the measured flow (ml/min) of exiting intestinal perfusate for the specified time interval calculated from the actual intestinal perfusate density (g/ml), which is determined by weighing the contents of a known volume of perfusate; Q is perfusion flow rate (0.25 ml/min); Cin and Cout were the concentration of drug in the effluent perfusate through the inlet and outlet tube (μg/ml), respectively. Meanwhile the 2πrl is the surface area of the intestinal segment concerned (cm2)
Statistical analyses used an unpaired Student’s t-test. The data were presented as mean with standard deviation (SD) for the each group. The p < 0.05 and p < 0.01 were considered as marginal significant and dramatic significant, respectively.
Results and discussions
Solubility study
Self-emulsifying formulations which consisted of the oil, surfactant, cosurfactant and drug should be clear and monophasic liquid at ambient temperature. Meanwhile it usually provides the advantage of increased capacity for loading more poorly water-soluble drugs when compared with lipid solutions. The surfactants which are used in the SEDDS improve the bioavailability by various mechanisms (Kommuru et al., Citation2001). So it is important to select the appropriate vehicle for the efficiency of self-emulsification. As the piperine could be clearly seen in the solution, the solubility of the piperine in the vehicles could be estimated by visual observations, and the data of solubility in the good vehicles should be determined. And then excipients with high solubility were selected to carry out the blank formulation compatibility experiment (). Among the tested that No.4 (ethyl oleate, Transcutol P, Tween 80) and No.10 (oleic acid, Transcutol P, Tween 80) blank formulations were not layered and formed uniform liquid. The solubility results of piperine in various vehicles were presented in . The piperine appeared to have higher solubility in ethyl oleate among three oils of soybean oils, oleic acid and ethyl oleate. Among the various cosurfactants, the Transcutol P showed highest drug solubility with 185.29 ± 0.16 mg/ml. It is important to select the appropriate vehicle for the piperine to improve the solubility. The Transcutol P had the best solubilizing capacity for piperine, so Transcutol P was extremely important for the formulation. The solubility of piperine in Cremophor RH40 and Tween 80 were 30.77 ± 0.11 and 70.71 ± 0.08 mg/ml, respectively (). Therefore, ethyl oleate, Transcutol P and Tween 80 were selected as the oil phase, cosurfactant and surfactant for constructing the pseudo-ternary phase diagram to optimize the formulation.
Table 2. Solubility of piperine in various vehicles at 25 °C (n = 3, mean ± SD; mg/ml).
Construction of pseudo-ternary phase diagram
Pseudo-ternary phase diagram were constructed to determine the isotropic clear self-microemulsifying regions and to obtain optimum concentration of components in the SEDDS formulations (Pouton, Citation1997). A series of blank formulation were prepared and their self-emulsifying properties were observed through naked eyes. The phase diagram of the systems was observed that increasing the concentration of the surfactants in formulation could increase the spontaneity of the self-emulsifying region of the self-emulsification process and decrease the mean droplet size (). The surfactant concentration in self-emulsifying formulations usually ranged from 30% to 70% which will form an emulsion state in the GI tract. Meanwhile the GI tract may be irritated by majority surfactants. Thus 55% surfactant was considered for the formulation. Surfactants can reduce the energy from the interfacial to form a layer around the emulsion droplets, stabilize the oil-water interface and provide a mechanical barrier to coalescence (Craig et al., Citation1995). Therefore, there will be more self-emulsifying region in phase diagrams with more cosurfactant in the formulation. Previous study indicated that the mean droplet size will be declined when the emulsification ability of the system increased (Devani et al., Citation2004; Li et al., Citation2005). That is, the cosurfactant with the highest solubilization capacities of piperine is a good candidate to fit the formulation. Therefore, a little Transcutol P was required which could not only dissolve more drugs but also reduced the interfacial energy between the oil and water interface to readily deformed oil droplets. In the study, there were no significant differences in the self-emulsifying region when the piperine was added into the boundary of blank prescription. Based on the in vitro experiment, the self-emulsifying region had a fine emulsion that was clear and slightly opaque in appearance ().
Figure 1. Pseudo-ternary phase diagrams indicating the efficient self-emulsification region with ethyl oleate as oil, Tween 80 as surfactant, and Transcutol P as the cosurfactant, with a drug loading of 2.5%. The solid outline represents the area explored for nano-emulsion region; and the filled squares represent the compositions which were evaluated.
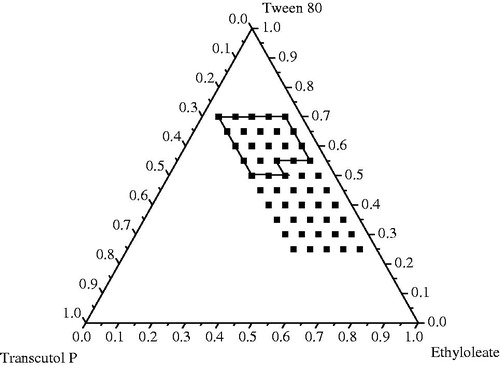
Finally, the formulation containing ethyl oleate (30%), Tween 80 (55%), Transcutol P (15%), and piperine (2.5%, w/w of the vehicle) were selected as the optimized formulation for the next experiments according to the results of pseudo-ternary phase diagram.
Droplet size of self-emulsifying formulation
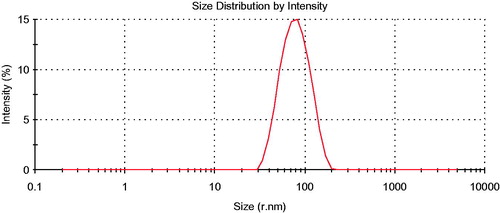
The distribution of the particle size is one of the most important characteristics of emulsion for it influences dissolution in vitro and the time of distribution in vivo which will affect the bioavailability (Pouton, Citation1997, Yap & Yuen, Citation2004). To evaluate the property of the SEDDS formulation, the droplet size of the self-emulsifying system was determined by the Zetasizer measurement. The mean droplet size of the piperine emulsions was 89.82 ± 2.16 nm (), which is a typical characteristic of SEDDS, suggesting small nanosized droplets were obtained.
In vitro dissolution study
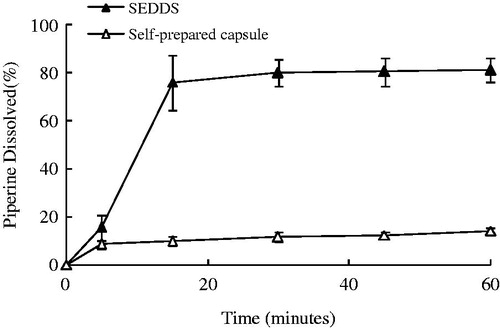
As the piperine has low solubility and high membrane permeable, dissolution rate is the primary limiting aspect to absorption (Six et al., Citation2004). The drug release profiles of piperine from self-emulsifying formulation and self-prepared capsules were compared (). For the SEDDDS system, the release rates of piperine were remarkably higher than those of the self-prepared capsules. The release percentages of piperine at 60 min were 80.27% and 13.60% in the dissolution medium from SEDDS formulation and the self-prepared capsules, respectively. Within 15 min, the total release amount of piperine from the SEDDS formulation rapidly reached more than 75%. The piperine from the SEDDS formulation could be released rapidly due to the spontaneous formation of emulsion and the resultant small droplet size, compared with that from the self-prepared capsules which was only arrived at 1.05%. The release profile of formulation indicated that SEDDS could increase the dissolution of piperine in the dissolution medium which may probably affect the bioavailability in rats.
In vivo study
An in vivo pharmacokinetic study was undertaken in rats in order to evaluate the bioavailability of the piperine SEDDS formulation after oral administration compared with the self-prepared capsules group. The resultant plasma concentration versus time profiles for the treatments of the oral SEDDS formulation and the self-prepared capsules were shown in , likewise the corresponding pharmacokinetic parameters were presented in . There was a significant improvement about the area under the curve of piperine from the SEDDS formulation compared with the self-prepared capsules. It suggested that the SEDDS can increase the dissolution of piperine in the dissolution medium and the GI absorption after oral administration. For the SEDDS formulation, it had a double-peaks phenomenon in the plasma concentration-time profiles (). The first piperine peak occurred at about 8 h after dosing, and the second peak occurred at about 26 h. Although the values of the Cmax1 and Cmax2 for the SEDDS formulation were 5-fold greater than those for the self-prepared capsules (p < 0.01), there were no significant difference between Tmax1 and Tmax2 for both plasmas peaks (p > 0.05), which indicated that the Tmax was not affected by formulation. In particular, the mean values of AUC0→60h estimated from the individual plasma drug concentration profiles of the SEDDS formulation was 416.64 µg/ml h and the self-prepared capsules was 79.80 µg/ml h (p < 0.05). The analyses for the value of t1/2 did not show apparent differences between of both formulations. As we all know, the double-peak phenomenon is often happened to the drug with the hepato-enteric circulation caused by the bile elimination. The earliest study also reported that the piperine was not detectable in urine at any time interval, however, piperine was excreted as such in the faeces over a period of 4 days orally administered to rats (Suresh & Srinivasan, Citation2010). It might signify that the most of piperine was excreted by bile and probably had hepato-enteric circulation, which could lead to part of drug was absorbed once again in the intestine. In present study, the SEDDS formulation had the smaller emulsion droplet, the faster drug release and the higher permeability in intestine, which could enhance the quantity of the absorption for piperine once again into the intestine (Porter et al., Citation2007). Meanwhile when the emulsion was excreted by the bile, it will have a smaller droplet (Zhang et al., Citation2013). In the SEDDS group, the initial peak was caused by the absorption of piperine in the GI tract, and the later peak was caused by the hepato-enteric circulation of piperine in the plasma concentration profile. The double-peak phenomenon of the self-prepared capsules was not conspicuous, maybe induced by the lower dissolution and absorption of piperine. Hence, the SEDDS would be a favorable formulation in clinical point of view as it could enhance the bioavailability of poorly water-soluble piperine.
Figure 4. Plasma concentration-time profiles of Pip from SEDDS and self-prepared capsule following oral administrations in rats. Each value is mean ± SE (n = 6).
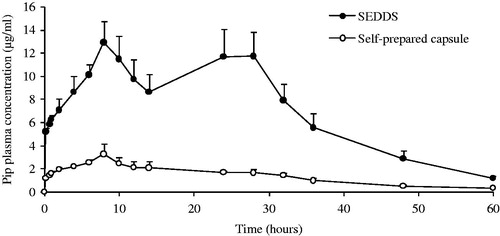
Table 3. Main pharmacokinetic parameters of piperine after a single oral dose of SEDDS or self-prepared capsules in rats (n = 6, mean ± SE, **p < 0.01; *p < 0.05).
As the piperine is widely used in the anti-tumor and overcoming multidrug resistance, it is important to make sure the time of first administration in order to affect the function of P-gp. For this property, it could probably reduce the administration time for anti-tumor and will provide some convenience for the patients. Thus the metabolism and transformation of piperine need to be deeply researched for widely using in clinic.
In situ intestinal absorption studies
There are two kinds of technique for the in situ intestinal perfusion method, recirculating perfusion model and single-pass perfusion model. The absorption in the recirculating perfusion model will be increased just because it has a long retention time for the solution in the intestine (Cao et al., Citation2013). The single-pass perfusion is suitable for drugs that are rapidly absorbed, meanwhile it could have a comparatively absorption just by its slowly single-pass rate (Luo et al., Citation2013). Thus it is widely used for the research of the intestinal absorption. The intestinal permeability of the piperine in different concentration of SEDDS or solution formulation was studied as a function in duodenum using the in situ SPIP technique. Three concentrations were selected for this part to determine whether the permeability of piperine was depended on concentration. And there was no significant difference among those concentrations for the two perfusion solutions (). Meanwhile Ka and Peff of piperine in the SEDDS were significantly higher than the control group (p < 0.01, p < 0.05) (). The intestinal permeability of piperine was increased about 1.3-times for the SEDDS formulation, compared to the control solution. As a result, the effect of SEDDS was dependent on the solubilized state of piperine. As the emulsion has a small droplet size that permits an easily access epithelial cells and enhances the absorption in intestine. This result was highly consistent with the dissolution study, and those results revealed that the transport mechanism may fit the active passive transport. The Peff values of piperine SEDDS formulation (5 µg/mL) in duodenum, jejunum and ileum were 0.64 × 10−3 ± 0.05 × 10−3 cm/s, 0.45 × 10−3 ± 0.04 × 10−3 cm/s and 0.39 × 10−3 ± 0.07 × 10−3 cm/s, respectively (), which was higher than the piperine solution. The absorption in duodenum was significantly better than other intestine segments (p < 0.01), not only for the piperine solution but also for the SEDDS formulation, and those results may caused as the duodenum has great amount of blood vessels to transform the drug for absorption. In addition, it indicated that the SEDDS formulation did not change the absorption segment of piperine. Numerous reports indicated that the surfactants could increase drug absorption by reversible injury in biological cell membranes (Neslihan Gursoy & Benita, Citation2004; Rahman et al., Citation2013). So Transcutol P and Tween 80 were selected for next study. The two excipients in the piperine solution had increased the absorption which was not extremely significant (p > 0.05) (). The SEDDS showed significant difference compared to the control solution (p < 0.01).
Figure 5. (A) Comparison of Peff and Ka of different concentration perfusion solutions determined by SPIP study in rat duodenum; (B) Effect of different intestine segments on compounds absorption; (C) Effect of adjuvant on absorption parameters of Pip solution. (n = 5, mean ± SD, #p < 0.05, ##p < 0.01 represented for Peff, *p < 0.05, **p < 0.01 represented for Ka).
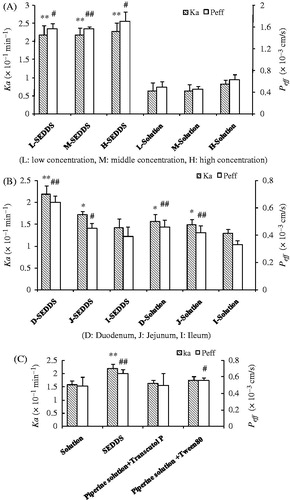
As a result, enhancement of the effect of SEDDS was depend on the forming small size of emulsions droplet which is provided a large interfacial surface area for drug absorption not only the alone role of complementary (Rahman et al., Citation2013).
In the dissolution study, the SEDDS formulation had significant greater extent of the dissolution and it had increased contact surface with dissolution media. In the intestinal absorption studies, the SEDDS formulation had higher permeability than the control reference. The above points may explain why the SEDDS formulation can improve the bioavailability of piperine in vivo.
Conclusions
The piperine is widely used to inhibit oxidative stress, anti-fungal, anti-inflammatory activity, anti-tumor and so on. Recently, most hot topics of researches on piperine are applied to enhance the bioavailability of many therapeutic drugs, so it is used as an overcome multidrug resistance agent. But it is not used widely in clinic just because it has the low solubility and bioavailability. In the present study, the piperine SEDDS formulation was successfully developed with an increased dissolution rate, bioavailability and permeability. The relative bioavailability of SEDDS formulation was 625.7% after oral administration compared to the self-prepared capsules. Therefore, the present study suggested that the SEDDS formulation could be a potential system to oral delivery of poorly water-soluble drugs, such as piperine.
Notice of Correction:
The version of this article published online ahead of print on 27 March 2014 was missing details on funding support. This has been added to the Declarations of interest section in this version.
Declarations of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. Financial support was provided by the Education Department of Heilongjiang Province, No. 12541482.
References
- Alzoubi K, Khabour O, Al-Azzam S, Mayyas F. (2013). The role of multidrug resistance-1 (MDR1) variants in response to fexofenadine among Jordanians. Int J Clin Pharmacol Ther 51:880–7
- Bezerra DP, de Castro FO, Alves AP, et al. (2008). In vitro and in vivo antitumor effect of 5-FU combined with piplartine and piperine. J Appl Toxicol 28:156–63
- Bhardwaj RK, Glaeser H, Becquemont L, et al. (2002). Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4. J Pharmacol Exp Ther 302:645–50
- Cao F, Gao Y, Wang M, et al. (2013). Propylene glycol-linked amino acid/dipeptide diester prodrugs of oleanolic acid for pepT1-mediated transport: synthesis, intestinal permeability, and pharmacokinetics. Mol Pharm 10:1378–87
- Craig D, Barker S, Banning D, Booth S. (1995). An investigation into the mechanisms of self-emulsification using particle size analysis and low frequency dielectric spectroscopy. Int J Pharm 114:103–10
- Devani M, Ashford M, Craig DQ. (2004). The emulsification and solubilisation properties of polyglycolysed oils in self-emulsifying formulations. J Pharm Pharmacol 56:307–16
- Ee GC, Lim CM, Rahmani M, et al. (2010). Pellitorine, a potential anti-cancer lead compound against HL6 and MCT-7 cell lines and microbial transformation of piperine from Piper nigrum. Molecules 15:2398–404
- Gao P, Morozowich W. (2006). Development of supersaturatable self-emulsifying drug delivery system formulations for improving the oral absorption of poorly soluble drugs. Expert Opin Drug Deliv 3:97–110
- Han Y, Chin Tan TM, Lim L-Y. (2008). In vitro and in vivo evaluation of the effects of piperine on P-gp function and expression. Toxicol Appl Pharmacol 230:283–9
- Huo X, Huang L, Liu S, et al. (2011). Study on the rat intestinal absorption of rapamycin formulated in self-microemulsifying drug delivery system by single-pass intestinal perfusion technique. Chin Hosp Pharm J 31:217–21
- Kim M-S, Kim J-S, Park HJ, et al. (2011). Enhanced bioavailability of sirolimus via preparation of solid dispersion nanoparticles using a supercritical antisolvent process. Int J Nanomed 6:2997–3009
- Kommuru T, Gurley B, Khan M, Reddy I. (2001). Self-emulsifying drug delivery systems (SEDDS) of coenzyme Q10: formulation development and bioavailability assessment. Int J Pharm 212:233–46
- Li P, Ghosh A, Wagner RF, et al. (2005). Effect of combined use of nonionic surfactant on formation of oil-in-water microemulsions. Int J Pharm 288:27–34
- Luo Z, Liu Y, Zhao B, et al. (2013). Ex vivo and In situ approaches used to study intestinal absorption. J Pharmacol Toxicol methods 68:208–16
- Neslihan Gursoy R, Benita S. (2004). Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother 58:173–82
- Pattanaik S, Hota D, Prabhakar S, et al. (2006). Effect of piperine on the steady-state pharmacokinetics of phenytoin in patients with epilepsy. Phytother Res 20:683–6
- Porter CJ, Trevaskis NL, Charman WN. (2007). Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov 6:231–48
- Pouton CW. (1985). Self-emulsifying drug delivery systems: assessment of the efficiency of emulsification. Int J Pharm 27:335–48
- Pouton CW. (1997). Formulation of self-emulsifying drug delivery systems. Adv Drug Delivery Rev 25:47–58
- Rahman MA, Hussain A, Hussain MS, et al. (2013). Role of excipients in successful development of self-emulsifying/microemulsifying drug delivery system (SEDDS/SMEDDS). Drug Dev Ind Pharm 39:1–19
- Rege BD, Kao JP, Polli JE. (2002). Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur J Pharm Sci 16:237–46
- Samiei N, Mangas-Sanjuan V, Gonz Lez-Álvarez I, et al. (2013). Ion-pair strategy for enabling Amifostine oral absorption: rat in situ and in vivo experiments. Eur J Pharm Sci 49:499–504
- Shao B, Tang J, Ji H, et al. (2010). Enhanced oral bioavailability of Wurenchun (Fructus Schisandrae Chinensis Extracts) by self-emulsifying drug delivery systems. Drug Dev Ind Pharm 36:1356–63
- Shoba G, Joy D, Joseph T, et al. (1998). Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta medica 64:353–6
- Singh DV, Godbole MM, Misra K. (2013). A plausible explanation for enhanced bioavailability of P-gp substrates in presence of piperine: simulation for next generation of P-gp inhibitors. J Mol Model 19:227–38
- Six K, Verreck G, Peeters J, et al. (2004). Increased physical stability and improved dissolution properties of itraconazole, a class II drug, by solid dispersions that combine fast- and slow-dissolving polymers. J Pharm Sci 93:124–31
- Srinivasan K. (2007). Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Crit Rev Food Sci Nutr 47:735–48
- Sunila E, Kuttan G. (2004). Immunomodulatory and antitumor activity of Piper longum Linn and piperine. J Ethnopharmacol 90:339–46
- Suresh D, Srinivasan K. (2007). Studies on the in vitro absorption of spice principles–Curcumin, capsaicin and piperine in rat intestines. Food Chem Toxicol 45:1437–42
- Suresh D, Srinivasan K. (2010). Tissue distribution, elimination of capsaicin, piperine, curcumin following oral intake in rats. Indian J Med Res 131:682–91
- Tang J, Sun J, Cui F, et al. (2008). Self-emulsifying drug delivery systems for improving oral absorption of ginkgo biloba extracts. Drug Deliv 15:477–84
- Tang J, Wang Y, Wang D, et al. (2013). Key Structure of Brij for Overcoming Multidrug Resistance in Cancer. Biomacromolecules 14:424–30
- Veerareddy P, Vobalaboina V. (2008). Pharmacokinetics and tissue distribution of piperine lipid nanospheres. Pharmazie 63:352–5
- Veerareddy P, Vobalaboina V, Nahid A. (2004). Formulation and evaluation of oil-in-water emulsions of piperine in visceral leishmaniasis. Pharmazie 59:194–7
- Wang X, Peng W, Zhang Q, et al. (2010). Pharmacokinetics of piperine capsules in healthy volunteers. J Cent South Univ T 8:513–16
- Wu Z, Xia X, Huang X. (2012). Determination of equilibrium solubility and apparent oil/water partition coefficient of piperine. J Jinan Univ 33:473–6
- Yap SP, Yuen KH. (2004). Influence of lipolysis and droplet size on tocotrienol absorption from self-emulsifying formulations. Int J Pharm 281:67–78
- Yoncheva K, Calleja P, Ag Eros M, et al. (2012). Stabilized micelles as delivery vehicles for paclitaxel. Int J Pharm 436:258–64
- Zhang P, Liu Y, Feng N, Xu J. (2008). Preparation and evaluation of self-microemulsifying drug delivery system of oridonin. Int J Pharm 355:269–76
- Zhang Z, Gao F, Jiang S, et al. (2013). Bile salts enhance the intestinal absorption of lipophilic drug loaded lipid nanocarriers: mechanism and effect in rats. Int J Pharm 452:374–81