Abstract
Several reports have indicated a positive correlation between the consumption of anthocyanins (ACN) and biomarkers relating to the improvement of type 2 diabetes (T2D). However, the results from in vitro studies often do not translate into clinical evidence. Potential causes of these discrepancies are experimental conditions that lack physiological relevancy; extensive degradation of these compounds in vivo due to changes in pH and metabolism; and a short residence time in the absorption window in relation to the absorption rate. Here, gastroretentive systems (GRS) are proposed as a strategy to overcome the limitations in ACN delivery and to reduce the existing bench-to-subject gap. This review summarizes recent literature on the use of ACN for the management and control of T2D, followed by GRS platforms to promote a sustained release of ACN for increased health benefits.
Introduction
Fruits are a rich source of essential micronutrients (such as vitamins and folic acid) (Brouwer et al., Citation1999; Proteggente et al., Citation2002) and other non-essential bioactive compounds, including anthocyanins (ACN) (Fischer et al., Citation2013; Faramarzi et al., Citation2015). ACN are members of the flavonoid class of secondary metabolites and important hydrophilic pigments in plants (Delgado-Vargas et al., Citation2000). In addition to their colorant properties, several studies have indicated a positive association between the consumption of ACNs and reduced risk of degenerative diseases (Wang & Stoner, Citation2008; Pascual-Teresa et al., Citation2010; Cassidy et al., Citation2011,Citation2013; Wallace Citation2011), including type 2 diabetes (T2D) (van Dam et al., Citation2013; Guo & Ling, Citation2015).
Nonetheless, there is still a considerable gap between the results obtained in vitro and those confirmed in vivo. One explanation is that the health-promoting effects are often from in vitro and/or in vivo studies, where the ACN compounds are administered in quantities that are above those commonly ingested through diet or that would be observed in vivo (Al-Awwadi et al., Citation2004; Heo & Lee, Citation2005; Tarozzi et al., Citation2007,Citation2010; Kelsey et al., Citation2011; Hidalgo et al., Citation2012). It is also possible that the experiments do not account for the accumulation and synergic effect of the compounds ingested and the metabolites produced in vivo (Kelsey et al., Citation2011). In addition to experimental design that has little physiological relevancy, another possible reason for this gap could include the extensive degradation of ACN due to variations in pH or as a result of metabolism, as demonstrated for cyanidin 3-glucoside (Ferrars et al., Citation2014).
The insufficient residence time in the upper gastrointestinal (GI) tract (stomach and initial portion of the small intestine) could be a factor that limits the absorption of the parent ACN, as it would contribute to degradation at higher pHs and due to metabolism. In this context, gastroretentive systems (GRS) are a promising strategy that could help to increase the retention time and modulate the release of ACN in portions of GI tract where they are absorbed. GRS differ from conventional delivery systems as the time they remain in the stomach is higher, prolonging the residence time of the bioactive compound in this organ (Joseph et al., Citation2002). For substances absorbed in the stomach, these systems can assist in increasing their absorption and improving their bioavailability. The development of a GRS can be achieved by different strategies that will be described in this review. Since these platforms have not been explored in depth by the food industry, information from the pharmaceutical sciences will be used as a reference when appropriate, then recommendations will be made for GRS for fruit-derived extracts that are rich in ACN and have potential as health-promoting compounds.
The purpose of this review is to present the use of GRS as a potential platform to modulate the release of ACNs in their absorption window (upper GI tract) to assist in the management of degenerative diseases. First, the evidence that correlates ACN with the management of degenerative diseases (using T2D as an example) is discussed. Then, the role of the stomach in the absorption of ACN is presented, followed by different classes of GRS based on information available from the pharmaceutical sciences and the potential application of GRS for the management of T2D.
Anthocyanins in the management of type 2 diabetes
Diabetes currently affects approximately 387 million people worldwide and it is estimated that this number will rise to 592 million by 2035 (International Diabetes Federation, Citation2013). T2D is characterized by insulin resistance and relative lack of insulin secretion, and accounts for more 90% of the cases reported (Kahn, Citation1998). Insulin plays a key role with glucagon in regulating the concentration of glucose in the blood and is secreted by β cells of the pancreatic islets of Langerhans as a response to high levels of glucose and fatty acids in the circulatory system (Gravena et al., Citation2002). Glucagon, on the other hand, is secreted by α cells of these islets, promoting the glycolysis of glycogen in the liver and an increase in blood glucose (Foster et al., Citation1993). Ishihara et al. (Citation2003) indicated that the activation of β cells leads to a suppression of the α cells. Current treatment agents available for T2D include insulin, sulfonylureas, and α-glucosidase inhibitors (Nathan et al., Citation2009). However, adverse effects have been commonly associated with these drugs, such as hypoglycemia (Krepinsky et al., Citation2000), weight gain (Nathan et al., Citation2009), and drug–drug interactions observed with the use of sulfonylureas (Schelleman et al., Citation2014). Research indicates that the consumption of polyphenols, such as ACN, can be associated with an improvement of biomarkers of T2D (), which could help with the prevention and management of diabetes.
Table 1. Summary of anti-diabetic activities promoted by ACN from different sources.
In vitro evidence
As highlighted in , there are in vitro studies associating ACNs with anti-diabetic properties. Rojo et al. (Citation2012) showed that ACN-rich extract from maqui berry could ameliorate diabetic conditions in vitro by reducing the production of glucose and increasing its cellular uptake. The authors used two systems to assess glucose production and uptake: H4IIE rat hepatoma cells and myotubes (differentiated from L6 myoblasts isolated from rat skeletal muscles). H4IIE cells are frequently used for the study of gluconeogenesis and as an in vitro model for obesity-related insulin resistance (T2D). The physiological relevance of these cells lies with their production of glucose, which is stimulated in vitro by glucagon and glucocorticoid simulators, and inhibited by insulin, in similar ways to what occurs in vivo (Hectors et al., Citation2012). Myotubes, on the other hand, are often used to evaluate the uptake of glucose (Nedachi & Kanzaki, Citation2006). When stimulated by insulin, skeletal muscle cells translocate the glucose transporter 4 (GLUT4) to the plasma membrane to enable glucose uptake, and this is observed also in the myotube model (Galante et al., Citation1995). Rojo et al. (Citation2012) found that ACN glycosides present in the maqui berry extract had a mild effect on the downregulation of glucose-6-phosphatase (G6Pase) gene compared to insulin and also observed that the concomitant administration of ACN and sub-optimal dose of insulin had a higher effect on the downregulation of G6Pase gene. G6Pase is involved with glucose homeostasis and its expression is strongly inhibited by insulin (Chen et al., Citation2000). They reported a significant increase of glucose uptake in the cells treated with ACNs and also those receiving ACNs and insulin. From this evidence, they suggested that ACNs were sensitizing the cells to insulin, resulting in the improved glucose uptake that was observed.
In a different study, Jayaprakasam et al. (Citation2005) evaluated the properties of purified ACN glycosides and aglycones as insulin secretagogues in β cells from rodents. Among the compounds investigated, purified glucosides of cyanidin and delphinidin were the most effective in inducing insulin secretion. It is important to note that ACN can be extensively metabolized by the gut microflora prior to absorption (Fang, Citation2014; Faria et al., Citation2014), and this was not considered in the in vitro studies by Rojo et al. (Citation2012) and Jayaprakasam et al. (2005). This can impact the significance of these findings, for example it is unlikely that ACN glycosides will reach the pancreatic β cells in their native form and exhibit the health-promoting effects in vivo.
In vivo evidence
Animal models have been frequently used to investigate the effect of ACNs on diabetes-related conditions. For example, Rojo et al. (Citation2012) evaluated the hypoglycemic effect and glucose tolerance in obese hyperglycemic C57BL/6J mice after administering ACN-rich extract from maqui berry. C57BL/6J mice develop T2D when treated with a high fat, high-single carbohydrate, low-fibre diet due to a genetic predisposition (Surwit et al., Citation1988). In this model, ACNs administered orally improved blood glucose concentrations and glucose tolerance and this effect was partially attributed to delphinidin 3-sambubioside-5-glucoside (Rojo et al., Citation2012). Other studies using C57BL/6J mice have shown that ACN has a positive effect in controlling weight gain, which would contribute to the management of T2D through the consumption of ACN from haskap berries (Wu et al., Citation2013).
In the study by Takikawa et al. (Citation2010), a positive effect on blood glucose and insulin sensitivity was observed in diabetic KK-Ay rats consuming bilberry extract for 5 weeks. These authors corroborated the in vitro results obtained by Martineau et al. (Citation2006) indicating that the effect of ACN would be through an insulin-independent mechanism.
In both models presented (C57BL/6J and KK-Ay mice), the animals were genetically predisposed for developing T2D-like conditions. Diabetes can also be chemically induced (by alloxan or streptozotocin) to simulate diabetes caused by insulin deficiency. Chemical treatment results in the loss of β cell mass and immunological activity in the islets (Zunino, Citation2009). Examples of studies that used a chemically induced diabetes model are provided in .
Clinical and epidemiological evidence
Studies conducted on healthy human volunteers have shown that the consumption of berries could be associated with an improvement of the postprandial plasma glucose, which can reduce the risks of developing T2D (Törrönen et al., Citation2010, Citation2012a,Citationb). In a cross-over study (randomized, single-blinded, 5-d wash-out period), the consumption of a sucrose-sweetened berry purée (prepared with bilberry, blackcurrant, cranberry, and strawberry) was compared to a control consisting only of sucrose. Plasma glucose levels were significantly lower in the first 30 min after ingestion of the berry purée compared to the sucrose control. There was also a delay to reach the maximum plasma glucose concentration: 45 and 30 min for the berry puree treatment and control meal, respectively, which could indicate a reduction in digestion and/or absorption of sucrose similar to in vivo results (Törrönen et al., Citation2010). However, the berry purée was not assessed for its polyphenol content. Based on literature values, the authors estimated that ACN were one of the major polyphenolic component in the berry puree; however, other compounds could have contributed to these results.
In a double-blind placebo-controlled trial, Stull et al. (Citation2010) evaluated the effect of blueberry consumption in 32 obese, non-diabetic, and insulin-resistant subjects. Although these volunteers were not diabetics, they presented high risk for development of T2D. The subjects were divided randomly into two groups: (a) treatment, consisting of freeze-dried blueberry powder (22.5 g) in a smoothie, and (b) placebo, consisting of a smoothie with equal macronutrient content and without blueberry powder. The smoothies were consumed twice a day (breakfast and dinner) for 6 weeks, and those containing blueberry powder represented approximately a cup of fresh whole berries per smoothie. The authors observed a significant improvement on the insulin sensitivity in subjects consuming the smoothie-containing blueberries (Stull et al., Citation2010).
In a study with obese T2D patients, the consumption of a capsule-containing concentrated bilberry extract reduced the area under curve (AUC) of glucose and insulin in comparison to a placebo. This double-blind, cross-over study consisted of: (a) treatment with one gelatin capsule filled with bilberry extract (containing 36% ACN), representing 50 g of fresh berries, and (b) placebo consisting of a capsule with microcrystalline cellulose (Hoggard et al., Citation2013). After ingesting the capsule with berry extract or placebo, the volunteers consumed a drink with 75 g of glucose and were evaluated for glucose metabolism, with a 2-week wash-out period. The authors suggested that the reduction in the AUC could be related to a reduction of the carbohydrate digestion and/or absorption. In addition, they reported a reduction of plasma insulin levels, possibly as a result of lower plasma glucose (reduction of approximately 18% of the AUC compared to the placebo) or due to an improvement on insulin sensitivity (Hoggard et al., Citation2013). A limitation of this study is the small number of participants enrolled (eight). However, it is the first demonstration of the beneficial effects of consuming a concentrated bilberry extract on T2D management.
Li et al. (Citation2015) also showed that the consumption of 160 mg of ACN purified from bilberries and blackcurrants split into two portions daily for 24 weeks had positive effects on 58 diabetic patients. Patients showed a reduction in fasting plasma glucose in comparison to the placebo group, in addition to improvement of dyslipidaemia.
In relation to epidemiological studies, Samieri et al. (Citation2014) studied the ingestion of different classes of flavonoids by 13 818 healthy women (>50 years old) for 15 years in an observational study, using questionnaires. Of those individuals who survived into their seventies, 1517 met the requirement of healthy aging (i.e. no chronic disease; high degree of cognitive, mental, and physical health) and the higher consumption of flavonoids, including ACNs, was associated with higher chances of health and well-being at older ages (Samieri et al., Citation2014).
Wedick et al. (Citation2012) also showed that higher intakes of ACN were inversely related to the development of T2D using data from three cohort studies consisting of more than 200 000 healthy subjects. The effect of other classes of flavonoids was not consistent between the cohort studies, which could indicate that they made little contribution to the reduced risk of T2D (Wedick et al., Citation2012). In addition, Muraki et al. (Citation2013) suggested that a higher risk of T2D could be associated with the higher consumption of fruit juices, possibly because of the higher glycaemic load and degradation of bioactive compounds during processing.
Possible use of gastroretentive systems for the management of type 2 diabetes
Several reports have indicated a positive association between the consumption of ACNs, reduced risk, and management of T2D (Stull et al., Citation2010; Törrönen et al., Citation2010, Citation2012a,Citationb; Wedick et al., Citation2012; Hoggard et al., Citation2013; Muraki et al., Citation2013; Samieri et al., Citation2014). However, most studies presented in this review used complex fruit extracts that contain several ACN in addition to other plant metabolites. For instance, Takikawa et al. (Citation2010) identified 15 ACN in the bilberry extract used in their study. In a different study, Rojo et al. (Citation2012) isolated and identified delphinidin 3-sambubioside-5-glucoside as the ACN partially responsible for improvement of glucose tolerance and blood concentration. However, this compound represented only 6.76% of the total ACN in the extract. In addition, it should be noted that the doses used in in vitro and in vivo studies with rodents to investigate the health-promoting effects of ACN are often above the quantities commonly consumed in a normal human diet, as in the study by Al-Awwadi et al. (Citation2004), Heo & Lee (Citation2005), Hidalgo et al. (Citation2012), and Tarozzi et al. (Citation2007, Citation2010).
The extrapolation of the results obtained in vitro are also limited, even though they can provide some indication of the mechanisms involved in the antidiabetic properties exhibited by polyphenols. ACNs are highly metabolized in vivo, and it is unlikely that the compounds that exert the activities are the ones found in the fruit. For example, Ferrars et al. (Citation2014) recently identified 25 metabolites of cyanidin 3-glucoside in body fluids. Thus, the presence of numerous ACNs (and other polyphenols) in the same extract can contribute to the complexity involved in establishing the association.
The specific absorption of ACN glycosides, which are the forms of ANC found in foods and studied in vitro, could be increased by prolonging their residence time in the upper GI tract and by providing a means for controlled release, which would also contribute to reduce their degradation due to changes in pH and metabolism.
ACN are generally stable under the acidic environment of the stomach (pH < 2) (McDougall et al., Citation2005; He et al., Citation2009; Liang et al., Citation2012; Stalmach et al., Citation2012; Liu et al., Citation2014) as they are likely to be found in the stable flavylium cation form (Brouillard & Dubois, Citation1977). Although the stomach is not often viewed as an absorption site, research has suggested that it could have a role in the absorption of ACNs (Felgines et al., Citation2007), which would help to explain the rapid detection of these compounds in the plasma (Vanzo et al., Citation2011). For example, the in situ gastric administration of purified ACNs extracted from blackberry and bilberry in anesthetized Wistar rats for 30 min resulted in the absorption of approximately 25% of the ACN monoglycosides (cyanidin and malvidin glucoside or galactoside). Cyanidin 3-glucoside metabolites were identified in the bile within 20 min (Talavéra et al., Citation2003). The study by He et al. (Citation2009) showed that ACNs were detected in urine within 30 min after stomach intubation of black raspberry extract. Studies have also shown that the absorption of ACNs is increased with phytic acid (Matsumoto et al., Citation2007) and delayed with dairy cream (Mullen et al., Citation2008), corroborating the involvement of the stomach with their absorption.
However, the flavylium cation form has limited chances of being absorbed by passive diffusion (Lipinski et al., Citation1997; Santos et al., Citation2011), and evidence has suggested that bilitranslocase (TCDB 2.A.65.1.1) (Saier, Citation2000) is involved in the absorption of ACNs in the stomach (Passamonti et al., Citation2003,Citation2005). Bilitranslocase is an ATP-independent uniporter carrier protein first identified in the liver (Sottocasa et al., Citation1989), where its role is in assisting the hepatic detoxification process and transporting organic anions from the blood into the hepatocytes. This protein has also been identified in rodent intestine (Battiston et al., Citation1999) and kidney (Elias et al., Citation1990), and the vascular endothelium of rats and humans (Maestro et al., Citation2010). It should be noted that under certain conditions, this carrier can reach saturation, i.e. increasing the concentration of ACN has resulted in an observable reduction in the amount absorbed (Talavéra et al., Citation2003; Kurilich et al., Citation2005; Fernandes et al., Citation2012).
The ACN that are not absorbed in the stomach are subject to extensive degradation by changes in intestinal pH (pH 7.5–8.0) (McDougall et al., Citation2005; Liu et al., Citation2014) and metabolism by intestinal microbiota (Fleschhut et al., Citation2005) and phase II enzymes (Fleschhut et al., Citation2005; Kay et al., Citation2005; Czank et al., Citation2013; Ferrars et al., Citation2014). As the pH is increased, the structure of ACN transforms into a pseudobase, quinoidal base, and then a chalcone, which is subject to nucleophilic attack by water (Brouillard & Delaporte, Citation1977; Brouillard & Dubois, Citation1977; Oliveira et al., Citation2006). These transformations would limit the amount of ACN glycosides that can reach the various tissues and sites in the body in their native form and, consequently, affect the translation of results from in vitro experiments into in vivo evidence.
Researchers have also shown that the administration of high concentrations of ACN often do not result in a proportionally higher bioavailability and biological effect (Charron et al., Citation2009; Adisakwattana et al., Citation2011; Banaszewski et al., Citation2013; Wei, Citation2014; Keane et al., Citation2015). For instance, Adisakwattana et al. (Citation2011) investigated the effects of three concentrations of cyanidin 3-rutinoside (30, 100, and 300 mg/kg) in lowering blood glucose concentration in Wistar rats in comparison to a control (distilled water), followed by the administration of a maltose or sucrose solution (3 g/kg). The highest concentrations (100 and 300 mg/kg) had a similar and significant effect on the AUC of glucose during the time of the experiment (180 min) in comparison to the control and prevented the increase in concentration potentially due to an inhibition of α-glucosidase (Adisakwattana et al., Citation2011). In healthy volunteers, Charron et al. (Citation2009) showed that the ingestion of different amounts of purple carrot juice (50, 150, and 250 mL) resulted in similar AUC for 150- and 250-mL treatments, which could indicate that the absorption could have been saturated. Keane et al. (Citation2015) also showed a similar AUC and maximum concentration of protocatechuic acid, an ACN metabolite (Vitaglione et al., Citation2007; Ferrars et al., Citation2014), after ingestion of 30 and 60 mL of Montmorency tart cherry concentrate by healthy volunteers.
In this context, GRS can be a promising delivery platform to modulate the release and absorption of ACN for the management and control of T2D and for increased health benefits. The sustained release of ACN glycosides would also prevent the saturation of carriers (such as bilitranslocase) and reduce the concentrations needed to observe an effect in vivo. As it has been shown that the uptake of parent ACN by bilitranslocase is rapid (Talavéra et al., Citation2003; Vanzo et al., Citation2011), the goal should be to control the release of ACN over time instead of increasing the concentration. Previous research has shown, for example, that GRS can improve the absorption and bioavailability of drugs with significantly higher AUC in comparison to conventional delivery systems, such as determined for ofloxacin (Shakyaa et al., Citation2013), theophylline (Miyazaki et al., Citation2008), and cefuroxime (Bomma & Veerabrahma, Citation2013).
This section provides an overview of the gastric motility and the different strategies that can be explored to increase the retention time of ACNs in the upper GI tract.
Gastric motility and emptying
The gastric motility pattern can be distinguished between fasted and fed states (Dooley et al., Citation1992). Undigested particles from ingested food and mucus residue remain in the stomach until approximately 2 h after the digested food has left this organ (Read et al., Citation1986). Migrating myoelectric complex (MMC) or housekeeper contractions are responsible for removing the residues left in the stomach after digestion through strong and cyclic contractions against an open pylorus. This cycle is divided into four phases based on the contraction strength and repeated every 2 h until a meal is eaten and digestion starts (Code & Marlett, Citation1975).
The time it takes to empty the stomach depends on the physical state of the food (Urbain et al., Citation1989; Vincent et al., Citation1995; Olausson et al., Citation2008), its quantity (Hunt & Spurrell, Citation1951), composition (Gentilcore et al., Citation2006), and caloric content (Moore et al., Citation1981). The presence of food will induce a fed pattern of motility, with regular tonic and peristaltic contractions (de Wever et al., Citation1978). At this stage, peristaltic waves will mix, grind, and empty the stomach simultaneously. Emptying is a critical step during digestion and occurs when the solids have been reduced to particles smaller than approximately 2 mm (Vincent et al., Citation1995). It has been suggested that particles up to 10 mm can be emptied from the stomach in the fed state in humans (Davis, Citation2005).
In relation to the food consistency, Clark et al. (Citation1993) evaluated the effect of isotopically labeled solid, semisolid, and liquid meals on gastric pH and emptying time. They reported that the rate of gastric emptying was higher for liquid and semisolid meals, varying from 9.8 min to 103.3 min (mean 35.6 min) and 33.5–120 min (mean 47.4 min), respectively, while for solid meals the range was 45.0–103.8 min (mean 72.0 min) (Clark et al., Citation1993). Liquid emptying occurs as first-order kinetics, with emptying being directly proportional to the volume present in the stomach (Hunt & Spurrell, Citation1951). In the case of solids, studies have shown a biphasic pattern: little emptying occurs initially (lag phase, possibly the time required to reduce the particle size), followed by linear emptying (independent of gastric volume) (Fallatah et al., Citation2013).
In relation to the composition and caloric density, approximately 2–4 kcal are delivered to the duodenum by the stomach per minute. Meals with similar energy content are emptied from the stomach at similar rates, and this rate is unlikely to be affected by consistency or composition (Faas et al., Citation2002). It has also been suggested that food could form layers in the body of the stomach when an upright position is adopted and if the meal contains a sufficient amount of fat (Wiggins & Dawson, Citation1961) – this would be due to density differences and weak peristaltic contractions, which would separate fat from water. Another explanation would be the lower rate of fat absorption in the intestine (Lin et al., Citation1996).
The rate of gastric emptying is also influenced by biological factors, such as body mass index (Brogna et al., Citation1998; Jackson et al., Citation2004), hormonal factors (Hutson et al., Citation1989), gender (Lorena et al., Citation2000), glycemia (Woerle et al., Citation2008), posture (Steingoetter et al., Citation2006), stress (Mistiaen et al., Citation2001; Ochi et al., Citation2008), and pathological states (Hardoff et al., Citation2001; Buckles et al., Citation2004). For instance, Hardoff et al. (Citation2001) showed that the emptying time is delayed in patients with Parkinson’s disease in comparison with healthy volunteers.
Classes of gastroretentive systems
GRS differ from conventional delivery systems because they remain in the stomach for a longer period of time, prolonging the residence time of the bioactive in the upper GI tract (Joseph et al., Citation2002). Longer gastric retention can be achieved by different strategies, such as muco-adhesion (Md et al., Citation2011), flotation (Ichikawa et al., Citation1991), high density systems (Bechgaard & Ladefoged, Citation1978), and expansion (Urquhart & Theeuwes, Citation1984). An overview of these different classes of GRS, highlighting the ones that are interesting for food applications, is presented in . This section will review different strategies that can be used for the development of GRS that may be of interest for the food industry, based on information described in the pharmaceutical literature.
Figure 1. Overview of different GRS. GRS applicable for food applications are highlighted with double lines. The other approaches (dotted lines) will not be discussed in this review as they may involve the use of non-GRAS solvents or polymers and be less suitable for the food industry.
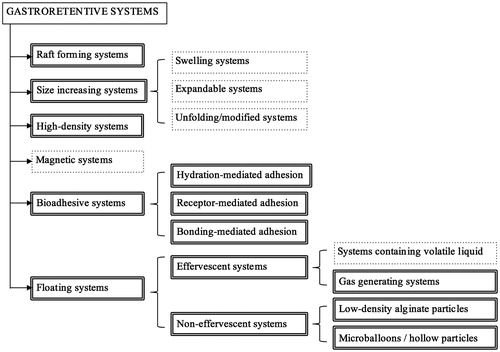
Raft-forming systems
These systems consist of liquid hydrogels (e.g. alginates) that gel when in contact with body fluids or changes in pH (Kubo et al., Citation2004), forming a continuous layer that is termed the raft. The systems are often formulated with gas-generating compounds (e.g. carbonate salts) that release CO2 in the acidic conditions of the stomach. The gas is entrapped in the gel matrix, making the system less dense and contributing to its buoyancy in relation to the gastric content (Tang et al., Citation2010), allowing it to float. For example, Ibrahim (Citation2009) showed that a system consisting of sodium alginate and a gas-generating compound (release of CO2 for buoyancy) gelled instantaneously once in contact with simulated gastric fluid (0.1 N HCl) and remained intact and buoyant for more than 48 h.
High-density (or sinking) systems
Hoelzel (Citation1930) provided the first evidence that the GI transit time could be prolonged by increasing the density of a dosage form. These systems have a density higher than the gastric fluids, which is designed to delay gastric emptying (Clark et al., Citation1993). Clarke et al. (Citation1995) proposed that the critical density to observe a prolonged residence time in the stomach is between 2.4 and 2.8 g cm−3. Devereux et al. (Citation1990) showed that pellets with density of 2.8 g cm−3 stayed longer in the stomach that were either in fed and fasted states than pellets with 1.5 g cm−3 density. The density of the GRS can be increased by coating it with a heavy inert material (e.g. zinc oxide) (Singh & Kim, Citation2000); however, the use of high-density GRS as a platform to increase the bioavailability of certain compounds has been questioned (Rouge et al., Citation1998).
Bioadhesive systems
In the context of this review, bioadhesion refers to the binding of a natural or synthetic polymer (in the form of a delivery system) to a biological substrate, such as a mucous layer (hence the term mucoadhesion) (Henriksen et al., Citation1996). Different theories proposed to explain the adhesion process have been reviewed (Boddupalli et al., Citation2010; Shaikh et al., Citation2011).
Low-density (or floating) systems
One of the first descriptions of floating systems was made by Davis (Citation1968), who employed pills with density below 1.0 g ml−1 to overcome issues reported by patients when swallowing medicinal pills. However, it has also been reported that sufficient liquid, such as a glassful of water (200–250 mL), is necessary to facilitate the floatability of these systems (Soppimath et al., Citation2001). The presence of liquid will result in emptying the stomach following a first-order process with half-time of ∼30–50 min. If no liquid is ingested during this period, after 2 h there will be insufficient fluid remaining within the stomach on which the unit can float (Davis et al., Citation1986), which would result in the elimination of the GRS.
To promote buoyancy, both effervescent and non-effervescent systems have been used. Effervescent floating drug delivery system (FDDS) combines swellable polymers, such as chitosan, and effervescent compounds (sodium bicarbonate, citric or tartaric acid). For instance, the optimal stoichiometric ratio of citric acid and sodium bicarbonate has been determined as 0.76:1 (Saxena et al., Citation2014). Another approach is the use of resin beads loaded with the gas-generating agent, which is later coated with ethyl cellulose. Since this polymer is insoluble but permeable, it will allow the permeation of water and entrapment of carbon dioxide (Saxena et al., Citation2014).
Non-effervescent FDDS are based on gel-forming or swellable polymers, such as hydrocolloids, polysaccharides, and matrix-forming compounds (e.g. polycarbonate). It is expected that these polymers will swell once in contact with gastric pH and trap gas within their structure, maintaining their integrity (Singh & Kim, Citation2000).
Although GRS could potentially improve the delivery of ACN for the management of diseases, such as T2D, more research is needed to determine the most appropriate GRS platforms for the delivery of ACN and their implications on degenerative diseases.
Conclusions
There is evidence that ACN could potentially be used to effectively manage and control degenerative diseases such as T2D (Stull et al., Citation2010; Törrönen et al., Citation2010, Citation2012a,Citationb; Wedick et al., Citation2012; Hoggard et al., Citation2013; Muraki et al., Citation2013; Samieri et al., Citation2014). GRS could potentially deliver and maintain ACN glycosides obtained from plant sources in the upper GI tract where their stability and absorption are favored. As discussed in this review, there are different categories of GRS that may be applicable to the food industry, using materials that are GRAS (generally recognized as safe). More research is needed in the formulation and characterization of the best GRS to be used for the administration of ACN for T2D and other degenerative diseases. In addition, GRS may be a suitable vehicle for other food-derived extracts that are rich in bioactive compounds, to increase bioavailability and maximize the potential health benefits, and potentially reducing the current gap when translating in vitro effects into clinical responses.
Acknowledgements
The authors acknowledge the National Council for Research and Development (CNPq – Brazil) and the Natural Sciences and Engineering Research Council (NSERC) of Canada for financial support.
Declaration of interest
The authors report no declarations of interest.
References
- Adisakwattana S, Yibchok-Anun S, Charoenlertkul P, Wongsasiripat N. (2011). Cyanidin-3-rutinoside alleviates postprandial hyperglycemia and its synergism with acarbose by inhibition of intestinal α-glucosidase. J Clin Biochem Nutr 49:36–41
- Al-Awwadi N, Azay J, Poucheret P, et al. (2004). Antidiabetic activity of red wine polyphenolic extract, ethanol, or both in streptozotocin-treated rats. J Agric Food Chem 52:1008–16
- Asgary S, RafieianKopaei M, Sahebkar A, et al. (2016). Anti-hyperglycemic and anti-hyperlipidemic effects of Vaccinium myrtillus fruit in experimentally induced diabetes (antidiabetic effect of Vaccinium mirtillus fruit). J Sci Food Agric 96:764–8
- Ataie-Jafari A, Hosseini S, Karimi F, Pajouhi M. (2008). Effects of sour cherry juice on blood glucose and some cardiovascular risk factors improvements in diabetic women: a pilot study. Nutr Food Sci 38:355–60
- Banaszewski K, Park E, Edirisinghe I, et al. (2013). A pilot study to investigate bioavailability of strawberry anthocyanins and characterize postprandial plasma polyphenols absorption patterns by Q-TOF LC/MS in humans. J Berry Res 3:113–26
- Banini AE, Boyd LC, Allen JC, et al. (2006). Muscadine grape products intake, diet and blood constituents of non-diabetic and type 2 diabetic subjects. Nutrition 22:1137–45
- Battiston L, Macagno A, Passamonti S, et al. (1999). Specific sequence-directed anti-bilitranslocase antibodies as a tool to detect potentially bilirubin-binding proteins in different tissues of the rat. FEBS Lett 453:351–5
- Bechgaard H, Ladefoged K. (1978). Distribution of pellets in the gastrointestinal tract. The influence on transit time exerted by the density or diameter of pellets. J Pharm Pharmacol 30:690–2
- Boddupalli BM, Mohammed ZNK, Nath RA, Banji D. (2010). Mucoadhesive drug delivery system: an overview. J Adv Pharm Technol Res 1:381–7
- Bomma R, Veerabrahma K. (2013). Development of gastroretentive drug delivery system for cefuroxime axetil: in vitro and in vivo evaluation in human volunteers. Pharm Dev Technol 18:1230–7
- Brogna A, Ferrara R, Bucceri AM, et al. (1998). Gastric emptying rates of solid food in relation to body mass index: an ultrasonographic and scintigraphic study. Eur J Radiol 27:258–63
- Brouillard R, Delaporte B. (1977). Chemistry of anthocyanin pigment. 2. Kinetic and thermodynamic study of proton transfer, hydration, and tautomeric reactions of malvidin 3-glucoside. J Am Chem Soc 99:8461–8
- Brouillard R, Dubois J-E. (1977). Mechanisms of the structural transformations of anthocyanins in acidic media. J Am Chem Soc 99:1359–64
- Brouwer IA, van Dusseldorp M, West CE, et al. (1999). Dietary folate from vegetables and citrus fruit decreases plasma homocysteine concentrations in humans in a dietary controlled trial. J Nutr 129:1135–9
- Buckles DC, Sarosiek I, McMillin C, McCallum RW. (2004). Delayed gastric emptying in gastroesophageal reflux disease: reassessment with new methods and symptomatic correlations. Am J Med Sci 327:1–4
- Cassidy A, Mukamal KJ, Liu L, et al. (2013). High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 127:188–96
- Cassidy A, O’Reilly ÉJ, Kay C, et al. (2011). Habitual intake of flavonoid subclasses and incident hypertension in adults. Am J Clin Nutr 93:338–47
- Chambers BK, Camire ME. (2003). Can cranberry supplementation benefit adults with type 2 diabetes? Diabetes Care 26:2695–6
- Charron CS, Kurilich AC, Clevidence BA, et al. (2009). Bioavailability of anthocyanins from purple carrot juice: effects of acylation and plant matrix. J Agric Food Chem 57:1226–30
- Chen R, Meseck M, McEvoy RC, Woo SLC. (2000). Glucose-stimulated and self-limiting insulin production by glucose 6-phosphatase promoter driven insulin expression in hepatoma cells. Gene Ther 7:1802–9
- Choi M-K, Park S-J, Eom SH, Kang M-H. (2013). Anti-diabetic and hypolipidemic effects of purple-fleshed potato in streptozotocin-induced diabetic rats. Food Sci Biotechnol 22:1–6
- Clark GWB, Jamieson IR, Hinder RA, et al. (1993). The relationship between gastric pH and the emptying of solid, semisolid and liquid meals. Neurogastroenterol Motil 5:273–9
- Clarke GM, Newton JM, Short MB. (1995). Comparative gastrointestinal transit of pellet systems of varying density. Int J Pharm 114:1–11
- Code CF, Marlett JA. (1975). The interdigestive myo-electric complex of the stomach and small bowel of dogs. J Physiol (Lond) 246:289–309
- Czank C, Cassidy A, Zhang Q, et al. (2013). Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: a 13C-tracer study. Am J Clin Nutr 97:995–1003
- Davis DW. (1968). Method of swallowing a pill, US Patent No. 3,418,999. Washington (DC): US Patent and Trademark Office
- Davis SS. (2005). Formulation strategies for absorption windows. Drug Discov Today 10:249–57
- Davis SS, Stockwell AF, Taylor MJ, et al. (1986). The effect of density on the gastric emptying of single- and multiple-unit dosage forms. Pharm Res 3:208–13
- de Wever I, Eeckhout C, Vantrappen G, Hellemans J. (1978). Disruptive effect of test meals on interdigestive motor complex in dogs. Am J Physiol 235:E661–5
- Delgado-Vargas F, Jiménez AR, Paredes-López O. (2000). Natural pigments: carotenoids, anthocyanins, and betalains-characteristics, biosynthesis, processing, and stability. Crit Rev Food Sci Nutr 40:173–289
- Devereux JE, Newton JM, Short MB. (1990). The influence of density on the gastrointestinal transit of pellets. J Pharm Pharmacol 42:500–1
- Dooley CP, Di Lorenzo C, Valenzuela JE. (1992). Variability of migrating motor complex in humans. Dig Dis Sci 37:723–8
- Elias MM, Lunazzi GC, Passamonti S, et al. (1990). Bilitranslocase localization and function in basolateral plasma membrane of renal proximal tubule in rat. Am J Physiol 259:F559–64
- Faas H, Steingoetter A, Feinle C, et al. (2002). Effects of meal consistency and ingested fluid volume on the intragastric distribution of a drug model in humans – a magnetic resonance imaging study. Alim Pharmacol Ther 16:217–24
- Fallatah B, AzizShehry A, Abdelsamad L, et al. (2013). Comparison study of gastric emptying after performing sleeve gastrectomy with two differente techniques. Global J Surg 1:53–6
- Fang J. (2014). Some anthocyanins could be efficiently absorbed across the gastrointestinal mucosa: extensive presystemic metabolism reduces apparent bioavailability. J Agric Food Chem 62:3904–11
- Faramarzi S, Pacifico S, Yadollahi A, et al. (2015). Red-fleshed apples: old autochthonous fruits as a novel source of anthocyanin antioxidants. Plant Foods Hum Nutr 70:324–30
- Faria A, Fernandes I, Norberto S, et al. (2014). Interplay between anthocyanins and gut microbiota. J Agric Food Chem 62:6898–902
- Felgines C, Texier O, Besson C, et al. (2007). Strawberry pelargonidin glycosides are excreted in urine as intact glycosides and glucuronidated pelargonidin derivatives in rats. Br J Nutr 98:1126–31
- Fernandes I, de Freitas V, Reis C, Mateus N. (2012). A new approach on the gastric absorption of anthocyanins. Food Funct 3:508–16
- Ferrars RM, Czank C, Zhang Q, et al. (2014). The pharmacokinetics of anthocyanins and their metabolites in humans. Br J Pharmacol 171:3268–82
- Fischer UA, Jaksch AV, Carle R, Kammerer DR. (2013). Influence of origin source, different fruit tissue and juice extraction methods on anthocyanin, phenolic acid, hydrolysable tannin and isolariciresinol contents of pomegranate (Punica granatum L.) fruits and juices. Eur Food Res Technol 237:209–21
- Fleschhut J, Kratzer F, Rechkemmer G, Kulling SE. (2005). Stability and biotransformation of various dietary anthocyanins in vitro. Eur J Nutr 45:7–18
- Foster MC, Leapman RD, Li MX, Atwater I. (1993). Elemental composition of secretory granules in pancreatic islets of Langerhans. Biophys J 64:525–32
- Galante P, Mosthaf L, Kellerer M, et al. (1995). Acute hyperglycemia provides an insulin-independent inducer for GLUT4 translocation in C2C12 myotubes and rat skeletal muscle. Diabetes 44:646–51
- Gentilcore D, Chaikomin R, Jones KL, et al. (2006). Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J Clin Endocrinol Metab 91:2062–7
- Grace MH, Ribnicky DM, Kuhn P, et al. (2009). Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine 16:406–15
- Gravena C, Mathias PC, Ashcroft SJH. (2002). Acute effects of fatty acids on insulin secretion from rat and human islets of Langerhans. J Endocrinol 173:73–80
- Guo H, Ling W. (2015). The update of anthocyanins on obesity and type 2 diabetes: experimental evidence and clinical perspectives. Rev Endocr Metab Disord 16:1–13
- Guo H, Ling W, Wang Q, et al. (2007). Effect of anthocyanin-rich extract from black rice (Oryza sativa L. indica) on hyperlipidemia and insulin resistance in fructose-fed rats. Plant Foods Hum Nutr 62:1–6
- Hardoff R, Sula M, Tamir A, et al. (2001). Gastric emptying time and gastric motility in patients with Parkinson’s disease. Mov Disord 16:1041–7
- He J, Wallace TC, Keatley KE, et al. (2009). Stability of black raspberry anthocyanins in the digestive tract lumen and transport efficiency into gastric and small intestinal tissues in the rat. J Agric Food Chem 57:3141–8
- Hectors TLM, Vanparys C, Pereira-Fernandes A, et al. (2012). Mechanistic evaluation of the insulin response in H4IIE hepatoma cells: new endpoints for toxicity testing? Toxicol Lett 212:180–9
- Henriksen I, Green KL, Smart JD, et al. (1996). Bioadhesion of hydrated chitosans: an in vitro and in vivo study. Int J Pharm 145:231–40
- Heo HJ, Lee CY. (2005). Strawberry and its anthocyanins reduce oxidative stress-induced apoptosis in PC12 cells. J Agric Food Chem 53:1984–9
- Hidalgo M, Martin-Santamaria S, Recio I, et al. (2012). Potential anti-inflammatory, anti-adhesive, anti/estrogenic, and angiotensin-converting enzyme inhibitory activities of anthocyanins and their gut metabolites. Genes Nutr 7:295–306
- Hoelzel F. (1930). The rate of passage of inert materials through the digestive tract. Am J Physiol 92:466–97
- Hoggard N, Cruickshank M, Moar K-M, et al. (2013). A single supplement of a standardised bilberry (Vaccinium myrtillus L.) extract (36% wet weight anthocyanins) modified glycaemic response in individuals with type 2 diabetes controlled by diet and lifestyle. J Nutr Sci 2:e22
- Hunt JN, Spurrell WR. (1951). The pattern of emptying of the human stomach. J Physiol (Lond) 113:157–68
- Hutson WR, Roehrkasse RL, Wald A. (1989). Influence of gender and menopause on gastric emptying and motility. Gastroenterology 96:11–17
- Ibrahim HK. (2009). A novel liquid effervescent floating delivery system for sustained drug delivery. Drug Discov Ther 3:168–75
- Ichikawa M, Kato T, Kawahara M, et al. (1991). A new multiple-unit oral floating dosage system. II: in vivo evaluation of floating and sustained-release characteristics with p-aminobenzoic acid and isosorbide dinitrate as model drugs. J Pharm Sci 80:1153–6
- International Diabetes Federation. (2013). IDF Diabetes Atlas. Brussels: Author
- Ishihara H, Maechler P, Gjinovci A, et al. (2003). Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nat Cell Biol 5:330–5
- Iwai K, Onodera A, Matsue H. (2004). Inhibitory effects of Viburnum dilatatum Thunb. (Gamazumi) on oxidation and hyperglycemia in rats with streptozotocin-induced diabetes. J Agric Food Chem 52:1002–7
- Jackson SJ, Leahy FE, McGowan AA, et al. (2004). Delayed gastric emptying in the obese: an assessment using the non-invasive (13)C-octanoic acid breath test. Diabetes Obes Metab 6:264–70
- Jankowski A, Jankowska A, Niedworok J. (2000). The effect of anthocyanin dye from grapes on experimental diabetes. Folia Med Cracov 41:5–15
- Jayaprakasam B, Olson LK, Schutzki RE, et al. (2006). Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in cornelian cherry (Cornus mas). J Agric Food Chem 54:243–8
- Jayaprakasam B, Vareed SK, Olson LK, Nair MG. (2005). Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J Agric Food Chem 53:28–31
- Joseph NJ, Lakshmi S, Jayakrishnan A. (2002). A floating-type oral dosage form for piroxicam based on hollow polycarbonate microspheres: in vitro and in vivo evaluation in rabbits. J Control Release 79:71–9
- Kahn BB. (1998). Type 2 diabetes: when insulin secretion fails to compensate for insulin resistance. Cell 92:593–6
- Kay CD, Mazza G, Holub BJ. (2005). Anthocyanins exist in the circulation primarily as metabolites in adult men. J Nutr 135:2582–8
- Keane KM, Bell PG, Lodge JK, et al. (2015). Phytochemical uptake following human consumption of Montmorency tart cherry (L. Prunus cerasus) and influence of phenolic acids on vascular smooth muscle cells in vitro. Eur J Nutr. [Epub ahead of print]. doi: 10.1007/s00394-015-0988-9
- Kelsey N, Hulick W, Winter A, et al. (2011). Neuroprotective effects of anthocyanins on apoptosis induced by mitochondrial oxidative stress. Nutr Neurosci 14:249–59
- Krepinsky J, Ingram AJ, Clase CM. (2000). Prolonged sulfonylurea-induced hypoglycemia in diabetic patients with end-stage renal disease. Am J Kidney Dis 35:500–5
- Kubo W, Konno Y, Miyazaki S, Attwood D. (2004). In situ gelling pectin formulations for oral sustained delivery of paracetamol. Drug Dev Ind Pharm 30:593–9
- Kurilich AC, Clevidence BA, Britz SJ, et al. (2005). Plasma and urine responses are lower for acylated vs nonacylated anthocyanins from raw and cooked purple carrots. J Agric Food Chem 53:6537–42
- Li D, Zhang Y, Liu Y, et al. (2015). Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J Nutr 145:742–8
- Liang L, Wu X, Zhao T, et al. (2012). In vitro bioaccessibility and antioxidant activity of anthocyanins from mulberry (Morus atropurpurea Roxb.) following simulated gastro-intestinal digestion. Food Res Int 46:76–82
- Lin HC, Zhao X-T, Wang L. (1996). Fat absorption is not complete by midgut but is dependent on load of fat. Am J Physiol 271:G62–7
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. (1997). Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Delivery Rev 23:3–25
- Liu Y, Zhang D, Wu Y, et al. (2014). Stability and absorption of anthocyanins from blueberries subjected to a simulated digestion process. Int J Food Sci Nutr 65:440–8
- Lorena SLS, Tinois E, Hirata ES, et al. (2000). Scintigraphic study of gastric emptying and intragastric distribution of a solid meal: gender differences. Arq Gastroenterol 37:102–6
- Maestro A, Terdoslavich M, Vanzo A, et al. (2010). Expression of bilitranslocase in the vascular endothelium and its function as a flavonoid transporter. Cardiovasc Res 85:175–83
- Martineau LC, Couture A, Spoor D, et al. (2006). Anti-diabetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomedicine 13:612–23
- Matsui T, Ebuchi S, Kobayashi M, et al. (2002). Anti-hyperglycemic effect of diacylated anthocyanin derived from Ipomoea batatas cultivar Ayamurasaki can be achieved through the alpha-glucosidase inhibitory action. J Agric Food Chem 50:7244–8
- Matsumoto H, Ito K, Yonekura K, et al. (2007). Enhanced absorption of anthocyanins after oral administration of phytic acid in rats and humans. J Agric Food Chem 55:2489–96
- McDougall GJ, Dobson P, Smith P, et al. (2005). Assessing potential bioavailability of raspberry anthocyanins using an in vitro digestion system. J Agric Food Chem 53:5896–904
- Md S, Ahuja A, Khar RK, et al. (2011). Gastroretentive drug delivery system of acyclovir-loaded alginate mucoadhesive microspheres: formulation and evaluation. Drug Deliv 18:255–64
- Mistiaen W, Blockx P, Van Hee R, et al. (2001). The effect of stress on gastric emptying rate measured with a radionuclide tracer. Hepatogastroenterology 49:1457–60
- Miyazaki Y, Yakou S, Takayama K. (2008). Comparison of gastroretentive microspheres and sustained-release preparations using theophylline pharmacokinetics. J Pharm Pharmacol 60:693–8
- Moore JG, Christian PE, Coleman RE. (1981). Gastric emptying of varying meal weight and composition in man. Dig Dis Sci 26:16–22
- Mullen W, Edwards CA, Serafini M, Crozier A. (2008). Bioavailability of pelargonidin-3-O-glucoside and its metabolites in humans following the ingestion of strawberries with and without cream. J Agric Food Chem 56:713–19
- Muraki I, Imamura F, Manson JE, et al. (2013). Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ 347:f5001
- Nathan DM, Buse JB, Davidson MB, et al. (2009). Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 32:193–203
- Nedachi T, Kanzaki M. (2006). Regulation of glucose transporters by insulin and extracellular glucose in C2C12 myotubes. Am J Physiol Endocrinol Metab 291:E817–28
- Ochi M, Tominaga K, Tanaka F, et al. (2008). Effect of chronic stress on gastric emptying and plasma ghrelin levels in rats. Life Sci 82:862–8
- Olausson EA, Alpsten M, Larsson A, et al. (2008). Small particle size of a solid meal increases gastric emptying and late postprandial glycaemic response in diabetic subjects with gastroparesis. Diabetes Res Clin Pract 80:231–7
- Oliveira J, Fernandes V, Miranda C, et al. (2006). Color properties of four cyanidin-pyruvic acid adducts. J Agric Food Chem 54:6894–903
- Pascual-Teresa S, Moreno DA, García-Viguera C. (2010). Flavanols and anthocyanins in cardiovascular health: a review of current evidence. Int J Mol Sci 11:1679–703
- Passamonti S, Terdoslavich M, Margon A, et al. (2005). Uptake of bilirubin into HepG2 cells assayed by thermal lens spectroscopy. Function of bilitranslocase. FEBS J 272:5522–35
- Passamonti S, Vrhovsek U, Vanzo A, Mattivi F. (2003). The stomach as a site for anthocyanins absorption from food. FEBS Lett 544:210–13
- Proteggente AR, Pannala AS, Paganga G, et al. (2002). The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Rad Res 36:217–33
- Read NW, Al-Janabi MN, Holgate AM, et al. (1986). Simultaneous measurement of gastric emptying, small bowel residence and colonic filling of a solid meal by the use of the gamma camera. Gut 27:300–8
- Rojo LE, Ribnicky D, Logendra S, et al. (2012). In vitro and in vivo anti-diabetic effects of anthocyanins from maqui berry (Aristotelia chilensis). Food Chem 131:387–96
- Rouge N, Allémann E, Gex-Fabry M, et al. (1998). Comparative pharmacokinetic study of a floating multiple-unit capsule, a high-density multiple-unit capsule and an immediate-release tablet containing 25 mg atenolol. Pharm Acta Helv 73:81–7
- Saier MH. (2000). A functional-phylogenetic classification system for transmembrane solute transporters. Microbiol Mol Biol Rev 64:354–411
- Samieri C, Sun Q, Townsend MK, et al. (2014). Dietary flavonoid intake at midlife and healthy aging in women. Am J Clin Nutr 100:1489–97
- Santos AC, Veiga F, Ribeiro AJ. (2011). New delivery systems to improve the bioavailability of resveratrol. Expert Opin Drug Deliv 8:973–90
- Sasaki R, Nishimura N, Hoshino H, et al. (2007). Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochem Pharmacol 74:1619–27
- Saxena A, Gaur K, Singh V, et al. (2014). Floating microspheres as drug delivery system. AJPPS 1:27–36
- Schelleman H, Han X, Brensinger CM, et al. (2014). Pharmacoepidemiologic and in vitro evaluation of potential drug-drug interactions of sulfonylureas with fibrates and statins. Br J Clin Pharmacol 78:639–48
- Seymour EM, Singer AAM, Kirakosyan A, et al. (2008). Altered hyperlipidemia, hepatic steatosis, and hepatic peroxisome proliferator-activated receptors in rats with intake of tart cherry. J Med Food 11:252–9
- Shaikh R, Singh TRR, Garland MJ, et al. (2011). Mucoadhesive drug delivery systems. J Pharm BioAllied Sci 3:89–100
- Shakyaa R, Thapa P, Saha RN. (2013). In vitro and in vivo evaluation of gastroretentive floating drug delivery system of ofloxacin. Asian J Pharm Sci 8:191–8
- Singh BN, Kim KH. (2000). Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J Control Release 63:235–59
- Soppimath KS, Kulkarni AR, Rudzinski WE, Aminabhavi TM. (2001). Microspheres as floating drug-delivery systems to increase gastric retention of drugs. Drug Metab Rev 33:149–60
- Sottocasa GL, Lunazzi GC, Tiribelli C. (1989). Isolation of bilitranslocase, the anion transporter from liver plasma membrane for bilirubin and other organic anions. Meth Enzymol 174:50–7
- Stalmach A, Edwards CA, Wightman JD, Crozier A. (2012). Gastrointestinal stability and bioavailability of (poly)phenolic compounds following ingestion of Concord grape juice by humans. Mol Nutr Food Res 56:497–509
- Steingoetter A, Fox M, Treier R, et al. (2006). Effects of posture on the physiology of gastric emptying: a magnetic resonance imaging study. Scand J Gastroenterol 41:1155–64
- Stull AJ, Cash KC, Johnson WD, et al. (2010). Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J Nutr 140:1764–8
- Surwit RS, Kuhn CM, Cochrane C, et al. (1988). Diet-induced type II diabetes in C57BL/6J mice. Diabetes 37:1163–7
- Törrönen R, Kolehmainen M, Sarkkinen E, et al. (2012a). Postprandial glucose, insulin, and free fatty acid responses to sucrose consumed with blackcurrants and lingonberries in healthy women. Am J Clin Nutr 96:527–33
- Törrönen R, McDougall GJ, Dobson G, et al. (2012b). Fortification of blackcurrant juice with crowberry: impact on polyphenol composition, urinary phenolic metabolites, and postprandial glycemic response in healthy subjects. J Funct Foods 4:746–56
- Törrönen R, Sarkkinen E, Tapola N, et al. (2010). Berries modify the postprandial plasma glucose response to sucrose in healthy subjects. Br J Nutr 103:1094–7
- Takahashi A, Okazaki Y, Nakamoto A, et al. (2014). Dietary anthocyanin-rich haskap phytochemicals inhibit postprandial hyperlipidemia and hyperglycemia in rats. J Oleo Sci 63:201–9
- Takikawa M, Inoue S, Horio F, Tsuda T. (2010). Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J Nutr 140:527–33
- Talavéra S, Felgines C, Texier O, et al. (2003). Anthocyanins are efficiently absorbed from the stomach in anesthetized rats. J Nutr 133:4178–82
- Tang M, Alvani K, Tester RF. (2010). Production and utilisation of gastric rafts from polysaccharide combinations to induce satiety: a preliminary study. Nutr Food Sci 40:155–65
- Tarozzi A, Morroni F, Hrelia S, et al. (2007). Neuroprotective effects of anthocyanins and their in vivo metabolites in SH-SY5Y cells. Neurosci Lett 424:36–40
- Tarozzi A, Morroni F, Merlicco A, et al. (2010). Neuroprotective effects of cyanidin 3-O-glucopyranoside on amyloid beta (25-35) oligomer-induced toxicity. Neurosci Lett 473:72–6
- Tsuda T, Horio F, Uchida K, et al. (2003). Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J Nutr 133:2125–30
- Urbain J-LC, Siegel JA, Charkes ND, et al. (1989). The two-component stomach: effects of meal particle size on fundal and antral emptying. Eur J Nucl Med 15:254–9
- Urquhart J, Theeuwes F. (1984). Drug delivery system comprising a reservoir containing a pluratility of tiny pills, US Patent No. 4,434,153. Washington (DC): US Patent and Trademark Office
- van Dam RM, Naidoo N, Landberg R. (2013). Dietary flavonoids and the development of type 2 diabetes and cardiovascular diseases: review of recent findings. Curr Opin Lipidol 24:25–33
- Vanzo A, Vrhovsek U, Tramer F, et al. (2011). Exceptionally fast uptake and metabolism of cyanidin 3-glucoside by rat kidneys and liver. J Nat Prod 74:1049–54
- Vincent R, Roberts A, Frier M, et al. (1995). Effect of bran particle size on gastric emptying and small bowel transit in humans: a scintigraphic study. Gut 37:216–19
- Vitaglione P, Donnarumma G, Napolitano A, et al. (2007). Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J Nutr 137:2043–8
- Wallace TC. (2011). Anthocyanins in cardiovascular disease. Adv Nutr 2:1–7
- Wang L-S, Stoner GD. (2008). Anthocyanins and their role in cancer prevention. Cancer Lett 269:281–90
- Wedick NM, Pan A, Cassidy A, et al. (2012). Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am J Clin Nutr 95:925–33
- Wei H. (2014). Postprandial plasma polyphenol profile and bioavailability of anthocyanins in insulin resistant humans after consuming multiple doses of strawberries beverage with a meal. Chicago (IL): Illinois Institute of Technology
- Wiggins HS, Dawson AM. (1961). An evaluation of unabsorbable markers in the study of fat absorption. Gut 2:373–6
- Woerle HJ, Albrecht M, Linke R, et al. (2008). Impaired hyperglycemia-induced delay in gastric emptying in patients with type 1 diabetes deficient for islet amyloid polypeptide. Diabetes Care 31:2325–31
- Wu T, Yu Z, Tang Q, et al. (2013). Honeysuckle anthocyanin supplementation prevents diet-induced obesity in C57BL/6 mice. Food Funct 4:1654–61
- Zunino SJ. (2009). Type 2 diabetes and glycemic response to grapes or grape products. J Nutr 139:1794S–800S

