Abstract
Objective. To report on the primary care consultation rates and clinical characteristics of patients with gastro-oesophageal reflux disease (GERD) as part of the RANGE (Retrospective ANalysis of GERD) observational study. Methods. RANGE was conducted at 134 primary care centres across six European countries. All subjects who consulted their primary care physician during a 4-month identification period were screened retrospectively. Those consulting for GERD-related reasons were identified, and a randomly selected cohort underwent clinical interview. Results. Out of 373,610 consultations in the six countries, 12,815 (3.4%) were for GERD-related reasons (inter-country range: 1.4–7.4%). From 2678 patients interviewed (24.7% of whom had been previously diagnosed with reflux oesophagitis), symptom recurrence following remission was the most common reason for primary care consultation (35.1%; range: 22.3–51.7%). Some 12.7% of patients (range: 9.1–21.4%) consulted due to persistence of previous symptoms, and 16.2% (range: 8.2–35.6%) had never consulted before regarding GERD-related symptoms.
Conclusion: Consultation rates for GERD-related reasons, and the clinical characteristics of consulting patients, vary widely across Europe. Symptom recurrence after an initial period of remission, and persistent symptoms, were important reasons for consultation, emphasizing the need for improved management of primary care patients with GERD across Europe.
Introduction
Gastro-oesophageal reflux disease (GERD) is defined as a condition in which the reflux of stomach contents causes troublesome symptoms and/or complications (Citation1). The cardinal symptoms of GERD are heartburn and regurgitation (Citation1), which can markedly impair health-related quality of life (Citation2–4) and impact on work and leisure-time productivity (Citation2,Citation4).
The results of population-based studies indicate that GERD is very common, with prevalence rates of 10–20% being reported in Europe (Citation5–7). Despite the associated burden of GERD and its high prevalence, management in primary care remains far from optimal, with around one-half of patients with a GERD diagnosis still experiencing symptoms after treatment (Citation4). The RANGE (Retrospective ANalysis of GERD) study was, therefore, designed to investigate the symptom profile, diagnosis, management, and response to treatment of patients with GERD in several European countries, with focus on inter-country variability. This is of particular interest, especially in terms of patient characteristics and management, as differences may indicate countries where an unmet medical need exists that could probably be improved with a more appropriate management approach.
As part of the RANGE study, we sought to determine the proportion of patients who consulted their primary care physician (PCP) for GERD-related reasons and their clinical characteristics, along with inter-country variability.
Patients and Methods
Study design and subjects
The design of the RANGE study (AstraZeneca study code: D9612L00114) has been described in full elsewhere (Citation8). In brief, RANGE was an observational study conducted as a series of parallel local studies at 134 primary care centres across six European countries (Germany, Greece, Norway, Spain, Sweden, and the UK). The study was approved by local ethics committees in all participating countries and conducted in accordance with the ethical principles outlined in the Declaration of Helsinki.
The identification of potential subjects for this study was carried out retrospectively by review of medical records. Identification strategies were anticipated to vary depending on the facilities available at each study centre (e.g., the availability and nature of computerized tools), but local adaptations to protocols were assumed to ensure that all eligible subjects were included. Where possible, computerized systems were used.
Initially, over a 4-month period, a population was identified comprising all subjects aged ≥18 years who had consulted their PCP for any reason. From this total population, based on a retrospective review of medical records, patients who had consulted their PCP for GERD-related reasons (with or without treatment, and regardless of whether GERD was the main reason for the visit) were selected. Patients were considered to have consulted their PCP for GERD if at least one of the following criteria existed at the initial consultation: they reported troublesome heartburn and/or regurgitation; GERD had been previously diagnosed by endoscopy (presence of oesophagitis or GERD-related complications, including haemorrhage, stricture, or Barrett's metaplasia), oesophageal pH monitoring (pathological oesophageal pH), or by the presence of symptoms only (heartburn and/or regurgitation); or they received a prescription for acid-suppressive medication (proton pump inhibitors [PPIs], H2-receptor antagonists) and/or antacids specifically for GERD. Patients were included irrespective of the number of times they had visited a PCP over the initial identification period. To fulfil the inclusion criteria, patients also had to provide informed consent and demonstrate an ability to complete patient-reported outcome instruments. Exclusion criteria were as follows: PPI use to heal or prevent peptic ulcers in patients taking non-steroidal anti-inflammatory drugs; PPI treatment as part of an Helicobacter pylori eradication regimen; and participation in another clinical study.
From the GERD patients identified, a sample of patients (selected using an Excel-generated random sequence list at each study centre) was invited to participate in the study. Those who agreed to participate were asked to attend a clinic visit at which the following data were collected during interview with the physician and from the subject's medical records: demographics; medical history; reason for initial consultation (e.g., new symptoms in patients who had never experienced GERD symptoms previously, recurrent or persistent symptoms, follow-up visit in an asymptomatic patient); GERD symptoms during the previous 7 days (frequency [absent, present for 1 day, ≥2 days, or daily] and intensity [mild, moderate, or severe]); previous diagnosis and diagnostic procedures utilized; and GERD complications.
Statistical methods
The objectives of this study were descriptive in nature, and there were no hypotheses to test using statistical methods. Consequently, sample sizes for the randomized and prospectively investigated patients for each country were predetermined to allow comparisons between countries and to reduce the sampling error to below 5%. Sample sizes for each country were as follows: Germany, Greece, Norway, and Spain, 500 patients each; Sweden and the UK, 300 patients each. All data are presented descriptively. For continuous variables, mean and standard deviation (SD) were calculated. For categorical variables, percentages and 95% confidence intervals (CI) were determined.
Results
Over the 4-month identification period, 373,610 subjects consulted their PCP; of these, 12,815 consulted at least once for GERD-related reasons (study population). Thus, the overall proportion of GERD-related consultations was 3.4% (95% CI: 3.37–3.49%). Across individual countries, GERD-related consultation rates were: 7.4% in Germany; 7.1% in Greece; 2.2% in Norway; 2.7% in Spain; 4.2% in Sweden; and 1.4% in the UK. From the study population, 4845 patients met the eligibility criteria and were randomly selected to participate; of these, 2678 were contactable and included in the study (the remainder were either non-contactable [n = 612], non-attendees [n = 196], declined participation [n = 340], or were not invited [n = 1019] on the basis that required by-country sample sizes were reached). Overall, 1537 patients (57.4%) were women, and the mean age was 56.8 years. Demographic characteristics of participating patients, stratified by country of residence, are summarized in . Overall, patient characteristics were generally similar across the countries surveyed.
Table I. Demographic and endoscopic characteristics of participating patients, stratified by country of residence.
Reasons for consultation
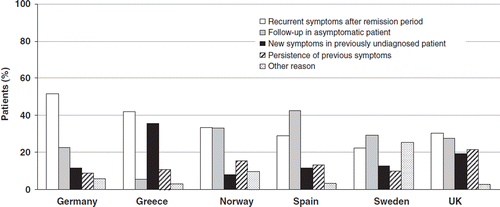
In general, the most common reasons for GERD-related PCP consultation were recurrent symptoms following an initial period of remission (35.1% of patients, ranging from 22.3% in Sweden to 51.7% in Germany) and follow-up in asymptomatic patients (26.0%, ranging from 5.5% in Greece to 42.7% in Spain). Some 12.7% of patients consulted their PCP due to persistence of previous symptoms, and 16.2% had never consulted before regarding GERD-related symptoms ().
Symptoms
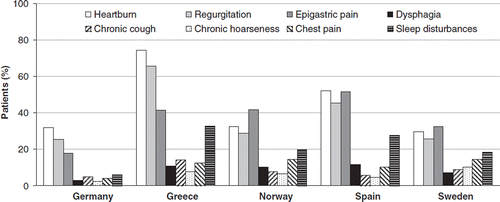
UK patients were not included in the analysis of GERD symptom frequency and intensity due to missing data. Among the remaining countries, heartburn (44.7% of patients, ranging from 29.6% in Sweden to 74.3% in Spain) and regurgitation (38.7%, ranging from 25.3% in Germany to 65.5% in Greece) were generally the most common symptoms, with frequency ≥2 days in the 7 days prior to consultation (). Some between-country differences were observed, with epigastric pain being the most common symptom in patients in Norway and Sweden.
Figure 2. Profile of symptoms at consultation (symptom frequency ≥2 days/week), stratified by country of residence (data not available for the UK)
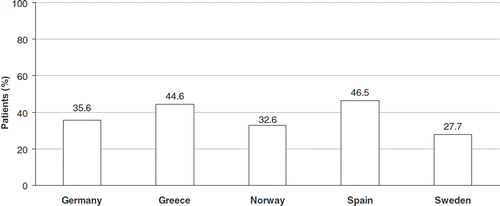
With regard to symptom intensity, the overall proportion of patients reporting moderate or severe heartburn was 41.4% (ranging from 25.8% in Sweden to 68.7% in Greece). Overall, 35.2% of patients reported moderate or severe regurgitation (ranging from 24.2% in Norway to 57.2% in Greece). The proportion of patients with significant GERD symptom load (defined as heartburn or regurgitation with frequency ≥2 days/week and of moderate or severe intensity) ranged from 27.7% in Sweden (95% CI: 23.2–32.6%) to 46.5% in Spain (95% CI: 42.0–51.1%) ().
Previous diagnosis of GERD and endoscopic findings
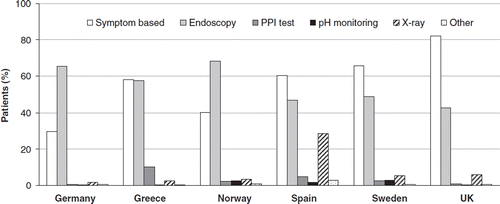
Based on medical record review, a previous diagnosis of GERD was most commonly made on the basis of symptoms alone in Greece, Spain, Sweden, and the UK (ranging from 58.2% [95% CI: 53.8–62.6%] to 82.1% [95% CI: 77.4–86.3%] for these countries), whereas endoscopy was employed far more frequently than symptom-based diagnosis in Germany (65.5% [95% CI: 61.1–69.6%] vs 29.9% [95% CI: 25.9–34.1%]) and Norway (68.4% [95% CI: 64.2–72.3%] vs 40.2% [95% CI: 36.0–44.5%]). Diagnosis was based on PPI response test, pH monitoring, or X-rays in only a small proportion of patients ().
Where endoscopy had been performed, reflux oesophagitis was the most common endoscopic finding (24.7% of patients, ranging from 9.5% in Sweden to 52.1% in Germany). Other complications of GERD (haemorrhage, Barrett's metaplasia, stricture, and adenocarcinoma) were infrequent and were observed in <3% of patients overall ().
Discussion
In view of its design and observational nature, the RANGE study provides an important “real world” insight into the primary care management of GERD and is noteworthy in that it allows for comparison of inter-country differences. During the 4-month identification period, for example, 3.4% of PCP consultations across participating countries were for GERD-related reasons, and this value ranged from 1.4% of consultations in the UK to 7.4% in Germany. A number of factors may account for this. The perception of GERD among PCPs may differ between countries, leading to variation in management. In addition, differences in healthcare systems between countries could mean that patients may be more (or less) likely to routinely visit a PCP for treatment. For example, in some countries, patients may receive repeat prescriptions of maintenance therapy without having to re-consult their PCP. Cultural differences (e.g., levels of stoicism) will also play a part in affecting consultation rates. Seifert et al., in their international survey of the management of gastrointestinal disorders across Europe, also concluded that these political and cultural factors were important (Citation9).
The comprehensive inclusion criteria employed by our study (designed to reflect the day-to-day reality of primary care) meant that patients were included irrespectively of the frequency or intensity of their symptoms, or whether they had new or existing GERD. Comparison with GERD incidence results from other studies is, therefore, not possible. However, prevalence comparisons may be more meaningful, but should still be considered cautiously, since epidemiological studies may define GERD more specifically than our study. When compared with GERD prevalence rates of 10–20% reported in European epidemiological studies (Citation5–7), for example, our consultation rate of 3.4% seems rather low. One possible explanation for this is that many community-based patients who experience symptoms of GERD do not perceive themselves to have a serious condition, use on-demand, over-the-counter therapies, and may, therefore, not consult their PCP (Citation10).
Our study noted that one of the most common reasons for GERD-related PCP consultation was treatment for recurrent symptoms following an initial period of remission. This type of consultation was most frequent in Germany (51.7%) and least frequent in Sweden (22.3%). This contrast is interesting, as it again highlights differing management strategies between countries. For example, it could indicate that GERD is perceived more as a chronic disease in Sweden, and more patients receive maintenance therapy to prevent symptom recurrence; in Germany, perhaps GERD is perceived as an acute, intermittent disease, so maintenance treatment is less widespread, leading to the observed higher rates of consultation for recurrent symptoms.
Overall, patients presented most commonly with heartburn and/or regurgitation, the cardinal symptoms of GERD (Citation1). Based on medical record review, up to one-half of patients consulted with their PCP because of recurrent symptoms, 10% cited persistent symptoms, and 16% had new symptoms; again, findings were subject to marked inter-country differences. Furthermore, over one-quarter of patients reported moderate or severe symptoms at consultation. Indeed, previous studies in primary care patients with GERD have reported that, despite medication, a substantial proportion of patients still experience symptoms (Citation4,Citation11). Together, such findings highlight that current management of GERD in primary care is far from optimal.
Differences in diagnostic methods were observed between countries in this study. Endoscopy had been used most frequently in Norway and Germany, and least frequently in the UK and Spain. Spain, notably, used X-rays as a diagnostic technique more frequently than any other country in this study. These differences are most likely accounted for by variations in healthcare systems between countries. Such differences aside, symptoms alone were used as the diagnostic basis in up to 80% of patients in this study. This approach is inherently subjective and may be associated with a risk of misdiagnosis, but since it is apparently so widely used, subjectivity could be reduced by standardizing symptom-based diagnosis. This has been addressed by the recently developed GerdQ questionnaire (Citation12), which is based on a combination of items from the Reflux Disease Questionnaire (Citation13), Gastrointestinal Symptom Rating Scale (Citation14), and the GERD Impact Scale (Citation15). While still subjective, this tool has been found to be both relatively sensitive and specific when used to diagnose GERD (65% and 71%, respectively), and can, therefore, facilitate diagnosis and management of GERD in routine clinical practice.
The management of GERD is complicated further by H. pylori infection, and its relationship with GERD symptoms. Where patients with GERD are receiving long-term PPI therapy, H. pylori infection appears to lessen the severity of reflux symptoms (Citation16,Citation17), indicating that the infection confers some kind of protective effect. This suggests that eradication of H. pylori would worsen reflux symptoms; yet this does not appear to be the case (Citation18). Although patients taking PPIs as part of an eradication regimen for H. pylori infection were actively excluded from our study, a proportion would likely to have been carrying infection, and it would be interesting to consider whether between-country variations in GERD symptom severity and consultation rates for relapse were linked with similar variations in the prevalence of H. pylori infection. Unfortunately, studies into national H. pylori seroprevalence rates are scarce (Citation19), and this was not monitored as part of the RANGE study.
One limitation of the RANGE study could be that those patients with a higher symptom load may have been over-represented in the sample, compared with those with less impactful disease, as patients with more frequent and/or severe symptoms would have been more likely to visit their physician (in Greece, for example, approximately 70% of patients reported severe heartburn). However, we consider the potential selection bias in the RANGE study to be low. The other limitation of the study is the potential recall bias inherent to retrospective designs. Despite this, we consider our study to be a valid investigation into the symptom profile and diagnosis of GERD in primary care, which allows for meaningful comparison of inter-country differences.
Conclusions
There is considerable variation across European countries (Germany, Greece, Norway, Spain, Sweden, and the UK) in consultation rates for GERD-related reasons, as well as in the clinical profile of patients and the means of diagnosis. This variation is most likely due to cultural and political differences, though differing perceptions of GERD by PCPs and patients may also be important. Recurrence of symptoms following an initial period of remission, and persistent symptoms, were important reasons for consulting a PCP. Together, these findings emphasize the need for improved management of patients with GERD across Europe.
Acknowledgements
This study was supported by AstraZeneca. The authors thank Claire Byrne and Anna Mett, from Wolters Kluwer Pharma Solutions (Auckland, New Zealand), who provided medical writing support funded by AstraZeneca.
Disclosures: Dr J. P. Gisbert has received educational/research grants and consulting fees from AstraZeneca; Dr A. Cooper has no competing interests to declare; Dr D. Karagiannis has received research grants from Abbott, and speaker fees from Janssen, AstraZeneca, and Falk (Galenica); Dr J. Hatlebakk has received speaker fees from AstraZeneca; Dr L. Agréus has received research grants and speaker fees from AstraZeneca, and is a former advisory board member for Orexo AB; Dr H. Jablonowski has received speaker fees from AstraZeneca; Dr M. Tafalla is an employee of AstraZeneca.
References
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101:1900–20.
- Wiklund I. Review of the quality of life and burden of illness in gastroesophageal reflux disease. Dig Dis 2004; 22:108–14.
- Wiklund I, Carlsson J, Vakil N. Gastroesophageal reflux symptoms and well-being in a random sample of the general population of a Swedish community. Am J Gastroenterol 2006; 101:18–28.
- Jones R, Liker HR, Ducrotté P. Relationship between symptoms, subjective well-being and medication use in gastro-oesophageal reflux disease. Int J Clin Pract 2007; 61:1301–7.
- Ronkainen J, Aro P, Storskrubb T, Lind T, Bolling-Sternevald E, Junghard O, . Gastro-oesophageal reflux symptoms and health-related quality of life in the adult general population – the Kalixanda study. Aliment Pharmacol Ther 2006; 23:1725–33.
- Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2005; 54:710–7.
- Valle C, Broglia F, Pistorio A, Tinelli C, Perego M. Prevalence and impact of symptoms suggestive of gastroesophageal reflux disease. Dig Dis Sci 1999; 44:1848–52.
- Gisbert JP, Cooper A, Karagiannis D, Hatlebakk J, Agréus L, Jablonowski H, . Impact of gastroesophageal reflux disease on patients’ daily lives: a European observational study in the primary care setting. Health Qual Life Outcomes 2009; 7:60–.
- Seifert B, Rubin G, de Wit N, Lionis C, Hall N, Hungin P, . The management of common gastrointestinal disorders in general practice. A survey by the European Society for Primary Care Gastroenterology (ESPCG) in six European countries. Dig Liver Dis 2008; 40:659–66.
- Ducrotté P, Liker HR. How do people with gastro-oesophageal reflux disease perceive their disease? Results of a multinational survey. Curr Med Res Opin 2007; 23:2857–65.
- Jones R, Armstrong D, Malfertheiner P, Ducrotté P. Does the treatment of gastroesophageal reflux disease (GERD) meet patients’ needs? A survey-based study. Curr Med Res Opin 2006; 22:657–62.
- Dent J, Jones R, Vakil N, Halling K, Junghard O, Wernersson B, . A management strategy for GERD based on the Gastroesophageal Reflux Disease Questionnaire (GerdQ) [abstract]. Scand J Gastroenterol 2008; 43:34–5.
- Shaw MJ, Talley NJ, Beebe TJ, Rockwood T, Carlsson R, Adlis S, . Initial validation of a diagnostic questionnaire for gastroesophageal reflux disease. Am J Gastroenterol 2001; 96:52–7.
- Svedlund J, Sjodin I, Dotevall G. GSRS – a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci 1988; 33:129–34.
- Jones R, Coyne K, Wiklund I. The gastro-oesophageal reflux disease impact scale: a patient management tool for primary care. Aliment Pharmacol Ther 2007; 25:1451–9.
- Jakobs R, Riemann JF. cagA-positive Helicobacter pyloristrains and gastro-oesophageal reflux disease: still puzzling?. Eur J Gastroenterol Hepatol 2004; 16:635–7.
- Raghunath A, Hungin AP, Wooff D, Childs S. Prevalence of Helicobacter pyloriin patients with gastro-oesophageal reflux disease: systematic review. BMJ 2003; 326:737.
- Raghunath AS, Hungin AP, Mason J, Jackson W. Helicobacter pylorieradication in long-term proton pump inhibitor users in primary care: a randomized controlled trial. Aliment Pharmacol Ther 2007; 25:585–92.
- The EUROGAST Study Group. Epidemiology of, and risk factors for, Helicobacter pylori infection among 3194 asymptomatic subjects in 17 populations Gut. 1993; 34:1672–6.