Abstract
Background: The ADA 2010 guidelines added HbA1c ≥ 6.5% as a criterion for diagnosing diabetes mellitus type 2.
Objective: To evaluate the HbA1c test in predicting type 2 diabetes in a high risk population.
Methods: A community-based historic cohort study was conducted including 10 201 patients, who had not been diagnosed with diabetes, and who underwent HbA1c test during the years 2002–2005. Data was retrieved on diabetes risk factors and the onset of diabetes (according to the ADA 2003 criteria), during a follow-up period of five-to-eight years.
Results: Mean age was 58.25 ± 15.58 years; mean HbA1c level was 5.59 ± 0.55% and 76.8% had a BMI > 25 kg/m2 (mean: 30.74 ± 8.30). In a Cox proportional hazards regression model, the risk of developing type 2 diabetes was 2.49 (95% CI: 1.29–3.71) for 5.5% ≤ HbA1c < 6% at baseline, 4.82 (95% CI: 2.83–8.20) for 6% ≤ HbA1c < 6.5% at baseline and 7.57 (95% CI: 4.43–12.93) for 6.5% ≤ HbA1c < 7% at baseline, compared to HbA1c < 4.5%. The risk of developing diabetes was 1.14 (95% CI: 1.05–1.25) for male gender, 1.16 (95% CI: 1.04–1.28) for cardiovascular diseases and 2.06 (95% CI: 1.80–2.35) for overweight (BMI > 25 kg/m2) at baseline. Neither age nor low socio-economic status was associated with increased risk of diabetes.
Conclusion: Levels of HbA1c ≥ 5.5% were associated with increased risk of type 2 diabetes during a five-to-eight-year follow-up period. Findings support the use of HbA1c testing as a screening tool in populations at risk of developing diabetes.
KEY MESSAGE:
The current study supports the association of HbA1c levels above 5.5% with increased risk of type 2 diabetes.
HbA1c testing of patients at risk of developing diabetes may promote stratification of a target population for prevention and early diagnosis of type 2 diabetes.
Introduction
For years, the fasting plasma glucose (FPG) test and oral glucose tolerance tests (OGTT) served as the definitive measures for diabetes diagnosis.
Elevated haemoglobin A1c (HbA1c, glycated haemoglobin) levels have been shown to associate with the development of diabetic retinopathy and contribute to the prediction and diagnosis of type 2 diabetes (Citation1–7). The American Diabetes Association (ADA) in 2010 and the World Health Organisation (WHO) in 2011 added an HbA1c level of ≥ 6.5% to the criteria for diagnosing diabetes (Citation8,Citation9). HbA1c testing has some of advantages over fasting glucose testing. Blood samples for HbA1c testing can be taken at any time of the day, and not only after fasting. Since HbA1c reflects the long-term exposure to hyperglycaemia (over a two-to-three-month period), it is not affected by fluctuations in glucose values that may immediately precede the test. Using the Rohlfing formula, HbA1c values can be converted to the mean plasma glucose value for the period tested (Citation10). These advantages of the HbA1c test, especially the perception that HbA1c is more reliable than FPG regarding long term exposure to hyperglycaemia, led physicians at Clalit Health Services, the largest health care provider and insurer in Israel, to use it as a screening tool in non-diabetic patients, years before official guidelines were established (Citation11).
From our experience in family practice in Israel, reasons for using HbA1c as a means of screening for diabetes relate to clinical suspicion of undiagnosed diabetes (overweight, symptoms and manifestations compatible with diabetes, etc.).
The aim of the current study was to evaluate, in individuals without diabetes, associations of HbA1c levels, as well as other risk factors, with the future development of type 2 diabetes.
Methods
Study design
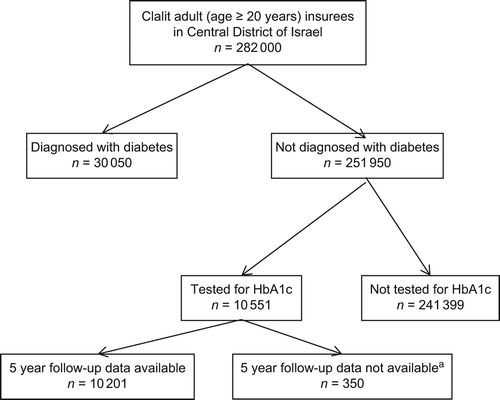
This is a community based historic cohort study of adult (age ≥ 20 years) individuals insured by ‘Clalit Health Services’ (CHS) in the central district of Israel, who underwent an HbA1c test during the years 2002–2005, and who were not diagnosed with diabetes prior to the test or 30 days following the test.
The central district of CHS serves a population of about 500 000 people. The CHS database contains laboratory results, records of medications purchased, medical diagnoses and socio-demographic details. The data are under extensive ongoing internal and external quality surveillance; for example, these data serve the National Program for Quality Indicators in Community Healthcare with its’ strict internal and external auditing (Citation12).
The study was approved by the Clalit Health Services ethics committee at the ‘Meir’ Medical Centre, Kfar-Saba.
Inclusion criteria
During the study period, the Israeli and the CHS guidelines stipulated HbA1c testing only for the evaluation of diabetes control (‘monitoring’). Nevertheless, there were no administrative limitations on HbA1c testing, and many HbA1c tests were performed on individuals without diagnosed diabetes, as a means of screening for diabetes. Individuals with data on HbA1c testing comprised the study cohort if five-year follow-up data were available. Every eligible patient was included once, according to the earliest HbA1c test performed during the study period.
Exclusion criteria
Exclusion criteria were: diagnosis of type 1 or type 2 diabetes prior to performance of the HbA1c test, a laboratory test result compatible with diabetes before the HbA1c test, treatment with oral hypoglycaemic agents or insulin or follow-up of less than five years (350 individuals). Diabetes was diagnosed according to one or more of the following: FPG ≥ 126 mg/dl (7.0 mmol/l) after an eight hour fast, OGTT result ≥ 200 mg/dl (11.1 mmol/l), or random plasma glucose ≥ 200 mg/dl (11.1 mmol/l).
Additionally, we excluded individuals for whom we had an indication of diabetes diagnosis or one of the above exclusion criteria during the 30 days following performance of the HbA1c test. Individuals with an HbA1c level of 7% and above were excluded from the cohort under the assumption that they most probably met criteria for diabetes diagnosis.
The selection procedure of the study group is shown in .
Data and variables
HbA1c. All HbA1c tests were performed in the district’s central laboratory. The HbA1c assay used in the CHS central district during the study period was immunoturbidimetry (Roche® COBAS INTEGRA®), DCCT aligned. At baseline, the study cohort was classified into six sub-groups, according to the first HbA1c value measured on the index day as follows: reference group: HbA1c < 4.5%; sub-group #1: 4.5% ≤ HbA1c < 5%; sub-group #2: 5% ≤ HbA1c < 5.5%; sub-group #3: 5.5% ≤ HbA1c < 6%; sub-group #4: 6% ≤ HbA1c < 6.5%; sub-group #5: 6.5% ≤ HbA1c < 7%.
Patient characteristics. Baseline patient characteristics retrieved from the database included: gender, age on the index day (i.e. date of HbA1c test), body mass index (BMI), socio-economic status (low socio-economic status was defined as indication in the database of exemption from National Insurance Institute of Israel contributions payments) and the presentation of cardiovascular diseases (hypertension, ischaemic heart disease and peripheral vascular disease), according to the chronic diseases register of CHS.
Type 2 diabetes (dependent variable). The dependent variable was the progression to type 2 diabetes during the follow-up period, according to criteria recommended by the ADA in 2010, excluding HbA1c ≥ 6.5% (HbA1c ≥ 6.5% was not a criterion for progression to diabetes in the current study because this was the predictor that was tested).
Follow-up time. The follow-up period for each patient started at the date of the first HbA1c test in the years 2002–2005. Follow up ended at the date of diagnosis of diabetes (event) or the end of the study period (31 October 2010).
Statistical analysis
In addition to descriptive statistics, a one-way analysis of variance (Kaplan–Meier estimator) was used to investigate the correlation between HbA1c and the development of diabetes. To examine the relationship between baseline HbA1c, age, gender, BMI, socio- economic status, history or present cardiovascular diseases and the development of diabetes, a multivariable analysis (Cox proportional hazards regression) was used. The hazard ratio was considered statistically significant when P ≤ 0.05.
All statistical analysis was performed using STATA 8 and Microsoft Excel 2010 software.
Results
Baseline characteristics
The study cohort comprised 10 201 individuals. The mean age on the index day was 58.25 ± 15.58 years. The mean HbA1c level was 5.59 ± 0.55 %. The mean BMI value was 30.74 ± 8.30 kg/m2; presents the baseline characteristics of the cohort. shows the distribution of patients according to the six sub-groups defined by HbA1c.
Table 1. Baseline characteristic of the cohort.
Table 2. Distribution of HbA1c values in the study cohort (n = 10 201).
Outcome: Development of diabetes
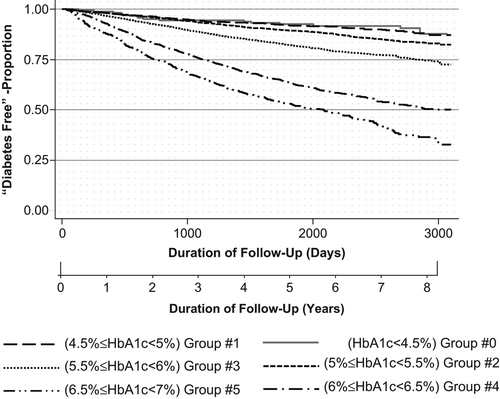
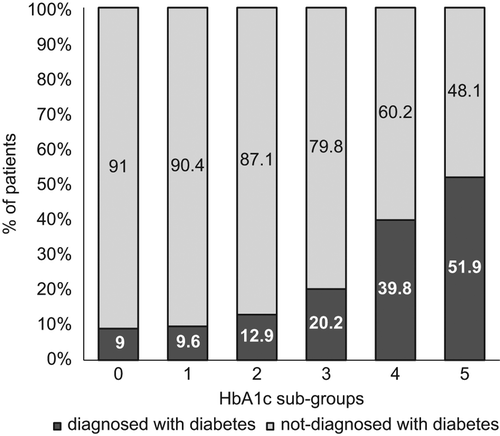
Overall, 22.5% of the cohort developed type 2 diabetes. shows the proportions of individuals who developed diabetes according to HbA1c subgroups. shows the rate of diagnosis at the end of follow-up, according to HbA1c sub-group at baseline (using the Kaplan–Meier curve). The proportion of patients that developed diabetes increased over time as baseline HbA1c values increased; increases were greatest for sub-groups 4 and 5. For sub-group 4 (6% ≤ HbA1c < 6.5%), the proportion of patients that stayed disease-free over a follow-up period of up to eight years was about 50%, compared to 30% for sub-group 5 (6.5% ≤ HbA1c < 7%). For sub-groups 1 and 2, the increase in the proportion of patients who developed diabetes was not statistically significant (P = 0.69, 0.08, respectively).
Figure 2. Diabetes diagnosis at the end of follow-up, by HbA1c sub-group. For each HbA1c subgroup, the percentage of patients who were diagnosed with diabetes appears in dark colour, and the percentage not diagnosed in light colour. The six sub-groups were determined according to the first HbA1c value measured on the index day, as follows: Reference group: HbA1c < 4.5%; sub-group #1: 4.5% ≤ HbA1c < 5%; sub-group #2: 5% ≤ HbA1c < 5.5%; sub-group #3: 5.5% ≤ HbA1c < 6%; sub-group #4: 6% ≤ HbA1c < 6.5%; sub-group #5: 6.5% ≤ HbA1c < 7%.
Multivariable associations
In a multivariable model (using Cox regression, ) with HbA1c < 4.5% as the reference, neither increased age nor low socio-economic status were associated with increased risk of diabetes. Male gender and baseline presence of cardiovascular diseases were associated with a slight, though statistically significant increase in risk. The risk of diabetes increased by 2.06 (95% CI: 1.80–2.35) for BMI > 25 kg/m2. The risk to develop type 2 diabetes was exponential, almost doubling with each increase of 0.5% of HbA1c.
Table 3. Cox regression analysis of the hazard ratio of HbA1c category for the development of type 2 diabetes (n = 10 201).
Discussion
Main findings
This study demonstrated increased risk for type 2 diabetes over a five-to-eight-year follow-up period for individuals with HbA1c levels below 6.5%, the threshold level of disease diagnosis, according to the ADA guidelines of 2010. Compared with other risk factors investigated, HbA1c level was most strongly associated with an increased hazard of diabetes development. The risk of developing diabetes increased with increased HbA1c values. The risk doubled with every increase of 0.5% in HbA1c level.
Strengths and limitations
The current study has several strengths. The availability of data from an administrative database enabled evaluation of a large cohort over a period of more than five years. The study cohort, which comprised more than 10 000 individuals, is large particularly in light of the prolonged follow-up period, and compared to that of other studies (Citation3,Citation4,Citation13). Conducting the study in Israel ensures uniform health services, including accessibility of free of charge laboratory tests to all citizens, under the National Health Insurance Law.
Characteristics of the cohort of the current study were compatible with those of populations at risk for type 2 diabetes: the majority was overweight (BMI > 25 kg/m2)—the main risk factor for type 2 diabetes (Citation14). The high rate of cardiovascular co-morbidities of type 2 diabetes is well known (Citation15).
This study also has some limitations. We did not retrieve data regarding factors possibly influencing HbA1c levels (haemoglobin disorders, malaria, etc.). However, most of these factors are very uncommon in Israel.
The study does not include the 350 patients (only 3.3% of the study population) whose follow-up was discontinued due to death or transfer to a different health insurance organization. The fasting plasma glucose tests were not performed systematically. Yet there were no patients without at least one fasting plasma glucose test result after five-to-eight-years follow-up. The fact that the majority of the cohort was defined overweight (BMI > 25 kg/m2) entails a selection bias resulting from the more common testing of HbA1c among individuals at risk for developing diabetes, primarily overweight patients. However, since overweight individuals are at increased risk for diabetes, confining our conclusions to this population may be appropriate. Nevertheless, we did not compare the study population to the population that was not tested for HbA1c, nor do we do have a systematic documentation of the reasons doctors performed the HbA1c test.
Secondary outcomes: Risk factors for diabetes
In a multivariable model, overweight (BMI > 25 kg/m2) carried a two-fold risk of developing diabetes and demonstrated the greatest prediction of risk, after HbA1c. This concurs with current literature, and with the direct association that has been observed between overweight and insulin resistance, a main contributor to diabetes and cardiovascular related morbidity (Citation14,Citation16).
In the present study, age and low socio-economic status, after controlling for baseline HbA1c and overweight, were not found to associate with the progression to diabetes. This is despite the increased prevalence of diabetes that has been observed with age, exceeding 20% of the population in Israel for the ages 65–74. The incidence continues to rise by 0.2% for each increase in age of one year (Citation17). A possible explanation for these findings lies in the fact that in these high-risk patients, the sub-group with elevated HbA1c levels is in higher risk of developing diabetes. Age and low socio-economic status are associated with a rise in HBA1c; once this is taken into account in the multivariate analysis the relation disappears.
Interpretation of main findings
It appears from findings in the current study that not only HbA1c testing may be beneficial not only in diagnosing diabetes, but also in predicting future development of the disease in populations at risk of diabetes development. In this study, rates of progression to diabetes increased exponentially with 0.5% increases in HbA1c. Apparently, the high rate of diabetes diagnosed during a five-to-eight-year follow-up period, 22.5% overall, is consequent to the increased rate of HbA1c testing among high risk patients, as evident from the characteristics of the study cohort. Similarly, screening strategies implemented in the FIN-D2D and ADDITION studies resulted in relatively high rates of conversion to diabetes (Citation18,Citation19). While screening increases rates of diagnosis, it is apparently not a reliable means of assessing the prevalence of diabetes in a given population. Further, in the ADDITION study, screening was shown not to reduce all-cause, cardiovascular, or diabetes-related mortality (Citation20). The recently published and most intriguing findings of the Oregon Experiment showed that increased diagnosis of diabetes did not associate with improved HbA1c levels two years later (Citation21). While HbA1c was the means of screening in the current study, FPG and OGTT were the primary means of assessing progression to diabetes, which was the study outcome. Several studies have shown the discordance between glucose measures and HbA1c in the diagnosis of diabetes (Citation22,Citation23).
Implications: The role of HbA1c
In recent years, even prior to the publication of the ADA guidelines (in 2010), HbA1c testing became common among individuals without diabetes, following clinical suspicion of the disease or the presence of risk factors or suspected disease symptoms. In Israel, national guidelines for the use of HbA1c in screening and diagnosing diabetes have not been established.
The current study supports the association of HbA1c levels above 5.5%, with increased risk of type 2 diabetes. As expected, overweight was shown to be an important, independent risk factor for type 2 diabetes, supporting early screening among overweight individuals.
Findings in the current study support recently published studies that have demonstrated a benefit to close follow-up of individuals with HbA1c levels in the range of 5.5–6.5% (Citation1,Citation24). Along this line, the ADA 2010 guidelines defined HbA1c in the range of 5.7–6.4% as indicative of pre-diabetes; i.e. increased risk of developing diabetes in the future. The ADA 2013 recommendations (B level evidence) includes the use of HbA1c testing, in addition to measures of fasting plasma glucose and 75-g 2-h oral glucose tolerance test for diagnosing pre-diabetes, as well as diabetes, in asymptomatic patients (Citation25).
In contrast, WHO guidelines of 2011 stated that there is currently insufficient evidence for the interpretation of HbA1c levels below 6.5% (Citation9). As a commentary to the WHO report, the German Diabetes Association proposed an algorithm of diabetes diagnosis, (Citation26) by which scoring of the risk for type 2 diabetes uses the FINDRISC questionnaire, with HbA1c as a second-step screening test (Citation27). Accordingly, assuming that individuals with HbA1c < 5.7% are very unlikely to have diabetes, and those with HbA1c > 6.5% have a significant abnormality of glucose metabolism, plasma glucose-based criteria are applied to the remaining individuals, i.e. those with HbA1c in the range of 5.7–6.4%. For patients who are diagnosed with no diabetes, the algorithm suggests they should be given information about the risk for diabetes, lifestyle intervention and therapy of risk factors. Additionally, the algorithm suggests that risk determination and HbA1c test should be repeated after one year.
Conclusion
Elevated levels of HbA1c, above 5.5%, were associated with increased risk of progression to type 2 diabetes during a five-to-eight-year follow-up period. We suggest HbA1c testing of patients at risk of developing diabetes (for example, according to BMI and history of CVD) to promote stratification of a target population for disease prevention and early diagnosis.
ACKNOWLEDGEMENTS
This study was performed as part of fulfilment of the M.D. thesis requirements of Nataly Lerner at the Sackler Faculty of Medicine, Tel-Aviv University.
The study was presented in the annual 28th conference of the Israel Diabetes Association, on 14 May 2011, Air-Port City, Israel, and in the annual Israel Family Physicians Organization Conference, on 27 March 2012, Jerusalem, Israel.
Declaration of interest: No relevant conflicts of interest or financial disclosures to report. The authors alone are responsible for the content and writing of the paper.
References
- Bonora E, Kiechl S, Mayr A, Zoppini G, Targher G, Bonadonna RC, et al. High-normal HbA1c is a strong predictor of type 2 diabetes in the general population. Diabetes Care 2011;34:1038–40.
- Colagiuri S, Borch-Johnsen K. DETECT-2: Early detection of type 2 diabetes and IGT. Diabetes Voice 2003;48:11–3.
- Sabanayagam C, Liew G, Tai ES, Shankar A, Lim SC, Subramaniam T, et al. Relationship between glycated haemoglobin and microvascular complications: Is there a natural cut-off point for the diagnosis of diabetes?. Diabetologia 2009;52:1279–89.
- Lu ZX, Walker KZ, O’Dea K, Sikaris KA, Shaw JE. HbA1c for screening and diagnosis of type 2 diabetes in routine clinical practice. Diabetes Care 2010;33:817–9.
- International Expert Committee. International expert committee report on the role of the A1c assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–34.
- Pradhan AD, Rifai N, Buring JE, Ridker PM. Hemoglobin A1c predicts diabetes but not cardiovascular disease in nondiabetic women. Am J Med. 2007;120:720–27.
- Droumaguet C, Balkau B, Simon D, Caces E, Tichet J, Charles MA, et al. Use of HbA1c in predicting progression to diabetes in French men and women: Data from an epidemiological study on the insulin resistance syndrome (DESIR). Diabetes Care 2006;29: 1619–25.
- American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care 2010;33Suppl 1:S11–61.
- Report of a World Health Organization Consultation, Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. Diabetes Res Clin Pract. 2011;93:312–3.
- Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and Hba (1c): Analysis of glucose profiles and HbA(1c) in the diabetes control and complications trial. Diabetes Care 2002;25:275–8.
- S. Vinker, R. Yahalom, A. Elhayani. Long term intervention to reduce utilization of selected laboratory tests in the community. The 13th Annual Conference of the Israel Society of Quality in Medicine, Tel-Aviv, 2006.
- Manor O, Shmueli A, Ben-Yehuda A, Paltiel O, Calderon R, Jaffe DH. National Program for Quality Indicators in Community Healthcare in Israel Report, 2008–2010. School of Public Health and Community Medicine, Hebrew University-Hadassah. Jerusalem, Israel; 2012.
- Nakagami T, Tominaga M, Nishimura R, Yoshiike N, Daimon M, Oizumi T, et al. Is the measurement of glycated hemoglobin A1c alone an efficient screening test for undiagnosed diabetes?. Japan National Diabetes Survey. Diabetes Res Clin Pract. 2007; 76:251–6.
- Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factor. J Am Med Assoc. 2003;289:76–9.
- Srikanth S, Deedwania P. Primary and secondary prevention strategy for cardiovascular disease in diabetes mellitus. Cardiol Clin. 2011;29:47–70.
- Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: A summary of the evidence. Diabetes Care 2005;28:1769–78.
- Porath A, Rabinowitz G, Raskin-Segal A, Weitzman R, Ben-Said S. Quality indicators for community health care in Israel, 2005–2007 Public Report. Israel Ministry of Health, Israel National Institute for Health Policy and Health Services Research, 2008.
- Saaristo T, Moilanen L, Korpi-Hyövälti E, Vanhala M, Saltevo J, Niskanen L, et al. Lifestyle intervention for prevention of type 2 diabetes in primary health care: One-year follow-up of the Finnish National Diabetes Prevention Program (FIN-D2D). Diabetes Care 2010;33:2146–51.
- Rasmussen SS, Glümer C, Sandbaek A, Lauritzen T, Borch-Johnsen K. Determinants of progression from impaired fasting glucose and impaired glucose tolerance to diabetes in a high-risk screened population: 3 Year follow-up in the ADDITION Study, Denmark. Diabetologia 2008;51:249–57.
- Simmons RK, Echouffo-Tcheugui JB, Sharp SJ, Sargeant LA, Williams KM, Prevost AT, et al. Screening for type 2 diabetes and population mortality over 10 years (ADDITION-Cambridge): A cluster-randomised controlled trial. Lancet 2012;380: 1741–8.
- Baicker K, Taubman SL, Allen HL, Bernstein M, Gruber JH, Newhouse JP, et al. The Oregon experiment—effects of medicaid on clinical outcomes. N Engl J Med. 2013;368:1713–22.
- Malkani S, Mordes JP. Implications of using hemoglobin A1C for diagnosing diabetes mellitus. Am J Med. 2011;124:395–401.
- Saltevo JT, Kautiainen H, Niskanen L, Oksa H, Puolijoki H, Sundvall J, et al. Ageing and associations of fasting plasma glucose and 2 h plasma glucose with HbA(1C) in apparently healthy population. ‘FIN-D2D’ study. Diabetes Res Clin Pract. 2011;93:344–9.
- Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:800–11.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013;36:(Suppl. 1)S11–S66.
- Kowall B, Rathmann W, Landgraf R. Is HbA1c a valid and feasible tool for the diagnosis of diabetes?. Diabetes Res Clin Pract. 2011;93:314–6.
- Bergmann A, Li J, Wang L, Schulze J, Bornstein SR, Schwarz PE. A simplified Finnish diabetes risk score to predict type 2 diabetes risk and disease evolution in a German population. Horm Metab Res. 2007;39:677–82.