Abstract
Background: Taking the family history helps the doctor in estimating the probability of disease in individual patients. However, significant barriers to obtaining adequate family history information remain. Tools overcoming these barriers might support family physicians in this task.
Objective: To review systematically the characteristics of existing family history tools and discuss their potential use in primary care.
Methods: Studies were identified through searches of PubMed, Embase and Cinahl from 1 January 2002 until May 2012. All authors independently screened studies and included original research papers on family history tools of which assessment had been performed or was planned. We reviewed diseases for which family history information was collected, study setting, tool design, type of family history collection, presence of risk-assessment and recommendations for management, and assessment (categorized as either validity or benefit).
Results: Eighteen family history tools were identified: six generic, two on cardiovascular disease and ten on cancer. The six generic tools were partly tested in primary care (3x), are mainly computerized (4x), rarely include management recommendations for the physician (1x) and were partly validated against a reference standard (genetic counsellor) (3x, plus one planned). Of the five specific tools studied in primary care, none was validated. No family history tool allows electronic transfer of family history information to electronic medical record systems. Use of a family history tool improved identification of patients at risk for disease.
Conclusion: Several promising family history tools for primary care have been developed but large-scale implementation cannot be advised yet, based on available validation studies.
Keywords::
KEY MESSAGE:
Eighteen family history tools were identified: six generic, two on cardiovascular disease and ten on cancer.
Using a family history tool identified a higher number of patients at increased risk for disease.
For future implementation of family history tools in primary care, more validation and management studies are required.
INTRODUCTION
The family history reflects genetic, environmental and behavioural aspects of family health (Citation1). In primary care, family history is an inexpensive, non-invasive aid for diagnosis and risk-assessment in medical genetics (Citation2,Citation3). Traditionally, information on family health is mainly used for diagnostic objectives, referral to specialist care and insight into family dynamics (Citation4). The collection of family history data may also be used in risk-assessment for the prevention of common chronic diseases such as cardiovascular disease. Higher family risk may lead to customized interventions and the improvement of patient's motivation to change their behaviour (Citation5–7).
In a previous systematic review our group has shown that family physicians consider taking a family history to be their responsibility. However, observational studies of consultations and analyses of medical records showed wide variability and a low degree of regular updating (Citation8). We confirmed the findings of the review by Rich et al., that lack of time, limited knowledge and skills and poor reimbursement are experienced as barriers to family history taking in primary care (Citation2,Citation8). Tools overcoming these barriers might help family physicians in adequate family history taking. A family history tool should collect more data than a simple question (‘Does disease X run in your family?’); yet practically, it cannot be as comprehensive as a complete pedigree-interview. A recent review revealed that few family health questionnaires (FHQs) have been formally evaluated and that there were no—simple, short—generic FHQs suitable for use in primary care practice (Citation9). Another recent review reported that various organizations are developing family history tools, which should have decision support capabilities and should be compatible with electronic health records (Citation10).
We wondered whether progress had been made in this area since the publication of these reviews (Citation2,Citation8–10). The objective of the current systematic review is to explore the current state of the art regarding family history tools that might be suitable for primary care. What tools exist and what are their characteristics? We discuss their potential use in primary care.
METHODS
Search strategy
We conducted a systematic literature review with reporting according to PRISMA guidelines (Citation11) in PubMed, Embase and Cinahl, using a combination of a set of key terms family history, family health history, tool*, medical record system, computer-assisted decision making, general practi*, family medicine, family practi*, family physician*, primary care, primary health care, health, score*, instrument* and the MeSH-terms pedigree, genetic testing, genetic predisposition to disease, medical history taking, medical records systems, computerized, electronic health records, decision support techniques and decision making, computer-assisted. Limits used were Humans and Publication date 1 January 2002 (i.e. 1 January 2002–May 2012). As the oldest previously published review was dated 2004, limiting our search from 1 January 2002 onwards seemed safe in order not to miss any relevant publications. A full overview of the search strategy is provided in the Supplementary Appendix to be found online at http://www.informahealthcare.com/doi/abs/10.3109/13814788.2013.840825.
Inclusion and exclusion criteria
Included were (a) original research papers; (b) describing the existence or characteristics of family history tools that (c) are being used or—in the opinion of the authors of this review—could possibly be applied in primary care; and (d) of which assessment of validity or benefit (specified in the section on data extraction) had been performed or was planned. We included articles describing generic and disease-specific (e.g. on cancer, or cardiovascular disease) family history tools. Articles on specialist tools for specific diseases (e.g. a cardiology tool for specific types of cardiomyopathy) were excluded as were articles describing tools that did not primarily focus on family history. Articles describing a simple family history question, (‘Does disease X run in your family? Yes/No’) were also excluded since broader collection of family history information was aimed for.
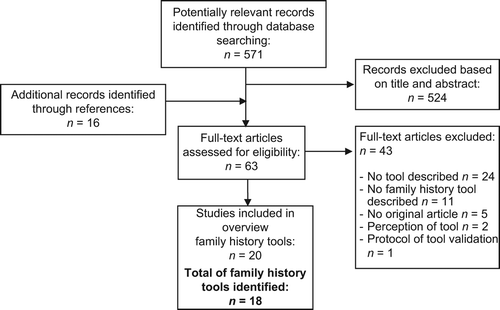
Selection of studies ()
The search identified 571 titles. Initial screening of papers based on title and abstract was independently performed by all authors (C.H., P.P. and H.S.). Subsequently, C.H., P.P. and H.S. independently reviewed full text copies of all potentially relevant articles. Additional references were retrieved by manually reviewing the reference lists of these papers (C.H.). Disagreement on papers was resolved by discussion until consensus was reached.
After consideration of title and abstract, 524 articles were excluded. By manually screening reference lists, 16 additional records were identified, leading to a total of 63 potentially relevant full text articles. Of these, 24 papers did not describe a tool, 11 described no family history tool (but for instance a decision aid or risk- assessment tool), five papers were not an original article (editorial, commentary), two papers were studies on perception of a tool and one paper was the protocol of a tool validation study. The remaining 20 studies were included in this review.
Data extraction and analysis
We categorized and summarized the tools and their characteristics as ‘generic’ (multiple diseases, ) or ‘disease-specific’ (one disease or disease group, ).
Table 1. Studies reporting on generic family history tools.
Table 2. Studies reporting on disease-specific family history tools.
We evaluated the setting in which the tool had been tested (primary care or other), the (number of) diseases addressed by the tool and its design (paper questionnaire/ computerized tool/web-based tool; to be completed by patient or physician). We distinguished disease- from pedigree-oriented types of family history collection; disease-oriented family history collection comprises merely stating the presence or absence of a disease within a family when showing a list of diseases to the proband, whereas pedigree-oriented family history collection attempts to generate a complete overview of family members (pedigree) and subsequently to assign diseases to each relative (genogram). In addition, presence of risk-assessment and recommendations for management for either doctor or patient were determined.
We categorized tool assessment into ‘validation’ (i.e. comparison with a reference standard; outcomes: sensitivity, specificity and/or agreement) and ‘benefit’ (i.e. advantages brought by the tool, e.g. identification of more patients at high risk or eligible for genetic screening, revision of probability estimate, psychological or behavioural effects in patients), respectively.
RESULTS
Included papers
In 20 included papers, 18 family history tools were identified. Six generic family history tools were described in eight papers () (Citation1,Citation12–18). The remaining 12 papers described 12 disease-specific family history tools () (Citation19–30).
Generic family history tools ()
The six generic family history tools included four computerized tools (MeTree, Health Heritage©, My Family Health Portrait and Family Healthware™, three of which were web based), and two paper-based FHQs () (Citation1,Citation12–16). The computerized tools had been developed quite recently. All tools are patient-completed and have a pedigree-oriented type of family history collection. The number of involved conditions ranges from 6–89. The main concerned diseases are (coronary) heart disease, stroke, diabetes and ovarian, breast and colorectal cancer. Only Health Heritage© (89 conditions) reported on the time it took to complete the collection of a full family history, ranging from 1–120 h (median around six hours) (Citation12). Each generic family history tool contains risk assessment; in MeTree this is provided for five out of 48 conditions (Citation15). Notably, to stratify disease risk, pedigrees acquired by My Family Health Portrait, are entered into an extra software program, Family Healthware™ (Citation1,Citation13).
Patient-specific recommendations for management are given in four tools (Citation1,Citation12,Citation14, Citation15). Only in My Family Health Portrait patients are advised to show their results to their physician (Citation13). Most recommendations for management are aimed at the patient; doctor specific recommendations or decision aids are rarely provided in currently existing generic family history tools (Citation14,Citation15).
Health Heritage© (Citation12), My Family Health Portrait (Citation13) and the FHQ described by Qureshi et al., in 2005 (Citation16) were formally validated; for MeTree a validation study has been planned (Citation15). Taking assessment by a genetic counsellor as the reference standard, sensitivity ranged from 67 to 100%, specificity from 92 to 100%, agreement was 77% (Citation13, Citation16). A tool outperformed usual care (Citation12). Taking clinical benefit (additional high-risk patients identified, behaviour change) as the outcome variable, using a tool was valuable too (Citation17,Citation18).
Three generic family history tools were or will be studied in a primary care setting: MeTree (Citation15), Family Healthware™ (Citation1,Citation17,Citation18), and the FHQ described by Qureshi et al. (Citation16).
Disease-specific family history tools ()
Two disease-specific tools concern coronary heart disease and cardiovascular disease respectively (Citation27,Citation29); one of them, the FHQ by Qureshi 2012 is designed for use in primary care (Citation29). The other 10 disease-specific family history tools collect family history of cancer, four of which were designed for use in primary care (Citation23,Citation25,Citation28,Citation30). All tools are patient-completed except GRAIDS, in which the physician enters information of the patient-completed FHQ into pedigree-drawing software (Citation23). Other features are described in . The time to complete the collection of family history is reported for the cardiovascular disease FHQ of Macleod et al., (15 min) (Citation27), GREAT (34 min (range: 8–55 min)) (Citation19), ‘Are you at risk for hereditary breast cancer?’ (< 10 min) (Citation22) and GRACE (30 min) (Citation21).
Risk-assessment is included in nine tools (Citation20,Citation21, Citation23–26,Citation28–30) and recommendations for management are given in six tools (Citation20–23,Citation26,Citation30); to the patient in four (Citation21–23,Citation26), to the doctor (referral for genetic testing) in one (Citation20) and to both doctor and patient in one (Citation30).
Formal validation of the family history tool with comparison to a reference standard (genetic counsellor pedigree/interview) has taken place in GREAT and the questionnaire by Fisher et al. 2003, with kappa 0.70 and agreement of 100% respectively (Citation19,Citation24). Benefit is described for 10 tools (Citation20–23,Citation25–30).
DISCUSSION
Main findings
In this systematic review, 18 family history tools were identified: six generic tools, two on cardiovascular disease, and 10 on cancer. The main findings are presented in and . The generic tools were partly tested in primary care (3x), are mainly computerized (4x), rarely include management recommendations for the physician (1x) and were partly validated against a reference standard (3x, plus one planned). Of the five specific tools two are computerized, and none has been validated. Where value for clinical practice had been assessed, family history tools improved identification of patients at risk compared to usual care. No family history tool was designed to allow electronic transfer of family history information to electronic medical record systems.
Table 3. Main findings: Summary overview of generic family history tools.
Table 4. Main findings: Summary overview of five specific family history tools for primary care.a
Strengths and limitations
Several reviews about family history tools have been published (Citation2,Citation8–10). This review has added value because of its focus on primary care and its systematic approach (Citation11). Multiple search strategies were tested for sensitivity before deciding on the final search strategy.
Potential benefit
The number of high-risk patients correctly identified by a tool in addition to those identified in usual care is an indicator of clinical benefit (Citation14,Citation17,Citation20,Citation27–30). The variation in results can be explained by differences in setting and the number identified in usual care. Identifying more patients at risk for disease or eligible for referral (Citation23) is beneficial only if these patients are ‘true positives’ (specificity), if there is enough capacity for further care, and if it does not increase anxiety or worry needlessly. Many tools have near-perfect specificity by definition because data interpretation is directly derived from current guidelines (Citation27–30). So far, there are no indications that use of a tool will increase cancer-related worries (Citation21).
Risk assessment
All generic family history tools included risk assessment. These tools focused on chronic diseases—heart disease, stroke, diabetes and ovarian, breast and colorectal cancer—characterized by ‘substantial public health burden, well-defined case definition, awareness of disease among relatives, accurate reporting by family members, family history being an established risk factor and effective interventions for primary and secondary prevention being available’ (Citation31).
In primary care, generic family history tools might be used as a screening strategy for every patient to provide the family physician with an idea of what medical risks run in the family. This could influence future clinical decisions (e.g. starting treatment in mild hypertension in a patient belonging to a family with high cardiovascular risk). If the family physician has a list of patients, the family history could be added to the medical record when the patient enters the practice (Citation8).
In case a patient expresses a specific concern for cancer or cardiovascular disease, a disease-specific tool could be used (e.g. a high risk profile for colorectal carcinoma might justify referral for colonoscopy). Within primary care, though, a disease-specific tool may have little added value; most relevant diseases are covered by generic tools as well, and the extra detail provided by some disease-specific tools will hardly support the decision to refer for diagnostic evaluation or not.
Barriers for taking a family history
The ‘lack-of-time’ barrier for the family physician can largely be taken away by having the patient complete the tool in his/her own time, and by providing risk assessment and management recommendations for the physician (Citation2,Citation8). The latter would also diminish another barrier for taking a family history in primary care, i.e. the perceived limited knowledge and skills of family physicians (Citation2,Citation8). Only one tool (i.e. MeTree) includes guideline recommendations for the physician (Citation15). Finally, none of the tools is designed to be integrated in a medical record system or to facilitate electronic information transfer (Citation32).
Ideal features
Ideally, a family history tool is:
self-administered by patients (computerized),
can be integrated with electronic health record systems (computerized),
is easy to use (by patient and health professional) whilst still collecting sufficient information to assess risk,
has an easy update functionality to follow family health over time (computerized),
comprises risk-assessment based on incorporated algorithms (computerized) and
contains evidence-based management (prevention) strategies for every familial risk level (Citation1,Citation12,Citation31).
These desirable features are mostly present in the more recent, computerized family history tools: MeTree, Health Heritage©, My Family Health Portrait and Family HealthwareTM (Citation1,Citation12,Citation13,Citation15), of which MeTree and Family HealthwareTM are designed for primary care, but not (yet) validated. In addition, the non-computerized FHQ by Qureshi is designed for primary care and has been validated (Citation16).
Future directions
Now that 18 family history tools have been identified, adaptation for use—and consequently validation—in primary care is the next step in developing user-friendly tools for patients and doctors (Citation9). All generic tools identified were developed in either the US or the UK, and many were designed from a public health perspective. Therefore, studies in primary care in other (European) countries with various health care systems should be performed.
Linking family history tools to clinical guidelines is necessary to enable recommendations for management by the family physician. Another crucial element for implementation of family history tools in primary care will be their integration with electronic medical record systems, where all other medical information is stored, available for clinical decision making (Citation10,Citation32). Finally, easy updating is a key feature, as both family information and scientific knowledge change over time.
Conclusion
Eighteen family history tools were identified: six generic, two on cardiovascular disease, and 10 on cancer. Use of a family history tool improved identification of patients with increased risk for disease. Despite these promising developments, more validation and management studies in primary care are needed before large scale implementation of family history tools can be advocated.
Supplementary Appendix
Download PDF (26.4 KB)Declaration of interest: The corresponding author reports that he also is Editor-in-Chief of this Journal. The other authors report no conflicts of interest.
REFERENCES
- Yoon PW, Scheuner MT, Jorgensen C, Khoury MJ. Developing family healthware, a family history screening tool to prevent common chronic diseases. Prev Chronic Dis. 2009;6:A33.
- Rich EC, Burke W, Heaton CJ, Haga S, Pinsky L, Short MP, et al. Reconsidering the family history in primary care. J Gen Intern Med. 2004;19:273–80.
- Wolpert CM, Speer MC. Harnessing the power of the pedigree. J Midwifery Womens Health. 2005;50:189–96.
- Claassen L, Henneman L, Janssens AC, Wijdenes-Pijl M, Qureshi N, Walter FM, et al. Using family history information to promote healthy lifestyles and prevent diseases; a discussion of the evidence. BMC Public Health. 2010;13;10:248.
- Audrain-McGovern J, Hughes C, Patterson F. Effecting behavior change: Awareness of family history. Am J Prev Med. 2003;24:183–9.
- Petruccio C, Mills Shaw KR, Boughman J, Fernandez C, Harlow I, Kruesi M, et al. Healthy choices through family history: A community approach to family history awareness. Community Genet. 2008;11:343–51.
- Walter FM, Emery J. Perceptions of family history across common diseases: A qualitative study in primary care. Fam Pract. 2006;23:472–80.
- Plat AW, Kroon AA, Van Schayck CP, De Leeuw PW, Stoffers HE. Obtaining the family history for common, multifactorial diseases by family physicians. A descriptive systematic review. Eur J Gen Pract. 2009;15:231–42.
- Reid GT, Walter FM, Brisbane JM, Emery JD. Family history questionnaires designed for clinical use: A systematic review. Public Health Genomics. 2009;12:73–83.
- Valdez R, Yoon PW, Qureshi N, Green RF, Khoury MJ. Family history in public health practice: A genomic tool for disease prevention and health promotion. Annu Rev Public Health. 2010;31:69–87.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J Clin Epidemiol. 2009;62:1006–12.
- Cohn WF, Ropka ME, Pelletier SL, Barrett JR, Kinzie MB, Harrison MB, et al. Health Heritage(c) a web-based tool for the collection and assessment of family health history: Initial user experience and analytic validity. Public Health Genomics. 2010;13:477–91.
- Facio FM, Feero WG, Linn A, Oden N, Manickam K, Biesecker LG. Validation of my family health portrait for six common heritable conditions. Genet Med. 2010;12:370–5.
- Frezzo TM, Rubinstein WS, Dunham D, Ormond KE. The genetic family history as a risk assessment tool in internal medicine. Genet Med. 2003;5:84–91.
- Orlando LA, Hauser ER, Christianson C, Powell KP, Buchanan AH, Chesnut B, et al. Protocol for implementation of family health history collection and decision support into primary care using a computerized family health history system. BMC Health Serv Res. 2011;11:264.
- Qureshi N, Bethea J, Modell B, Brennan P, Papageorgiou A, Raeburn S, et al. Collecting genetic information in primary care: Evaluating a new family history tool. Fam Pract. 2005; 22:663–9.
- Rubinstein WS, Acheson LS, O’Neill SM, Ruffin MT 4th, Wang C, Beaumont JL, et al. Clinical utility of family history for cancer screening and referral in primary care: a report from the Family Healthware Impact Trial. Genet Med. 2011;13:956–65.
- Ruffin MT, Nease DE, Jr, Sen A, Pace WD, Wang C, Acheson LS, et al. Effect of preventive messages tailored to family history on health behaviors: The Family Healthware Impact Trial. Ann Fam Med. 2011;9:3–11.
- Acheson LS, Zyzanski SJ, Stange KC, Deptowicz A, Wiesner GL. Validation of a self-administered, computerized tool for collecting and displaying the family history of cancer. J Clin Oncol. 2006;24:5395–402.
- Armel SR, McCuaig J, Finch A, Demsky R, Panzarella T, Murphy J, et al. The effectiveness of family history questionnaires in cancer genetic counseling. J Genet Couns. 2009;18:366–78.
- Braithwaite D, Sutton S, Mackay J, Stein J, Emery J. Development of a risk assessment tool for women with a family history of breast cancer. Cancer Detect Prev. 2005;29:433–9.
- Cohn WF, Jones SM, Miesfeldt S. ‘Are you at risk for hereditary breast cancer?‘: Development of a personal risk assessment tool for hereditary breast and ovarian cancer. J Genet Couns. 2008;17:64–78.
- Emery J, Morris H, Goodchild R, Fanshawe T, Prevost AT, Bobrow M, et al. The GRAIDS Trial: A cluster randomised controlled trial of computer decision support for the management of familial cancer risk in primary care. Br J Cancer. 2007;97:486–93.
- Fisher TJ, Kirk J, Hopper JL, Godding R, Burgemeister FC. A simple tool for identifying unaffected women at a moderately increased or potentially high risk of breast cancer based on their family history. Breast. 2003;12:120–7.
- Hughes KS, Roche C, Campbell CT, Siegel N, Salisbury L, Chekos A, et al. Prevalence of family history of breast and ovarian cancer in a single primary care practice using a self-administered questionnaire. Breast J. 2003;9:19–25.
- Kelly KM, Porter K, Remy A, Westman JA. Promotion of cancer family history awareness: Jameslink Cancer Risk Assessment Tool at community health fairs. J Genet Couns. 2008; 17:274–82.
- MacLeod HM, McNally EM. A pilot study of a family history risk assessment tool for cardiovascular disease. J Genet Couns. 2008;17:499–507.
- Murff HJ, Greevy RA, Syngal S. The comprehensiveness of family cancer history assessments in primary care. Community Genet. 2007;10:174–80.
- Qureshi N, Armstrong S, Dhiman P, Saukko P, Middlemass J, Evans PH, et al. Effect of adding systematic family history enquiry to cardiovascular disease risk assessment in primary care: A matched-pair, cluster randomized trial. Ann Intern Med. 2012; 21;156:253–62.
- Ozanne EM, Loberg A, Hughes S, Lawrence C, Drohan B, Semine A, et al. Identification and management of women at high risk for hereditary breast/ovarian cancer syndrome. Breast J. 2009;15:155–62.
- Yoon PW, Scheuner MT, Khoury MJ. Research priorities for evaluating family history in the prevention of common chronic diseases. Am J Prev Med. 2003;24:128–35.
- Feero WG, Bigley MB, Brinner KM. New standards and enhanced utility for family health history information in the electronic health record: An update from the American Health Information Community’s Family Health History Multi-Stakeholder Workgroup. J Am Med Inform Assoc. 2008; 15:723–8