Abstract
Background: In patients with superficial venous thrombosis (SVT) co-existence of deep venous thrombosis (DVT) can be present. Varicosities are considered as a risk factor for both SVT and DVT separately. However, current evidence is contradictory whether varicosities are associated with an increased or reduced prevalence of concomitant DVT in patients with SVT.
Objectives: To determine the diagnostic value of both presence and absence of varicosities in the detection of concomitant DVT in non-hospitalized patients with SVT.
Methods: In MEDLINE and EMBASE, a systematic search was performed to collect all published studies on this topic. The selected papers were critically appraised. By diagnostic 2 × 2 tables prior probabilities and predictive values were computed.
Results: Six relevant articles were identified. The prior probability of concomitant DVT in patients referred from primary care to the outpatient clinic varied between 13 and 34%. In five studies, absence of varicosities was related to a higher probability of concomitant DVT (33–44%) compared to a presence of varicosities (3–23%). The sixth study showed an inversed, non-significant association: DVT was present in 21% of patients with SVT on non-varicose veins versus in 35% of patients with SVT on varicose veins.
Conclusion: In five out of six studies on patients with SVT in outpatient settings, absence of varicosities was related to a higher probability of concomitant DVT. Further research is needed to determine whether an assessment of varicosities in general practice could result in an improved selection of patients who require additional imaging to detect or exclude DVT.
In five studies on patients with SVT in outpatient settings, the absence of varicosities was associated with a higher risk of concomitant DVT (33–44%).
Further research should indicate whether the assessment of varicosities could improve the selection of SVT patients who require additional imaging to exclude DVT.
INTRODUCTION
Superficial venous thrombosis (SVT) or ‘thrombophlebitis’ is a common disease. Yearly 125 000 SVT cases are reported in the United States, others report an annual incidence of 56 to 160 per 100 000 inhabitants per year and in the Netherlands the prevalence is reported to be 180 per 100 000 patients in primary care (Citation1–3). However, the true incidence is generally believed to be higher as many cases are unrecognized and unreported.
The inflammatory-thrombotic process in a superficial vein results in pain and a reddened, warm, tender cord extending along the vein. SVT is generally considered as a benign and self-limiting disease and is usually diagnosed by clinical presentation only (Citation4). However, SVT has been associated with a higher risk for deep venous thrombosis (DVT) and subsequent pulmonary embolism (PE), thus carrying considerable risks (Citation5,Citation6). Data for the prevalence of concomitant DVT vary greatly ranging from 2.7 to 53% (Citation7–10).
In primary care, a subset of patients with SVT is referred to outpatient clinics for additional imaging to exclude concomitant DVT or to differentiate from alternative diagnoses such as erysipelas, lymphangitis, tendonitis or Baker’s cyst. Patients with proximal SVT or SVT localized in the main stem vena saphena magna are considered to have the highest risk for concomitant DVT and are therefore more likely to be referred (Citation11–13). Moreover, clinical prediction rules are used to differentiate patients in high versus low probability for DVT. The Wells rule involves criteria as active cancer, paralysis/paresis or immobilization, localized tenderness, swelling of the calf/entire leg, pitting oedema, non-varicose collateral superficial veins or ‘alternative diagnosis more likely’ (Citation14). The AMUSE primary care rule involves the additional use of a rapid point-of-care D-dimer assay for low-probability patients in the Wells rule, and replaces ‘alternative diagnosis more likely’ with ‘absence of leg trauma’ (Citation15).
Importantly, none of the widely used clinical prediction rules mentioned above involve varicose veins as a criterion on which selection for referral should be based. Moreover, the Wells criteria are based on a population in which patients with SVT were excluded (Citation16).
Varicosities are considered as a risk factor for both SVT and DVT separately (Citation4,Citation17–19). Current evidence is contradictory about how varicosities are associated with concomitant DVT in patients with SVT, as both the presence and absence of varicosities have been linked to a higher prevalence of concomitant DVT (Citation20–22). Presence or absence of varicosities is easily established by physical examination. Therefore, it might serve as an easily implementable diagnostic tool resulting in a better selection of patients who require ultrasound imaging for confirmation of concomitant DVT.
The aim of this systematic review is to determine whether the presence or absence of varicosities in community-dwelling adults with clinically suspected SVT of the lower extremities improves the prediction of DVT.
METHODS
Search strategy and selection
A systematic search was performed in MEDLINE (via PubMed interface) and EMBASE to collect all published studies on the role of varicosities in the detection of DVT in patients clinically suspected for SVT. The search strategy used is detailed in Box 1. In short, for both the outcome (DVT) and determinant (varicosities) all synonyms were combined using the Boolean operators ‘OR’ and ‘AND.’ Synonyms for the domain (community-dwelling adults with clinically suspected SVT of the lower extremities) were not included in the search syntax as this might lead to missing articles.
#1 Determinant: (varicos*[tiab] OR varix[tiab] OR varices[tiab])
#2 Outcome: (deep[tiab] AND (vein[tiab] OR veinous[tiab] OR veins[tiab] OR venous[tiab]) AND (thrombosises[tiab] OR thrombosis[tiab] OR thromboses[tiab] OR thrombotic[tiab] OR thrombus[tiab] OR thrombi[tiab] OR thromboembolic[tiab] OR thromboembolism[tiab] OR thrombolism[tiab] OR trombosis[tiab] OR trombotic[tiab] OR trombus[tiab] OR trombi[tiab] OR tromboembolic[tiab] OR tromboembolism[tiab] OR obstruction[tiab] OR obstructions[tiab] OR occlusion[tiab] OR occlusions[tiab])) OR (deep[tiab] AND (venothrombosises[tiab] OR venothrombosis[tiab] OR venothrombotic[tiab] OR venothromboembolic[tiab] OR venothromboembolism[tiab])) OR DVT[tiab] OR VTE[tiab] OR (venous[tiab] AND thromboembolism[tiab]) OR (venous[tiab] AND tromboembolism[tiab]) OR (venous[tiab] AND thromboembolisms[tiab])
#3: #1 AND #2
Legend. #, search number.
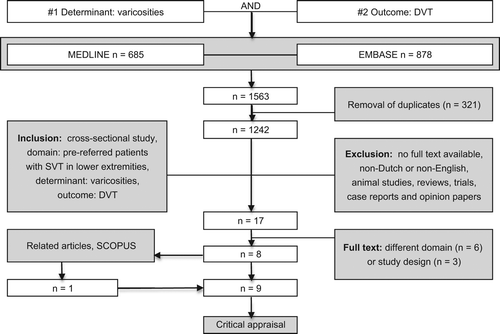
The selection process is shown as a flowchart in . In short, all titles and abstracts were searched and selected by one author independently. This work was divided between four authors. In case of doubt whether an article should be in- or excluded, the article was included and screened by four authors based on full-text. Studies were considered relevant for this review if they met the following inclusion criteria: a cross-sectional study design (or at least a cross-sectional part of the study), inclusion of pre-referred patients with SVT in the lower extremities, assessment of varicosities as determinant and presence of concomitant DVT as outcome. Articles were excluded if the study design was not applicable (e.g. reviews, trials, case reports and opinion papers), animal studies, non-Dutch or non-English, or if no full text was available after extensive effort, which included contact with study authors and retrieving possible paper versions in all academic libraries in the Netherlands. Reviews were checked for references and a second reference check was performed with SCOPUS, to avoid missing articles.
Critical appraisal
The selected papers were independently critically appraised by two authors on relevance and validity (risk of bias) according to standardized criteria, shown in . In case of discordant judgement, a third author was consulted to retrieve consensus.
Table 1. Critical appraisal: assessment of risk of bias of individual studies selected for qualitative synthesis. All studies mentioned in the table had a cross-sectional study design, or contained at least a cross-sectional element.
The risk of selection bias was determined by assessing completeness of data (varicosities assessed and ultrasound or phlebography performed in all patients). A greater weight was given to this criterion compared to blinding or standardization, as missing data could severely distort the calculated predictive values due to selection bias.
The risk of information bias was determined by judgement whether assessment of varicosities was blinded from the outcome. Reasonably, blinding for presence of varicosities is not possible during the ultrasound examination for DVT. Therefore, it was only critically appraised whether authors mentioned blinding for presence of DVT during the assessment of varicosities. Then, it was assessed to what extent the measurement of the determinant (presence or absence of varicosities) and outcome (DVT, by ultrasound or phlebography) was standardized and clearly described (Citation23).
Final analysis
For all studies eligible for final analysis, diagnostic 2 × 2 tables of all patients with SVT were created. The columns contained presence and absence of DVT and the rows contained presence and absence of varicosities; being considered as a diagnostic test. First, the prior probability (presence) of concomitant DVT in all SVT patients was calculated. Second, the predictive values of presence and absence of varicosities was determined for each study separately by calculating the percentage of patients with DVT for both subgroups (with and without varicosities). Third, 95% confidence intervals (CI) were calculated online by the interactive statistics page (Citation24).
RESULTS
The systematic search (April 2014) in three medical databases retrieved 1563 titles, which resulted in 1242 titles after removal of duplicates. The selection process is further detailed in the flowchart in . The reference check retrieved one extra article; therefore, after careful selection finally nine papers were qualified for further assessment.
Risk of bias
As shown in , critical appraisal led to the rejection of Skillman et al. and Lutter et al. due to insufficient completeness of data (Citation25,Citation26). As ultrasound or phlebography was not performed in all patients, selection bias could have taken place because in subgroups, the presence of (subclinical) DVT could have been missed. One study was sufficiently valid but not suitable for data-analysis as only odds ratios were mentioned; therefore, no diagnostic 2 × 2 tables could be recreated (Citation27). Blinding and standardization of assessment of varicosities was not described in any of the studies, most probably because that the presence of varicosities in relation to the presence of DVT was not the main study objective but part of certain ‘baseline characteristics.’
Finally, six studies were considered to be eligible for inclusion in qualitative synthesis (Citation21,Citation28–32). These studies included patients referred from primary care to the outpatient clinics; there was no study available that was performed in primary care prior to referral. All selected studies were in English. A systematic description of all studies is included in .
Table 2. Probability of concomitant DVT in patients with SVT: Prior, in absence and in presence of varicosities.
Bergqvist et al. included 56 consecutive patients with clinically suspected SVT, based on palpable elongated subcutaneous lumps along the axis of superficial veins, tenderness, swelling and redness (Citation28). Patients were consecutive patients coming to the emergency department, either spontaneously or sent in from the primary care.
Prountjos et al. analysed 57 consecutive patients admitted with clinically manifested SVT to a surgical department (Citation21). All patients underwent phlebography during their hospitalization. According to the authors, no evident signs of DVT were present in these patients.
Gorty et al. retrospectively reviewed 1815 venous duplex ultrasound scans performed in a three-year period in an outpatient private office for findings of SVT (Citation29). In total, 77 patients had ultrasound-documented SVT of whom 17 were excluded due to incomplete data.
In a cross-sectional and prospective study by Decousus et al. 844 patients with symptomatic and ultrasound-confirmed SVT were included (Citation30). Most patients were referred to a vascular specialist for compression ultrasonography to confirm the diagnosis of SVT and to exclude concomitant DVT.
Galanaud et al. studied 783 patients with symptomatic SVT objectively confirmed by compression ultrasound (Citation31). Patients were selected from 8256 in- and outpatients referred to vascular medicine physicians for clinically suspected VTE (SVT, DVT or pulmonary embolism).
Finally, Hirmerova et al. included 138 patients referred to a vascular clinic of a tertiary hospital with clinical and ultrasonographical signs of SVT of the legs (Citation32). In this cross-sectional study, it was observed if patients had SVT on a varicose vein (varicophlebitis) or on a non-varicose vein.
Of these six studies, the prior probabilities of concomitant DVT and predictive values of presence and absence of varicosities are shown in .
DISCUSSION
Main findings
In this hypothesis generating systematic review of available literature, the relationship between varicosities and concomitant DVT in patients with SVT was assessed. All studies reported a high prevalence of concomitant DVT in patients with SVT who were referred to the outpatient clinic, varying from 13 to 34%. In five of the six studies, concomitant DVT was more prevalent in patients without varicosities (33–44%) compared to patients with varicosities (3–23%) (Citation21,Citation28–31). The sixth study showed an inversed, non-significant result: DVT was present in 35% (95% CI: 27–45) of patients with SVT on non-varicose veins and in 21% (95% CI: 5–51) of patients with SVT on varicose veins (Citation32). Due to the heterogeneity of the assessed studies and inclusion of patients who were referred from primary care, a strong recommendation regarding the assessment of varicosities as a diagnostic tool in primary care cannot be made yet.
Proposed underlying mechanism
The pathophysiological mechanisms why patients with SVT and varicosities could have a lower incidence of concomitant DVT are unclear. Although it was outside the scope of this study, some underlying causes have been suggested. In patients with varicosities, stasis and wall damage could result in the development of thrombosis. In the absence of varicosities, systemic diseases such as thrombophilia or inflammation could play a larger role in the development of SVT, simultaneously leading to a higher risk of concomitant DVT (Citation25,Citation33).
Strengths and limitations
The selected articles in this systematic review included patients with SVT referred from primary care to a vascular medicine specialist for ultrasound imaging, or reviewed consecutive duplex ultrasounds in order to include patients with diagnosed SVT (Citation21,Citation28–32). Unfortunately, no study was performed in primary care, before the decision to refer or not. The perceived benign prognosis of SVT may explain underreporting and the scarcity of available data (Citation31). Because the studies only included patients with SVT who were referred from primary care, the prevalence of concomitant DVT found in this study may be much higher than in the whole population of patients with SVT seen in primary care. Moreover, this could lead to referral bias. The selection by general practitioners prior to referral might influence the found relation between varicosities and DVT in SVT patients. For instance, primary care physicians could refer SVT patients with varicosities for imaging more easily, as they might have a higher suspicion for DVT in these patients compared to patients without varicosities. Besides, the symptoms of varicosities can be in part similar to those as DVT; therefore, a larger proportion of patients with both varicosities and SVT could be referred for further diagnostics.
Key methodological limitations of this review include that only articles published in English and Dutch were included, screening was based on title and abstract and unpublished studies were not searched and analysed.
A fraction of the patients (7–16%) was hospitalized (Citation29–31) or phlebography was performed during hospitalization after primary admission due to SVT (Citation21). Prountjos et al. identified and excluded patients with clinical signs of DVT, which influences the prior probability of concomitant DVT (Citation21). In two studies outcomes were measured as venous thromboembolism (Citation30,Citation32). DVT and pulmonary embolism were not measured as separate outcomes in patients with varicosities, however, only a relatively small amount of patients with VTE was diagnosed with isolated pulmonary embolism (7–11%). Hirmerova et al. assessed if the SVT occurred on a varicose or non-varicose vein, instead of the presence or absence of varicosities in general (Citation32). Although all studies included patients referred to the hospital or outpatient clinic, these differences in severity of disease and patient characteristics most probably account for the wide range of concomitant DVT.
Due to the differences in study-design, intervention and patient population between the selected studies, we have chosen not to perform a meta-analysis in this hypothesis-generating review, as this would retrieve unreliable results (Citation34).
Implications for further research
The studies addressed in this review included patients who were referred from primary care. In the study of Hirmerova et al. only 10% of all patients (n = 14) presented with SVT on non-varicose veins (Citation32). This leads to very broad and widely overlapping confidence intervals; we therefore, agree with the authors that no relevant conclusion might be derived from this study.
Therefore, future research in large patient groups should be performed in primary care to indicate whether the assessment of varicosities could result in a better selection of SVT patients who should be referred for further imaging to exclude concomitant DVT. In addition to this, further research might indicate whether varicosities together with the current clinical prediction rules for primary care and additional clinical features as the presence of proximal SVT can be incorporated in an improved prediction rule for DVT in SVT patients (Citation11–16).
Conclusion
In five studies on patients with SVT in outpatient settings, the absence of varicosities is associated with a higher risk of concomitant DVT. Further research is needed to determine whether the assessment of varicosities could be of additional value in the selection of SVT patients in primary care who require additional imaging to detect or exclude DVT.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- de Wees M. Non-operative treatment of acute superficial thrombophlebitis and deep femoral thrombosis. In: Ernst CB, Stanley JC, editors. Current therapy in vascular surgery. Philadelphia: BC Decker; 1991. pp. 952–60.
- Anderson FA, Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991;151:933–8.
- van der Linden MW, Westert GP, de Bakker DH, Schellevis FG. Tweede nationale studie naar ziekten en verrichtingen in de huisartspraktijk. Klachten en aandoeningen in de bevolking en in de huisartspraktijk. (Second National Study into disease and treatment in family practice. Symptoms and ailments in the population and in the family practice). Utrecht/Bilthoven: NIVEL/RIVM; 2004. (In Dutch.) Available at: http://www.nivel.nl/nationalestudie/ (accessed 7 April 2014).
- Walma EP, Eekhof JAH, Nikkels J, Buis P, Jans PGW, Slok-Raymakers EAM, et al. NHG-Standaard Varices (tweede herziening). (Dutch College of General Practitioners’ (NHG) practice guideline ‘varicosities’ (second revision)). Huisarts Wet. 2009:52:391–402. (In Dutch.)
- van Weert H, Dolan G, Wichers I, de Vries C, ter Riet G, Buller H. Spontaneous superficial venous thrombophlebitis: Does it increase risk for thromboembolism? A historic follow-up study in primary care. J Fam Pract. 2006;55:52–7.
- ten Cate-Hoek AJ, van der Velde EF, Toll DB, van Weert HC, Moons KG, Büller HR, et al. Common alternative diagnoses in general practice when deep venous thrombosis is excluded. Neth J Med. 2012;70:130–5.
- Di Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev. 2007;CD004982.
- Bounameaux H, Reber-Wasem MA. Superficial thrombophlebitis and deep vein thrombosis. A controversial association. Arch Intern Med. 1997;157:1822–4.
- Schönauer V, Kyrle PA, Weltermann A, et al. Superficial thrombophlebitis and risk for recurrent venous thromboembolism. J Vasc Surg. 2003;37:834–8.
- Blättler W, Schwarzenbach B, Largiadér J. Superficial vein thrombophlebitis-serious concern or much ado about little? Vasa 2008;37:31–8.
- Timmermans SH, Wlazlo N, Mom EM, Stoffers HE. Thrombophlebitis of the leg: Diagnosis and treatment by the general practitioner. Ned Tijdschr Geneeskd. 2010;154:1098. (In Dutch.)
- Chengelis DL, Bendick PJ, Glover JL, Brown OW, Ranval TJ. Progression of superficial venous thrombosis to deep vein thrombosis. J Vasc Surg. 1996;24:745–9.
- Blumenberg RM, Barton E, Gelfand ML, Skudder P, Brennan J. Occult deep venous thrombosis complicating superficial thrombophlebitis. J Vasc Surg. 1998;27:338–43.
- Wells PS, Anderson DR, Bormanis J, Guy F, Mitchell M, Gray L, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997;350:1795–8.
- Büller HR, Ten Cate-Hoek AJ, Hoes AW, Joore MA, Moons KG, Oudega R, et al. AMUSE (Amsterdam Maastricht Utrecht Study on thromboembolism) Investigators. Safely ruling out deep venous thrombosis in primary care. Ann Intern Med. 2009;150:229–35.
- Wells PS, Hirsh J, Anderson DR, Lensing AW, Foster G, Kearon C, et al. Accuracy of clinical assessment of deep-vein thrombosis. Lancet 1995;345:1326–30.
- Leon L, Giannoukas AD, Dodd D, Chan P, Labropoulos N. Clinical significance of superficial vein thrombosis. Eur J Vasc Endovasc Surg. 2005;29:10–7.
- Crişan S, Vornicescu D, Crişan D, Pop T, Vesa S. Concomitant acute deep venous thrombosis and superficial thrombophlebitis of the lower limbs. Med Ultrason 2011;13:26–32.
- Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LI. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160:809–15.
- Jerkic Z, Karic A, Karic A. Clinically silent deep vein thrombosis in patients with superficial thrombophlebitis and varicose veins at legs. Med Arh. 2009;63:284–7.
- Prountjos P, Bastounis E, Hadjinikolaou L, Felekuras E, Balas P. Superficial venous thrombosis of the lower extremities co-existing with deep venous thrombosis. A phlebographic study on 57 cases. Int Angiol. 1991;10:63–5.
- Gillet JL, Allaert FA, Perrin M. Superficial thrombophlebitis in non-varicose veins of the lower limbs. A prospective analysis in 42 patients. J Mal Vasc. 2004;29:263–72.
- Hammer GP, du Prel JB, Blettner M. Avoiding bias in observational studies: Part 8 in a series of articles on evaluation of scientific publications. Dtsch Arztebl Int. 2009;106:664–8.
- Pezzullo JC. Statpages.org (Internet). Interactive statistics (updated 25 May2009). Available at: http://statpages.org/confint.html (accessed 7 April 2014).
- Skillman JJ, Kent KC, Porter DH, Kim D. Simultaneous occurrence of superficial and deep thrombophlebitis in the lower extremity. J Vasc Surg. 1990;11:818–24.
- Lutter KS, Kerr TM, Roedersheimer LR, Lohr JM, Sampson MG, Cranley JJ. Superficial thrombophlebitis diagnosed by duplex scanning. Surgery 1991;110:42–6.
- Sobreira ML, Maffei FH, Yoshida WB, Rollo HA, Lastoria S, Griva BL, et al. Prevalence of deep vein thrombosis and pulmonary embolism in superficial thrombophlebitis of the lower limbs: Prospective study of 60 cases. Int Angiol. 2009;28:400–8.
- Bergqvist D, Jaroszewski H. Deep vein thrombosis in patients with superficial thrombophlebitis of the leg. Br Med J. 1986;292:658–9.
- Gorty S, Patton-Adkins J, DaLanno M, Starr J, Dean S, Satiani B. Superficial venous thrombosis of the lower extremities: Analysis of risk factors, and recurrence and role of anticoagulation. Vasc Med. 2004;9:1–6.
- Decousus H, Quéré I, Presles E, Becker F, Barrellier MT, Chanut M, et al. POST (prospective observational superficial thrombophlebitis) study group. Superficial venous thrombosis and venous thromboembolism: A large, prospective epidemiologic study. Ann Intern Med. 2010;152:218–24.
- Galanaud JP, Genty C, Sevestre MA, Brisot D, Lausecker M, Gillet JL, et al. OPTIMEV SFMV investigators. Predictive factors for concurrent deep-vein thrombosis and symptomatic venous thromboembolic recurrence in case of superficial venous thrombosis. The OPTIMEV study. Thromb Haemost. 2011;105:31–9.
- Hirmerova J, Seidlerova J, Subrt I. Deep vein thrombosis and/or pulmonary embolism concurrent with superficial vein thrombosis of the legs: Cross-sectional single center study of prevalence and risk factors. Int Angiol. 2013;32:410–6.
- Milio G, Siragusa S, Miná C, Amato C, Corrado E, Grimaudo S, et al. Superficial venous thrombosis: Prevalence of common genetic risk factors and their role on spreading to deep veins. Thromb Res. 2008;123:194–9.
- Melsen WG, Bootsma MC, Rovers MM, Bonten MJ. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. 2014;20:123–9.