Abstract
Background: Patients visiting their GPs exceptionally often (frequent attenders, FAs) have high rates of somatic disease, emotional distress, psychiatric illnesses and social problems and require a disproportionate amount of their GPs’ time.
Objectives: To summarize which types of FA have been studied and what the effects of interventions were on quality of life (QoL), symptom severity of underlying illness(es) and consultation frequency. To discover when patients are considered FAs.
Methods: Systematic review of RCTs using a comprehensive search (MEDLINE, PsycINFO, CINAHL and EMBASE, from 1980 to August 2015) and no language restrictions. Two investigators extracted data. Results were summarized qualitatively.
Results: We included 17 RCTs. Heterogeneity at the level of populations, interventions and outcomes precluded statistical pooling. In-depth analysis by GPs assessing a patient’s reasons for frequent attendance decreased consultation frequency by four to six per year. A small effect on symptom severity was noted in depressed FAs, although this finding was not replicated in a recent trial. Multi-component therapy and medication in FAs with medically unexplained symptoms (MUS) improved QoL (SF36 odds ratio: 1.92; 95%CI: 1.08–3.40) and morbidity (CES-D 3.17; 95%CI: 1.27–5.08).
Conclusion: RCTs on intervention effects in frequent attenders to primary care used different patient populations, interventions, comparators and outcome measures. Consistent evidence on the effects of particular interventions in specific patient domains is lacking. A tailored approach based on in-depth analysis among GPs of potential reasons for frequent attendance may decrease consultation frequency. Research involving the screening and treating for FAs with MUS may be useful in future trials.
Key Messages
Frequent attenders require a disproportionate amount of GPs’ time and convincing evidence of effectiveness of treatments is lacking. Thirteen newly identified trials since 2008 did not change this situation much. Tailored treatment based on in-depth analysis of reasons for frequent attendance by a team of GPs may decrease consultation frequency.
Introduction
Since the 1960s, it has been observed that the general practitioner (GP) sees a proportion of his or her registered patients, frequently.[Citation1–3] In particular, GPs spend almost 40% of their time on 10% of their patients.[Citation4] Studies consistently report that the majority of these frequent attenders (FAs) have mental health problems, emotional distress and/or social difficulties, mostly on top of physical illness.[Citation5,Citation6] Chronic somatic and psychiatric illnesses are usually accepted reasons for frequent consultation. Temporary crises pass and may be a reason for brief periods of frequent consultation. Research shows that regression to the mean occurs, and only one out of seven patients remain FAs for three consecutive years.[Citation4] However, frequent attendance by multi-problem patients with undetected psychiatric morbidity may trigger many consultations and lead to ineffective healthcare and persistent frequent attendance.[Citation7] Persistent FAs (≥ 3 consecutive years of attendance ranking in the (sex-age adjusted) top 10%) make up about 1.6% of all enlisted patients,[Citation4] implying that in The Netherlands an average GP has some 40 patients who consult very often over extended periods of time. GPs may perceive this as an important burden. Moreover, persistent frequent attendance is associated with major healthcare spending in primary and specialist healthcare.[Citation8] If effective treatments exist, detection and treatment of morbidities could improve FAs’ quality of life (QoL) and lower the impact of frequent attendance on the healthcare system.[Citation4,Citation9] Unfortunately, interpretation and comparison of studies on frequent attendance are difficult because of their heterogeneous characteristics and different definitions.[Citation5]
Smits et al., in 2008, systematically reviewed interventions on frequent attenders in primary care and found five randomized controlled trials (RCTs) describing interventions on FAs.[Citation11] They found no convincing evidence that any intervention improves quality of life (QoL), morbidity or healthcare utilization of FAs. In the meantime, new RCTs have been published, and an updated review seems indicated. We wanted to answer the following questions:
Which interventions in FAs were studied in RCTs?
Which types of FAs were studied, and how were FAs defined?
What were the intervention effects on QoL, severity of symptoms or underlying illness(es) (morbidity) and consultation frequency?
Methods
We used acknowledged review methodology and reported according to PRISMA.[Citation12]
Eligibility criteria
We accepted all definitions of the term ‘frequent attender’. We defined primary care as all first points of consultation sites, not-in-hospital care. We included only RCTs. No language restrictions were used, and we accepted all possible interventions as long as a (usual care) control group was available.
Literature search
We searched EMBASE, MEDLINE, PsycINFO and CINAHL between 1 January 1980 and 13 August 2015 using a dedicated search strategy designed by a clinical librarian (FvE; see Appendix 1 in Supplementary Material, available online). Also, we checked the references of all included articles for additional relevant articles.
Selection of articles
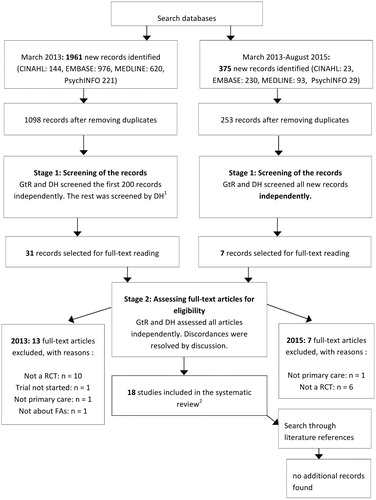
Stage 1. Two authors (GtR and DH) separately screened the first 200 hits and then discussed them, which made DH become familiar with this type of research. One author (DH) screened the rest of the titles and abstracts against the inclusion criteria. In the latest search, covering the period between January 1980 and August 2015, both authors evaluated all new hits independently. Disagreements were resolved by consensus. When there was doubt about the setting or the patient population, we assessed the full paper.
Stage 2. Full-text papers were retrieved for all studies considered to meet the inclusion criteria, and two authors (GtR and DH) independently assessed if they indeed met the inclusion criteria. Two authors (GtR and DH) independently extracted the data. Consensus resolved disagreements.
Quality assessment
One author (DH) appraised the quality of each RCT using a dedicated checklist.[Citation13] This checklist consisted of nine methodological items scored as yes, no, or uncertain (Table 21). A second author (GtR) independently checked the quality assessments, which DH indicated as difficult to assess. Disagreements were resolved by consensus.
Results
Selection of articles
The search yielded 1098 articles of which 17 were included. In an update of the search, an additional 253 articles were found, but no new eligible RCTs. shows a full flowchart.
Population and types of frequent attenders
The included studies targeted diverse types of FAs (). Three studies enrolled older FAs in health maintenance organizations (HMO) in the USA.[Citation16,Citation27,Citation29] Three authors targeted depressed FAs.[Citation17,Citation18,Citation26] One study targeted FAs with general mental health problems.[Citation24] Six trials studied FAs with medically unexplained symptoms (MUS) or somatization.[Citation19,Citation21–23,Citation30–31] Three studies did not define a specific type of FAs.[Citation20,Citation25,Citation28] One study used students attending a university health centre.[Citation14] Another study targeted the so-called ‘distressed FAs’ (those with a sum score 1 standard deviation above population mean on the symptom checklist—revised).[Citation15]
Definitions of frequent attenders
Definition of FA varied considerably. In particular, three studies used pre-set centiles of the attendance distribution. Thirteen used a cut-off number, ranging from 5–24 consultations per year (we normalized the number of consultations to a period of one year). Some authors chose this number based on the mean visit frequency for the (sub)group or clinic. One author used a ‘healthcare utilization algorithm’ to select FAs.[Citation29] The time window, in which the consultation frequencies were assessed, also differed from three months to three years.[Citation26,Citation28]
Interventions
shows a full list of interventions. Interventions varied considerably per subtype of FA and were listed by subtype of FAs.
Older FAs. Two out of three studies used a group intervention. Beck et al. used a group intervention led by a GP and a nurse consisting of health education, prevention measures such as house safety, exercise, nutrition, and mutual support.[Citation16] Haas et al. compared health education classes to enhanced mental benefits, including psychiatric evaluation, psychotherapy visits, and if needed, medication.[Citation27] Shannon et al. investigated referrals to home and community-based services (HCBS).[Citation29]
Depressed FAs. All three studies offered a depression management programme. Katzelnick and Simon refer to the same research programme.[Citation17,Citation18] They implemented a depression management programme, which consisted of a two-hour training, evaluation contacts, antidepressant medication, information material, and assignment of a treatment coordinator. In the third trial, Berghofer et al. assessed a depression management programme, consisting of a treatment algorithm, pharmacotherapy, standardized patient-provider education, and physician and patient counselling.[Citation26]
FAs with MUS or somatoform symptoms. Six studies targeted these FAs using patient or GP education, disclosures of events, acupuncture or cognitive therapy. Larisch targeted FAs with somatoform symptoms by offering six 20-min consultations with their GP over six months.[Citation21] The GPs received 12-h training about treating somatization (role play, video feedback, using a reattribution model). Schilte et al. treated FAs using disclosure of emotionally important events by FAs in two or three meetings with a trained ‘disclosure doctor’.[Citation19] Patients received screening questions and kept a diary about their experiences. Rasmussen used an hour-long ‘health enhancement consultation’ by a nurse with the GP present. Medical records were reviewed by two GPs to assess whether hospitalization was avoidable. Smith et al. intervened by conducting 12 nurse practitioner-led 20-min visits using multi-step patient-centred methods.[Citation22] Treatment included antidepressants, reduction of ineffective medications, exercise, relaxation/physical therapy, and physical disease management. Telephone contact was scheduled between visits. Van Ravesteijn applied eight 2.5-h mindfulness sessions.[Citation31] Patients received information about the sessions, homework assignments and forms to keep a record of their adherence, together with CDs with meditations and exercises. Paterson et al. applied 12 individual sessions (60 min, over a six-month period) of acupuncture by eight acupuncture practitioners.[Citation30]
Distressed FAs. In the only trial that focused on distressed FAs, a diagnostic Interview schedule and an interview was performed by a psychiatrist with the GP present after which a jointly formulated treatment plan was created for each FA [Citation15]. Schreuders et al., in FAs with general mental health problems, tested a ‘problem-solving treatment’ (four to six 2.5–4 h sessions) by trained nurses, to increase the patients’ understanding of the relationship between everyday problems and psychological symptoms.[Citation24]
No specific subtype of FAs. Four studies targeted all patients identified as FAs. Gidron et al. used a guided disclosure protocol.[Citation28] Patients wrote (15 min for three days) about their most stressful experience of the past five years. Controls wrote about neutral topics. Christensen et al. used a patient questionnaire and an invitation for the FAs to contact their GP for a status consultation; information about the project; GP education on frequent attending; and economic incentives for the GP to perform the status consultation.[Citation20] Bellón et al. applied a ‘7 hypothesis + team intervention’ after three GPs received 15-h training in the intervention.[Citation25] The GPs held meetings to share analyses and reflections on their FAs and to make tailored plans for each FA. GPs also received emotional support in these meetings and helped generate strategies to deal with FAs. Finally, Olbrisch compared an educational programme aimed at making students aware of the psychological and social factors that make people prone to illness and inappropriate use of healthcare. It included a question and answer period and a demonstration of systematic deep muscle relaxation of which a relaxation tape or an individual session was offered.[Citation14]
Quality assessment
shows an overview of the quality assessment. Appendix 2 in Supplementary Material, available online, lists the full quality assessments in narrative form.
Table 1. Quality assessment. Notes: The best possible score per study was (9 yes, 0 unclear, 0 no). (The scores can be found in the three columns on the far right). The bottom row lists the number of studies that met the criteria for each quality criterion.
Table 2. Study characteristics and effects.
Study characteristics and effects per outcome
Heterogeneity at the level of patient populations, interventions and outcomes precluded sensible statistical pooling of results (See for the characteristics per study.)
Quality of life
Eight studies measured QoL, of which four were of high quality (with concealed allocation, intention to treat analysis and overall high quality). Six studies used a generic short form scale. One study also used a visual analogue scale (VAS), two the EuroQol-5D. All studies targeted specific types of FAs. Two high-quality studies showed an effect on QoL. Smith et al. intervened by conducting visits using multi-step patient-centred methods including antidepressants, exercise, relaxation training, etc. They reported that the intervention group was more likely to improve (SF36) and the number needed to treat for one patient to improve was 6.4 (95%CI: 0.89–11.89).[Citation23] Katzelnick et al. intervening in depressed FAs, reported a beneficial effect of depression management at 12 months on social functioning, mental health and general health perceptions (SF-20, P < 0.05 for all), but not on physical and role functioning and pain perception.[Citation17] Participants in the mindfulness-based cognitive therapy (MBCT) group reported a greater improvement in mental functioning at the end of treatment (SF-6D: difference 3.9; 95%CI: 0.24–7.6), but between-group differences disappeared after nine months.[Citation31] The general health status (EQ-5D VAS) did not differ at the end of treatment. The other studies have been summarized in .
Morbidity
We found 10 studies that assessed symptoms and (severity of) mental health diseases using various scales, of which four studies were of high quality. Four authors reported modest effects in three research programmes (Katzelnick and Simon referred to the same research programme [Citation17,Citation18]). They reported that patients in the intervention group had on average 47/365 more depression-free days following depression management (95%CI: 26.6–68.2), showed improvements of 9.2 versus 5.6 on the Hamilton score at 12 months for intervention and usual care patients, respectively (P < 0.001). Berghöfer, in a more recent trial targeting depressed FAs, reported no differences on the HAMD-17 at six or 12 months. The intervention group had superior results after six months (56% reduction of the baseline B-PHQ-9 sum score versus 17% in controls; P < 0.002) but not at follow-up [Citation26]. Smith et al. also reported less depressive symptomatology in the treatment group.[Citation23] The calculated inter-group difference after one year was 1.44 (CES-D, 95%CI: –1.23–4.11) for treatment.
Frequency of attendance
Fifteen studies measured effects on the consultation frequency. Five RCTs were deemed to be of high quality. One RCT reported statistically significantly fewer primary care consultations.[Citation25] Bellón et al. reported that the ‘7 hypothesis + team (7H + T) intervention’ group (IG) consulted less compared to the two control groups (C1/C2) after one year.[Citation25] Their intervention consisted of a group of GPs assessing the reasons why patients frequently attend. C1 received usual care by different GPs. C2 received usual care by the GPs that also intervened (IG–C1: –6.27, P = 0.001; IG–C2: –3.62, P = 0.006). Although Shannon et al., who intervened in older FAs, reported that the intervention group was more likely than controls to use GP services (odds ratio (OR): 2.05; P < 0.001), the number of hospital admissions (OR: 0.43; P < 0.01) and hospital days (OR: 0.39, P < 0.05) were more stable in the intervention group which was offered referrals and regular assessments.[Citation29] Ravesteijn et al., who intervened in FAs with MUS using mindfulness-based cognitive therapy, found no difference between the consultation frequencies of both groups.[Citation31] Simon and Katzelnick reported that the depression management programme, which targeted depressed FAs, resulted in more attendance in the intervention group (+1.6 versus -2.0, P = 0.02).[Citation17,Citation18] For the effects on consultation frequency on the other studies, see .
Discussion
Main findings
Large heterogeneity at the levels of interventions, study populations and outcome measures precluded combination of results across 17 RCTs in FAs of primary care. A few trials stood out: Katzelnick et al. and Simon et al. reported modest improvements in QoL and depression-free days using a depression management programme, the effect on morbidity was however not replicated in a recent trial.[Citation17,Citation18,Citation26] Bellón et al., using a patient-tailored approach, reported a modest reduction of consultations.[Citation25] Smith et al., using multi-step patient-centred methods, reported modest improvement of QoL and morbidity in FAs with MUS.[Citation23]
Strengths and limitations
To our knowledge, this systematic review is currently the most comprehensive review of interventions on FAs. We used acknowledged methodology and reported according to PRISMA.[Citation12] We updated our previous systematic review using a more comprehensive search strategy developed by an experienced librarian (FvE) and found 13 additional trials.[Citation11] This could be attributed to not using proximity keywords in a previous review by our team. The enormous variation in FA definition, patient selection methods across trials and settings, however, precluded drawing strong conclusions. A limitation of this review is that we took the effects reported at face value and did not go into detail about the various conceptual models underlying the interventions used. Furthermore, theoretically, randomization takes care of prognostic factors across intervention groups, however, without formally defining an a priori set of relevant prognostic factors implies that post-randomization baseline imbalances in prognostic factors could always be excluded. Although a review protocol existed, at the time it was not disseminated beyond our research group. Another possible limitation of the current review is that only two investigators screened the first 200 search results obtained independently. However, we felt that these 200 were sufficient for DH to become an expert. In addition, the articles that were selected for detailed reading (n = 31) were assessed independently by two investigators against the inclusion criteria. The interrater reliability (Kappa) for this process was 0.718 (SEM: 0.131, 95%CI: 0.461–0.975), suggesting good agreement. In the update of the search, performed in August 2015, two independent investigators resulting in no discordances screened all new hits. Therefore, it is unlikely that we have missed many RCTs.
Comparison with existing literature
Based on a review in 2008 of five studies, Smits et al. concluded that ‘no convincing treatment existed that was effective.’ They noted a small effect in a subgroup of depressed patients in one trial. Recently, a trial looked at depressed patients but the (subgroup) effect reported by Katzelnick and Simon was not replicated.[Citation26] The current review confirms the considerable heterogeneity at the level of patients, interventions, and outcome measures reported previously.[Citation11]
Implications for research and practice
Most trials defined FAs using a cut-off based on an annual number of visits irrespective of sex, age and physician’s work style. Selection that takes into account these factors is more appropriate and avoids the risk of over-representing elderly women.[Citation5,Citation10] Thus, only selecting the top centiles of the attenders, and stratifying for age and sex may allow for meaningful comparisons between countries, interventions, and their generalization. In their statistical analyses, trialists’ should focus on between-group differences, not on differences between baseline and follow-up within groups. For instance, one study reported less depression in the treatment group (CES-D), but when we calculated the between-group difference, it was modest.[Citation23] Also, applying a follow-up period of at least a year seems indicated, considering that frequent attending does not always persist.[Citation4] Many FAs suffer from MUS and more research involving the selection of FAs and screening, and treating for MUS may be useful. More research is also needed to replicate and validate the modest results in the trials we found. Concerning the high costs of FAs in primary and secondary care, intervention studies should preferably include a cost-effectiveness analysis. A programme as tested by Bellón [Citation25] must be replicated in another RCT. The cost-effectiveness and cost-utility of this programme is currently being tested.[Citation32]. Smith et al. reported an effect in FAs with MUS.[Citation23] Although Katzelnick and Simon found a small effect in depressed FAs, their results have not been replicated.[Citation26]
Conclusion
Frequent attendance is a regular phenomenon in general practice and may be a burden to the GP, practice staff and work-flow. However, it does not seem to allow for a uniform approach or simple interventional procedures, but should perhaps be viewed as a trigger for stratification and differentiation according to individual patient’s underlying conditions, adaptive selection and design of interventions, and professional staff’s reflection on own attitudes and involvement. In-depth analysis among GPs assessing a particular patient’s reasons for frequent attendance and corresponding tailored actions may decrease consultation frequency. An effect might also be present in FAs with MUS; however, more rigorously designed trials are needed to establish this.
Supplementary Material for this article can be found online at http://dx.doi.org/10.3109/13814788.2016.1161751
Download PDF (114.3 KB)Acknowledgements
The authors thank Eric Verhoeff and Jeroen Kruidenier for their critical comments to earlier drafts of the manuscript.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Gill D, Sharpe M. Frequent consulters in general practice: a systematic review of studies of prevalence, associations and outcome. J Psychosom Res. 1999;47:115–130.
- Wamoscher Z. The returning patient: a survey of patients with high attendance rate. J Coll Gen Pract. 1966;11:166–173.
- Backett E, Heady J, Evans JCG. Studies of a general practice. Br Med J. 1954;110:109–114.
- Smits FT, Brouwer HJ, ter Riet G, et al. Epidemiology of frequent attenders: a 3-year historic cohort study comparing attendance, morbidity and prescriptions of one-year and persistent frequent attenders. BMC Public Health 2009;9:36.
- Vedsted P, Christensen MB. Frequent attenders in general practice care: a literature review with special reference to methodological considerations. Public Health 2005;119:118–137.
- Gill D, Sharpe M. Frequent consulters in general practice: a systematic review of studies of prevalence, associations and outcome. J Psychosom Res. 1999;47:115–130.
- Katon W, Von KM, Lin E, et al. Distressed high utilizers of medical care. DSM-III-R diagnoses and treatment needs. Gen Hosp Psychiatry 1990;12:355–362.
- Smits FT, Brouwer HJ, Zwinderman AH, et al. Morbidity and doctor characteristics only partly explain the substantial healthcare expenditures of frequent attenders: A record linkage study between patient data and reimbursements data. BMC Fam Pract. 2013;14:138.
- Dowrick CF, Bellón JA, Gomez MJ. GP frequent attendance in Liverpool and Granada: the impact of depressive symptoms. Br J Gen Pract. 2000;50:361–365.
- Smits FT, Mohrs JJ, Beem EE, et al. Defining frequent attendance in general practice. BMC Fam Pract. 2008;9:21.
- Smits FT, Wittkampf KA, Schene AH, et al. Interventions on frequent attenders in primary care. A systematic literature review. Scand J Prim Health Care .2008;26:111–116.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Br Med J. 2009 21;339:b2700.
- Khan KS, ter Riet G, Popay J, et al. Study quality assessment. Undertaking systematic reviews of research on effectiveness CRD’s guidance for carrying out or commissioning reviews: 2. NHS Centre for Reviews and Dissemination (CRD): University of York, York; 2001.
- Olbrisch ME. Evaluation of a stress management program for high utilizers of a prepaid university health service. Med Care 1981;19:153–159.
- Katon W, Von Korff M, Lin E, et al. Randomized trial of psychiatric consultation with distressed high utilizers. Gen Hosp Psychiatry 1992;14:86–98.
- Beck A, Scott J, Williams P, et al. A randomized trial of group outpatient visits for chronically ill older HMO members: the Cooperative Health Care Clinic. Gen Hosp Psychiatry 1990;12:355–362.
- Katzelnick DJ, Simon GE, Pearson SD, et al. Randomized trial of a depression management program in high utilizers of medical care. Arch Fam Med. 2000;9:345.
- Simon GE, Manning WG, Katzelnick DJ, et al. Cost effectiveness of systematic depression treatment for high utilizers of general medical care. Arch Gen Psychiatry 2001;58:181–187.
- Schilte AF, Portegijs PJ, Blankenstein AH, et al. Randomised controlled trial of disclosure of emotionally important events in somatisation in primary care. Br Med J. 2001;323:86.
- Christensen MB, Christensen B, Mortensen JT, et al. Intervention among frequent attenders of the out-of-hours service: a stratified cluster randomized controlled trial. Scand J Prim Health Care 2004;22:180–186.
- Larisch A, Fisch V, Fritzsche K. Cost-effectiveness of psychosocial interventions for somatising patients by the general practitioner. Z Klin Psychol Psychother. 34:282–290.
- Rasmussen NH, Furst JW, Swenson-Dravis DM, et al. Innovative reflecting interview: effect on high-utilizing patients with medically unexplained symptoms. Dis Manag. 2006;9:349–359.
- Smith RC, Lyles JS, Gardiner JC, et al. Primary care clinicians treat patients with medically unexplained symptoms: a randomized controlled trial. J Gen Intern Med. 2006;21:671–677.
- Schreuders B, van Marwijk H, Smit J, et al. Primary care patients with mental health problems: outcome of a randomised clinical trial. Br J Gen Pract. 2007;57:886.
- Bellón JA, Rodríguez-Bayón A, de Dios Luna J, et al. Successful GP intervention with frequent attenders in primary care: randomised controlled trial. Br J Gen Pract. 2008;58:324.
- Berghöfer A, Hartwich A, Bauer M, et al. Efficacy of a systematic depression management program in high utilizers of primary care: a randomized trial. BMC Health Serv Res. 2012;12:298.
- Haas LJ, Spendlove DC, Silver MP. Expanding mental health services to older high utilizing HMO patients: a pilot study. J Clin Psychol Med Settings 2001;8:189–197.
- Gidron Y, Duncan E, Lazar A, et al. Effects of guided written disclosure of stressful experiences on clinic visits and symptoms in frequent clinic attenders. Fam Pract. 2002;19:161–166.
- Shannon GR, Wilber KH, Allen D. Reductions in costly healthcare service utilization: findings from the care advocate program. J Am Geriatr Soc. 2006;54:1102–1107.
- Paterson C, Taylor RS, Griffiths P, et al. Acupuncture for ‘frequent attenders’ with medically unexplained symptoms: a randomised controlled trial (CACTUS study). Br J Gen Pract. 2011;61:e295–305.
- van Ravesteijn H, Lucassen P, Bor H, et al. Mindfulness-based cognitive therapy for patients with medically unexplained symptoms: a randomized controlled trial. Psychother Psychosom. 2013;82:299–310.
- Bellón JA. Effectiveness of the “7H” intervention to reduce frequent attendance in primary care [Internet]. Ongoing trial: clinicaltrials.gov Identifier: NCT01151969 [cited 2016 Jan 20]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01151969