Patients with hereditary retinoblastoma (Rb) have an increased risk of developing pineoblastoma.Citation1 Unfortunately, most patients with concurrent pineoblastoma do not survive; KiveläCitation2 has shown that only patients who were diagnosed by screening and had a tumor smaller than 15 mm had a chance of survival. However, not all pineal lesions found with screening at the time of retinoblastoma diagnosis are pineoblastomas. Pineal cysts are a relatively common finding in the general population. In children up to 5 years of age the prevalence of pineal cysts diagnosed with magnetic resonance imaging (MRI) is 2.0%.Citation3 Potentially, about 50% of pineoblastomas can be found at baseline screening and another 25% during the first year after Rb diagnosis.Citation2 Therefore, baseline screening of the brain in newly diagnosed retinoblastoma is currently advised in imaging guidelines.Citation4 In a recent study Rodjan and colleaguesCitation5 showed that the majority of pineoblastomas in Rb patients had a cystic appearance. Consequently baseline brain imaging with extended follow-up of pineal cysts might help early detection of pineoblastoma. We present a rare case of unilateral familial retinoblastoma and a suspicious cystic pineal gland on brain MR imaging that progressed into a pineoblastoma.
Case Report
Our patient, a 13-day-old girl, was diagnosed with familial retinoblastoma of the right eye. She underwent bilateral external beam radiotherapy (EBRT) over 5 weeks from the age of 1 month, fractioned at five times a week 2 Gy up to an aimed dose of 50 Gy per eye. The pineal gland was not in the radiation field, but unfortunately we could not obtain detailed dosimetric information. At the age of 19 months a new tumor in the right eye was treated with cryotherapy. An MRI examination – performed at that time for tumor staging of the eye tumor – also included T1-weighted sagittal images of the brain showing a prominent pineal gland measuring 9 × 6 mm with a multi-cystic anterior part and a solid posterior part (). The images revealed no other abnormalities. Due to the prominent size and the multi-cystic component of the pineal gland a follow-up MR examination was indicated. Because the patient was transferred to another hospital, a follow-up MRI examination was performed 9 months later. The follow-up scan showed an increase in size of the pineal gland to 12 × 8 mm, slightly compressing the tectal plate of the mesencephalon without hydrocephalus. Besides a multi-cystic part and a solid part – as was visible 9 months before – this time a fine nodular aspect of the cystic wall was identified on sagittal T2-weighted images (). The solid part of the pineal gland showed prominent contrast enhancement; there were no signs of other brain abnormalities (), particularly no intracranial or spinal leptomeningeal enhancement. Because of the increase in size of the pineal gland, progression of the solid component, the fine nodular aspect of the wall, and pronounced contrast enhancement, the lesion was suspicious for pineoblastoma. Analysis of the cerebrospinal fluid revealed no abnormalities.
FIGURE 1. Sagittal T1-weighted magnetic resonance image (A) of the brain, showing a prominent pineal gland (arrow) with a multi-cystic anterior part and a solid posterior part. Sagittal T2-weighted magnetic resonance image (B), showing a fine nodular aspect of the cystic wall (arrowheads). The T1-weighted magnetic resonance image (C) shows prominent contrast enhancement of the solid part of the pineal gland combined with an increase in diameter compared with image (A). The histopathologic view (D) of the pineal tumor tissue stained with hematoxylin and eosin shows no necrosis or microvascular proliferation (original magnification × 2.5). At a higher magnification (E) moderately polymorphous cells with little cytoplasm and relatively large nuclei were visible; cells in a mitotic stage could easily be seen (original magnification × 40).
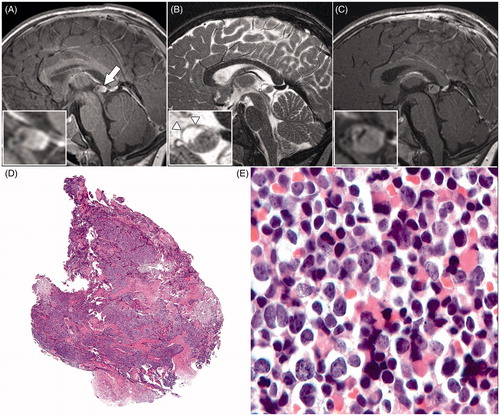
One month after the MRI examination the lesion was surgically removed. In the period until the resection the patient did not show any clinical symptoms. One day after the resection, magnetic resonance images showed a completely resected pineal gland. Histopathologically, the tumor showed no necrosis or microvascular proliferation (). The resected tumor consisted of moderately polymorphous cells with little cytoplasm and relatively large nuclei (). No rosettes (which are typical for pineocytoma) were visible. Cells in a mitotic stage could easily be found (). Tumor cells were negative for glial fibrillary acidic protein (GFAP), Cytokeratin AE1/AE3, and chromogranin, but strongly positive for synaptophysin. MIB-1 (cell proliferation marker) labeling was estimated at 30–40%. The tumor was diagnosed as a pineoblastoma WHO grade IV.Citation6
After surgery the patient subsequently received chemotherapy according to the ARET0321 protocol stage 4b,Citation7 consisting of four courses of vincristine, cisplatin, cyclophosphamide and etoposide followed by high doses of carboplatin, thiotepa and etoposide concluded with autologous stem cell reinfusion. The most recently performed MRI examination (35 months after resection) showed no tumor recurrence. Up until the last visit to the outpatient clinic (35 months since resection, 44 months since the first brain scan, and 63 months since Rb diagnosis) the patient was clinically doing well.
In our patient, the first MRI examination was not performed at the time of Rb diagnosis, but rather at a new retinoblastoma tumor 19 months later. This raises the question whether this pineal cyst was already present at Rb diagnosis or not, and if so would it have been visible on magnetic resonance images? In a registry-based study, Moll and co-authorsCitation8 have shown that out of seven pineoblastoma patients six were treated with EBRT for retinoblastoma before the age of 1 year suggesting that radiotherapy before this age might increase the risk of developing pineoblastoma. Maybe scattered X-rays from the EBRT in the first months triggered malignant deregulation of the pineal gland, but we are unsure if this could have happened in our patient.
It is a challenge to correctly distinguish a benign pineal cyst from a developing pineoblastoma. A normal pineal gland is no larger than 10 mm with a signal intensity on T1- and T2-weighted images comparable to that of grey matter.Citation9,Citation10 On MR images cystic pineal lesions present as isointense lesions relative to cerebrospinal fluid with contrast enhancement of the wall on T1-weighted images and hyperintense lesions on T2-weighted images. Pineoblastomas can either present as a completely solid lesion, as a completely cystic lesion, or as a combination of both.Citation5 Whereas benign pineal cysts usually present as a cystic pineal gland with a discrete rim contrast enhancement and a thin smooth wall, a pineal cyst is considered suspicious when the wall is irregularly thickened and more than 2 mm thick.Citation11,Citation12 Intracystic septations can be seen in both benign and malignant lesions, but a fine nodular aspect of the wall is considered to be a suspicious finding.Citation9,Citation10 If a pineal cyst remains stable on follow-up MR images, this is considered to be a benign cyst and no further follow-up is recommended.
In conclusion, follow-up is not long enough to declare this patient cured from pineoblastoma, but she is still in complete remission 32 months after the end of therapy, and if anything it underlines the importance of early detection followed by adequate treatment. If there is any suspicion concerning the pineal gland on magnetic resonance images of the brain in retinoblastoma patients we recommend performing follow-up MRI examination. The optimal follow-up schedule is still unknown. In a multi-center study we are investigating the value of performing follow-up MRI examination of pineal cysts 3 months after baseline brain screening (and if any doubt remains with a third MRI examination after another 3 months).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
FUNDING
M.C.J. is financially supported in part by grants from the ODAS Foundation, Delft, The Netherlands.
References
- Jakobiec FA, Tso MO, Zimmerman LE, Danis P. Retinoblastoma and intracranial malignancy. Cancer 1977;39:2048–2058
- Kivelä T. Trilateral retinoblastoma: a meta-analysis of hereditary retinoblastoma associated with primary ectopic intracranial retinoblastoma. J Clin Oncol 1999;17:1829–1837
- Al-Holou WN, Garton HJL, Muraszko KM, et al. Prevalence of pineal cysts in children and young adults. Clinical article. J Neurosurg Pediatr 2009;4:230–236
- De Graaf P, Göricke S, Rodjan F, et al. Guidelines for imaging retinoblastoma: imaging principles and MRI standardization. Pediatr Radiol 2012;42:2–14
- Rodjan F, de Graaf P, Brisse HJ, et al. Trilateral retinoblastoma: neuroimaging characteristics and value of routine brain screening on admission. J Neurooncol 2012;109:535–544
- Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97–109
- Children’s Oncology Group, National Cancer Institute. Combination chemotherapy, autologous stem cell transplant, and/or radiation therapy in treating young patients with extraocular retinoblastoma. In: ClinicalTrials.gov. 2007. Accessed February 4, 2013 http://clinicaltrials.gov/ct2/show/study/NCT00554788
- Moll AC, Imhof SM, Schouten-Van Meeteren AY, et al. Second primary tumors in hereditary retinoblastoma: a register-based study, 1945–1997. Ophthalmology 2001;108:1109–1114
- Lacroix-Boudhrioua V, Linglart A, Ancel PY, et al. Pineal cysts in children. Insights Imaging 2011;2:671–678
- Pastel DA, Mamourian AC, Duhaime A-C. Internal structure in pineal cysts on high-resolution magnetic resonance imaging: not a sign of malignancy. J Neurosurg Pediatr 2009;4:81–84
- Rodjan F, de Graaf P, Moll AC, et al. Brain abnormalities on MR imaging in patients with retinoblastoma. AJNR Am J Neuroradiol 2010;31:1385–1389
- Popovic MB, Balmer A, Maeder P, et al. Benign pineal cysts in children with bilateral retinoblastoma: a new variant of trilateral retinoblastoma? Pediatr Blood Cancer 2006;46:755–761

