Abstract
Chemical investigations from a foliar extract of Araucaria excelsa (Lamb.) (Araucariaceae) resulted in the identification of seven phenolic metabolites including one flavananol, one flavananol 3-O-glycoside, four C-glycoside flavonoids, and one phenolic acid. Structures were elucidated by spectral determination including: UV, NMR and MS analysis. Moderate antioxidant activity was observed with a 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging assay in comparison with the reference antioxidant ascorbic acid.
Introduction
Araucariaceae is an ancient family of conifers consisting of three genera with 41 extant species; the genus Araucaria consists of evergreen coniferous trees with the species Araucaria excelsa (Lamb.) commonly referred to as the Norfolk Island pine. Although not a true pine, the wood of the mature tree is used commercially and the sapling stage is grown worldwide as a houseplant.
The genus Araucaria is rich in essential oils (CitationSuresh, 1995), sesquiterpenes, isoflavones, phenylpropanoids, and biflavonoids (CitationAhmed et al., 2005). Biological activity from the genus includes antipyretic (CitationDhanasekaran et al., 1994), antinociceptive and anti-inflammatory activities (CitationDalvi et al., 1994) from an A. bidwillii (Hook.) ethanol extract; while anticoagulant (CitationSuresh et al., 1994), ulcero-protective (CitationOlawore & Ogunwande, 2005) and analgesic activities (CitationFonseca et al., 2000) were observed in petroleum ether and methanol extracts from members of the Araucaria genus. From the species A. excelsa essential oils, alkaloids, and a biflavone have been identified, although other flavonoid compounds have yet to be characterized (CitationIlyas et al.,, 1978). Here we report the identification of seven phenolic metabolites as well as extract antioxidant activity using a DPPH radical scavenging assay method.
Materials and methods
General experimental procedures
UV spectra were measured on a Shimadzu spectrophotometer model UV-240. 1H NMR (500 MHz, CD3OD or DMSO-d6) and 13C NMR (125 MHz, CD3OD or DMSO-d6) were recorded on Varian Inova-500 NMR (Palo Alto, CA), while two dimensional spectra (500 MHz, CD3OD or DMSO-d6) were measured on a Bruker spectrophotometer. The chemical shifts were given in δ values (ppm) with TMS as an internal standard. Electrospray ionization mass spectra (ESI-MS) were collected on a Finnigan MAT TSQ 70 spectrometer.
Column chromatography was performed with Sephadex LH-20 (Fluka). TLC was run on pre-coated silica gel type 60 (Merck) aluminum-backed plates. HPLC: C18 column, with Agilent Technologies Chemstation software, UV detector at 330 or 256 nm, flow rate 0.2 mL/min with an acetonitrile-water isocratic solvent system. PC: Whatman No. 1 and 3 MM using the solvent systems: 1) BAW (n-butanol: AcOH: H2O, 4:1:5, upper layer); 2) 15% AcOH (AcOH: H2O); (3) H2O.
Plant material
Araucaria excelsa shoots were collected from the National Research Centre Garden in the summer of 2006. A voucher specimen (A35) identified by Nabil H. El-Sayed was deposited in the National Research Centre Herbarium, Cairo, Egypt.
Extraction and isolation
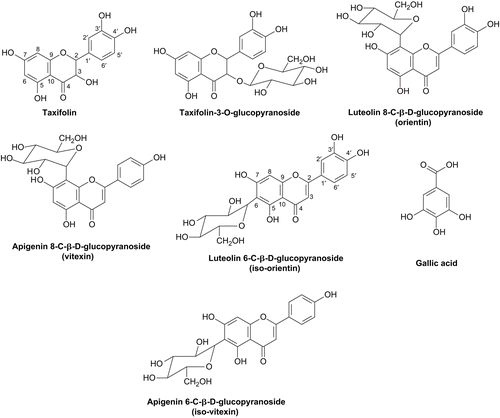
For bioassays, 10 g of dried needles were powdered and then successively extracted with analytical grade acetone, EtOAc, and MeOH (100 mL/solvent) in a Soxhlet extractor for 2 h; extracts were concentrated to dryness under reduced pressure at 45° ± 5°C to yield three oily-greenish brown extracts weighing 210, 200, and 370 mg respectively. All extracts were refrigerated until further use. For phytochemical analysis, 500 g of dried needles were extracted with 70% MeOH (3 L) in a Soxhlet extractor for 48 h; the extract was concentrated under reduced pressure to yield a brown solid weighing 3.6 g. The extract was applied to a Sephadex LH-20 column and eluted first with water then water/MeOH mixtures with increasing MeOH; the final purification was obtained using preparative paper chromatography and HPLC employing the respective solvent systems listed in the general experimental procedures. Seven compounds were purified and identified including taxifolin and its 3-O-glucoside, vitexin, isovitexin, orientin, iso-orientin, and gallic acid.
Total polyphenolic content determination
Total phenolic content for each extract was determined by a Folin-Ciocalteu method (CitationFolin & Ciocalteu, 1927). Briefly stated, an extract aliquot (10 µL, 1 mg/mL) was mixed with 50 µL of Folin Ciocalteu phenol reagent (10× concentration) and following 5 min, 20% saturated Na2CO3 (40 µL) was added; after 1 h dark incubation an absorbance measurement at 725 nm was taken using a microplate ELISA reader (Biorad, Hercules, CA); triplicate measurements for each extract were taken. A gallic acid standard curve was run to calibrate the phenolic content. The total polyphenol content (TPC) for each extract was expressed as mg gallic acid equivalents per g of plant material on a dry-weight basis.
1,1-Diphenyl-2-picryl hydrazyl (DPPH) assay
Antioxidant activity for plant extracts and an ascorbic acid standard were assessed on the basis of the radical scavenging effect of the stable DPPH free radical (CitationGamez et al., 1998). Weighed extracts were dissolved in distilled DMSO; since aqueous methanol extracts were not fully soluble in DMSO (even with a 5 min ultrasonication treatment), all extracts were filtered (0.22 µm pore size) with only the soluble portion collected for further analysis. The DMSO extract (10 μL) or an ascorbic acid aqueous standard (from 0 to 100 µg/mL) was added to 90 µL of 100 µM DPPH (Sigma, St. Louis, MO) in methanol solution in a 96-well micro-titer plate. After incubation in the dark at 37°C for 30 min, the decrease in absorbance of each solution was measured at 515 nm using an ELISA micro-plate reader (Bio Rad, model 550). Absorbance of a blank containing an equal volume of DMSO and DPPH solution was prepared and measured as well. Percentage DPPH radical scavenging activity = 1 - [Asample/Acontrol] × 100, where Asample and Acontrol are absorbance of sample and control respectively. The concentration of sample required to scavenge 50% of DPPH (SC50) was determined. Decreasing of the DPPH solution absorbance indicates an increase of the DPPH radical scavenging activity. The experiment was carried out in triplicate.
Taxifolin Rf-values × 100:78 (BAW), 59 (15% AcOH). UV (λmaxnm) in MeOH: 290, 326 sh. + NaOMe: 326, decomp. + NaOAc: 289 sh. 327 + NaOAc/H3BO3: 292,327 sh. + AlCl3: 280 sh. 312, 375 + AlCl3/HCl: 312, 375, sh = shoulder. ESI-MS (M)+ m/z = 304; isolated 17 mg. 1H NMR (CD3OD): δ (ppm) 6.96 (d, J = 1.8 Hz, H-2′); 6.8 (d, J = 7.1 Hz, H-5′); 6.76 (dd, J = 7.1 and 1.8, H-6′); 5.93 (d, J = 2.1 Hz, H-8); 5.9 (d, J = 2.4 Hz, H-6); 4.8 (d, J = 6.3 Hz, H-2); 4.16 (m, H-3). 13C NMR: 79.87 (C-2); 67.48 (C-3); 192.26 (C-4); 157.67 (C-5); 96.36 (C-6); 158 (C-7); 95.87 (C-8); 157.36 (C-9); 100.05 (C-10); 132.28 (C-1′); 115.31 (C-2′); 145.77 (C-3′); 145.94 (C-4′); 115.73 (C-5′); 119.38 (C-6′).
Taxifolin 3-O-glucopyranoside Rf-values × 100:64 (BAW), 71 (15 % AcOH). UV (λmaxnm) in MeOH: 292, 327 sh. + NaOMe: 346, 328 + NaOAc: 290 sh., 329 + NaOAc/H3BO3: 294, 335 sh. + AlCl3: 238, 316, 375 sh. + AlCl3/HCl: 287 sh. 314, 378. ESI-MS (M)+ m/z = 466; isolated 15 mg. 1H NMR (CD3OD): δ (ppm) 6.9 (d, J = 1.8 Hz, H-2′); 6.82 (d, J = 7.1 Hz, H-5′); 6.7 (dd, J = 7.1 and 1.8 Hz, H-6′); 5.53 (d, J = 1.8 Hz, H-8); 5.48 (d, J = 1.8 Hz, H-6); 5.3 (d, J = 6.6 Hz, H-1 of glucose), 4.63 (d, J = 6.3 Hz, H-2); 4.13 (m, H-3). 13C NMR: aglycone moiety: 79.43 (C-2); 73.12 (C-3); 189.26 (C-4); 160.67 (C-5); 95.36 (C-6); 162 (C-7); 95.17 (C-8); 159.36 (C-9); 100 (C-10); 126.28 (C-1′); 114.31 (C-2′); 144.77 (C-3′); 144.94 (C-4′); 115.33 (C-5′); 118.38 (C-6′); sugar moiety: 102.81 (C-1″); 73.44 (C-2″); 76.82 (C-3″); 69.91 (C-4″);76.75 (C-5″); 61.2 (C-6″).
Vitexin (apigenin 8-C-β-glucopyranoside) Rf-values × 100: 41 (BAW), 24 (15% AcOH), 06 (H2O).UV (λmaxnm) in MeOH: 271, 300 sh., 335 + NaOMe: 277, 329, 395 + NaOAc: 280, 300, 379 + NaOAc/H3BO3: 270, 329 sh., 345 + AlCl3: 275 sh., 306, 352, 386 + AlCl3/HCl: 278, 303,343, 383. ESI-MS (M)+ m/z = 432; isolated 23 mg. 1H NMR (DMSO): δ (ppm) 8.03 (d, J = 8 Hz, H-2′, 6′); 6.9 (d, J = 8 Hz, H-3′,5′); 6.77 (s, H-3); 6.27 (s, H-6); 4.63 (d, J = 9.5 Hz, H-1″ of glucose); 3.1-3.9 (m, rest of sugar protons). 13C NMR: 164.03 (C-2); 102.61 (C-3); 181.9 (C-4); 160.67 (C-5); 98.93 (C-6); 162.87 (C-7); 104.2 (C-8); 155.36 (C-9); 104.2 (C-10); 121,28 (C-1′); 128.31 (C-2′,6′); 160.94 (C-4′); 116.33 (C-3′, 5′); sugar moiety: 73.9 (C-1″); 71.42 (C-2″); 78.72 (C-3″); 69.91 (C-4″); 81.43 (C-5″); 61.5 (C-6″).
Iso-vitexin (apigenin 6-C-β-glucopyranoside) Rf-values × 100: 57 (BAW), 44 (15% AcOH), 16 (H2O). UV (λmaxnm) in MeOH: 273, 335, 335. + NaOMe: 275, 329, 396. + NaOAc: 280, 303, 385 + NaOAc/H3BO3: 274, 346, 406 sh. + AlCl3: 264 sh., 278, 304, 356, 384 + AlCl3/HCl: 260 sh, 280, 302, 344, 380. ESI-MS (M)+ m/z = 432; isolated 22 mg. 1H NMR (DMSO): δ (ppm) 8 (d, J = 8 Hz, H-2′, 6′); 6.93 (d, J = 8 Hz, H-3′, 5′); 6.78 (s, H-3); 6.5 (s, H-8); 4.68 (d, J = 9 Hz, H-1″ of glucose); 3.1-3.8 (m, rest of sugar protons). 13C NMR: 163.83 (C-2); 102.91 (C-3); 181.9 (C-4); 156.67 (C-5); 108.93 (C-6); 163.87 (C-7); 94.2 (C-8); 161.36 (C-9); 103.2 (C-10); 121,23 (C-1′); 128.41 (C-2′,6′); 160.64 (C-4′); 116.33 (C-3′,5′); sugar moiety: 79 (C-1″); 73.42 (C-2″); 70.72 (C-3″); 70.91 (C-4″); 81.33 (C-5″); 61.6 (C-6″).
Orientin (luteolin 8-C-β-glucopyranoside) Rf-values × 100: 32 (BAW), 17 (15% AcOH), 07 (H2O). UV (λmaxnm) in MeOH: 251, 369, 348 + NaOMe: 269, 278, 324 sh., 377 + NaOAc: 278, 323 sh., 385 + NaOA/H3BO3: 277, 375, 430 sh. + AlCl3: 275, 305 sh., 355, 425 +AlCl3/HCl: 265 sh, 278, 298, 356, 385. Isolated 12 mg. 1H NMR (DMSO): δ (ppm) 7.39 (dd, J = 2 and 8 Hz, H-6′); 7.43 (d, J = 2 Hz, H-2′); 6.93 (d, J = 8 Hz, H-5′); 6.45 (s, H-3); 6.1 (s, H-6); 4.7 (d, J = 10 Hz, H-1″ of glucose); 3.2-3.9 (m, rest of sugar protons). 13C NMR: 164.3 (C-2); 102.41 (C-3); 182 (C-4); 160.67 (C-5); 98.13 (C-6); 162.7 (C-7); 105.8 (C-8); 156.6 (C-9); 103.9 (C-10); 122,13 (C-1′); 114 (C-2′); 146.64 (C-3′); 149.33 (C-4′); 115.9 (C-5′); 119.31 (C-6′); sugar moiety: 72.9 (C-1″); 70.42 (C-2″); 78.72 (C-3″); 70.11 (C-4″); 81.33 (C-5″); 61.3 (C-6″).
Iso-orientin (luteolin 6-C-β-glucopyranoside) Rf-values × 100: 41 (BAW), 35 (15% AcOH), 09 (H2O). UV (λmaxnm) in MeOH: 242 sh., 255, 271, 349 + NaOMe: 267, 278 sh., 337 sh., 406 + NaOAc: 276, 323 sh., 393 + NaOA/H3BO3: 265, 377, 430 sh. + AlCl3: 278, 302 sh. 332, 429 + AlCl3/HCl: 265 sh. 279, 296 sh., 361, 384. Isolated 11 mg. 1H NMR (DMSO): δ (ppm) 7.39 (dd, J = 2.5 and 8 Hz, H-6′); 7.4 (d, J = 2.5 Hz, H-2′); 6.93 (d, J = 8 Hz, H-5′); 6.59 (s, H-3); 6.53 (s, H-8); 4.82 (d, J = 10 Hz, H-1″ of glucose); 3-3.9 (m, rest of sugar protons). 13C NMR: 163.3 (C-2); 102.71 (C-3); 181.7 (C-4); 160.57 (C-5); 108.7 (C-6); 163 (C-7); 93.4 (C-8); 156.2 (C-9); 103.3 (C-10); 121,30 (C-1′); 113.2(C-2′); 145.64 (C-3′); 149.53 (C-4′); 115.9 (C-5′); 118.83 (C-6′); sugar moiety: 78.9 (C-1″); 72.42 (C-2″); 70.82 (C-3″); 71.13 (C-4″); 81.2 (C-5″); 61.5 (C-6″).
Gallic acid Rf-values × 100: 78 (BAW), 56 (15% AcOH). UV (λmaxnm) in MeOH: 272 + NaOMe: decomposition + AlCl3: 277. Isolated 42 mg. 1H NMR: δ (ppm) 7.15 (s, H-2, H-6). 13C NMR: 122.5 (C-1); 110.7 (C-2 and 6); 139.4 (C-3 and 5); 146.5 (C-4); 164.7 (C-7).
Results and discussion
DPPH radical scavenging activity
Organic solvent extraction of Araucaria excels needles were assayed for radical scavenging activity using a DPPH colorimetric assay. The highest DPPH radical scavenging effect was detected in an aqueous methanol extract with a SC50 of 72.5 µg/mL although the antioxidant activity was lower than ascorbic acid (SC50 7.8 µg/mL) that is often used as a positive control because of its high antioxidant activity (). In examining radical scavenging capacity, a trend can be observed that the more polar protic solvents were more effective at extracting the antioxidant components in the Norfolk pine needle extract.
Table 1. Araucaria excelsa extract antioxidant and total polyphenol contents based on DPPH free radical scavenging and Folin-Ciocalteu phenol assay, respectively (mean ± SD of three independent experiments).
Total polyphenol contents (TPC)
As plant phenolics constitute one of the major groups of compounds acting as primary antioxidants or free radical terminators, the total amount of phenolic compounds in the selected plant extracts was determined using the Folin-Ciocalteu method. The aqueous methanol extract exhibited the highest level of polyphenols (). The Folin Ciocalteu phenol reagent was used to obtain an estimate of phenolic compounds present in the extract. Phenolic compounds undergo a complex redox reaction with phosphotungstic and phosphomolybdic acids present in the reagent. However, the assay has been shown not specific to polyphenols in that other oxidizing components can react with the Folin reagent (CitationEscarpa & Gonzalez, 2001; CitationSingleton et al., 1999). In addition, phenolic compounds, depending on the number of phenolic groups present, respond differently to the Folin-Ciocalteu reagent (CitationSingleton et al., 1999). Hence, this may explain the observation that for acetone and ethyl acetate extracts, high TPC values did not correspond to a high antioxidant activity.
Phytochemical analysis
To identify the metabolites responsible for the antioxidant activity observed in the crude extracts, a large scale MeOH extraction of Norfolk pine needles was performed. After chromatography purification, seven phenolic compounds were identified including: taxifolin, taxifolin 3-O-glucopyranoside, vitexin, isovitexin, orientin, iso-orientin, and gallic acid (); chemical structures were confirmed by retention time comparisons with authentic standards as well as spectral comparisons with literature values (CitationMarkham, 1982; CitationHarborne, 1993; CitationAgrawal, 1989).
Declaration of interest
This research was funded in part by the Welch Foundation (Grant D1478) and the Frasch Foundation for Chemical Research.
References
- Agrawal PK (1989): Carbon-13 NMR of Flavonoids. Amsterdam, Elsevier Press, pp. 1–564.
- Ahmed KN, Kumar V, Raja S, Mukherjee K, Mukherjee PK (2005): Anti-nociceptive and anti-inflammatory activity of Araucaria bidwillii Hook. Iran J Pharm Ther 4: 105–109.
- Dalvi SS, Nayak VK, Pohujani SM (1994): Effect of gugulipid on bioavailability of diltiazem and propranolol. J Assoc Phys India 14: 454–455.
- Dhanasekaran S, Ravisankar S, Sumitra SK, Suresh B, Sethuraman M, Rajan S (1994): Pharmacological studies of Araucaria bidwillii Hook. Geobios New Reports 13: 49–52.
- Escarpa A, Gonzalez MC (2001): Approach to the content of total extractable phenolic compounds from different food samples by comparison of chromatographic and spectrophotometric methods. Anal Chim Acta 427: 119–127.
- Folin O, Ciocalteu V (1927): On tyrosine and tryptophan determination in proteins. J Biol Chem 27: 627–650.
- Fonseca FN, Ferreira AJS, Sartorelli P, Lopes NP, Floh EIS, Handro W, Kato MJ (2000): Phenylpropanoid derivatives and biflavones at different stages of differentiation and development of Araucaria angustifolia. Phytochemistry 55: 575–580.
- Gamez EJ, Luyengi L, Lee SK, Zhou LF, Fong HH, Kinghorn AD (1998): Antioxidant flavonoid glycosides from Daphniphyllum calycinum. J Nat Prod 61: 706–708.
- Harborne JB (1993): The Flavonoids Advances in Research Since 1986. London, Chapman and Hall, pp. 1–658.
- Ilyas N, Ilyas M, Rahman W, Okigawa M, Kawano N (1978): Biflavones from leaves of Araucaria excelsa. Phytochemistry 17: 987–990.
- Markham KR (1982): Techniques of Flavonoids Identification. London, Academic Press, pp. 1–114.
- Olawore NO, Ogunwande IA (2005): Analysis of the leaf oil of Araucaria cunninghamii Sweet grown in Nigeria. J Essent Oil Res 459–461.
- Singleton VL, Orthofer R, Lamuela-Raventos RM (1999): Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol 29: 152–178.
- Suresh B, Dhanasekaran S, Elango K (1995): Antipyretic activity of some plants in female albino rats: A preliminary report. Ancient Sci Life 14: 253–257.
- Suresh B, Dhanasekaran S, Kumar RV, Balasubramanian S (1994): Ethnopharmacological studies on the medicinal plants of Nigeria. Indian Drugs 32: 340–352.